文章信息
- 抗CEA和CA19-9的单链抗体在HEK293细胞中的融合表达及亲和力鉴定
- Fusion Expression and Affinity Identification of Two scFvs Against CEA or CA19-9 in HEK293 Cells
- 肿瘤防治研究, 2019, 46(1): 7-13
- Cancer Research on Prevention and Treatment, 2019, 46(1): 7-13
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.0824
- 收稿日期: 2018-06-19
- 修回日期: 2018-10-03
2. 213003 常州,常州市第一人民医院,苏州大学附属第三医院肿瘤科
2. Department of Medical Oncology, The First People's Hospital of Changzhou, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China
近年,肿瘤免疫疗法发展迅速,尤其以嵌合抗原受体(CAR)-T为代表的过继性T细胞疗法。其中,CD19-CAR T更以令人惊叹的临床治疗效果获得了FDA批准[1]。在CAR改造T细胞所涉及的诸多重要环节中,选择肿瘤细胞表面特异性靶点和获取具有合适亲和力的单链抗体基因是最重要的基础要素[2]。癌胚抗原(carcino-embryonic antigen, CEA)是大肠癌组织产生的一种糖蛋白,常作为胃癌、胰腺癌、结直肠癌、乳腺癌、甲状腺髓样癌等肿瘤的标志物,是多种免疫疗法的消化道肿瘤特异性靶点[3-4]。CA19-9是唾液酸化lewis-a血型抗原,在胃肠道等多种癌症诊断中都有一定价值。目前,已有较多靶向CA19-9以提高诊断灵敏度和特异性的研究,但以此为靶点构建CAR-T细胞用于临床治疗的报道[5-6]不多。本研究拟利用基因工程技术,构建带有人IgG1 Fc标签的抗CEA scFv和抗CA19-9 scFv真核表达载体。另外,为提高抗体表达效率,在anti-CEA scFv上游加入人IL-2信号肽,形成三个不同单链抗体融合基因;脂质体转染后,在HEK293细胞中表达三个抗体-Fc融合蛋白,并进行表达检测和亲和力鉴定。以此获得anti-CEA scFv和anti-CA19-9 scFv基因并验证两种单链抗体亲和力,为后续开展以CEA和CA19-9为靶点的肿瘤免疫治疗提供理论和物质基础。
1 材料与方法 1.1 材料菌种E.coli BL21(DE3)和质粒pET-32a(+)购自Novagen公司(美国);Fc/pTT5为含有人IgG1 Fc段基因的pTT5载体,由本实验室保存。HEK293、SW480、CFPAC-1等细胞株均购自中科院上海细胞库(中国);EcoRⅠ、BamHⅠ、XhoⅠ、NcoⅠ等限制性内切酶和Taq酶、T4连接酶等工具酶均购自NEB公司(美国);rProtein-A FF亲和层析柱购自GE公司(美国);鼠抗人IgG Fc单克隆抗体、鼠抗人CEA单克隆抗体、鼠抗人CA19-9单克隆抗体、HRP标记山羊抗鼠IgG H & L多克隆抗体均购自Abcam公司(英国);基因及引物合成由金唯智公司(中国)完成;合成后测序验证由生工生物工程(上海)股份有限公司(中国)完成。以下为anti-CEA scFv上下游引物(P1和P2)和anti-CA19-9 scFv上下游引物(P3和P4),两端均引入酶切位点(5’-EcoRⅠ,3’-NcoⅠ);hIL-2信号肽上游引物(P5);hIL-2信号肽基因合成序列(5’端整合EcoRⅠ酶切位点,3’端引入anti-CEA scFv 5’端重复序列)。P1: 5’-AAGAATTCATGTCCTGGGTCAAAC-3’;P2: 5’-AACCATGGGGCGGCCCTGGGCTCCA-3’;P3: 5’-AAGAATTCATGGCCCAGGTGAAGCT-3’;P4: 5’-AACCATGGTCTTTTAATCTCCAGCT-3’;P5: 5’-AAGAATTCATGAGTGGTTTTGGAGT-3’;hIL-2信号肽:5’-AGAATTCATG AGTGGTTTTT GGAGTCAGTA CATTCTCTTT TCAAATCCTT CTCTGCCCCT TACTGGCAAT TCCTGGGTCA AACAGGCT-3’。
1.2 方法 1.2.1 基因合成和pET32a(+)表达载体构建从GenBank获取两种单链抗体基因序列:抗CEA scFv(AB001737.1)和抗CA19-9 scFv(BD455083.1);其中,AB001737.1为完整读码框;CA19-9 scFv(BD455083.1)需要删除两端多余密码子,并在3’段加入终止子。对两基因序列进行密码子优化,加入酶切位点(5’-BamHⅠ和3’-XhoⅠ)并送基因公司做全基因合成。基因合成后,将含有anti-CEA scFv/pET32a(+)和anti-CA19-9 scFv/pET32a(+)的两种E.coli DH5α在肉汤培养基(LB)抗性平板(50 μg/ml氨芐西林)上进行划线接种,37℃培养过夜。挑取抗性平板上的阳性单菌落,进行扩大培养;提取质粒,并经双酶切鉴定后,送基因公司进行测序验证。
1.2.2 scFv/pET32a(+)转化BL21和表达经测序验证,将纯化后的阳性重组质粒导入感受态E.coli BL21(DE3)中。LB抗性平板筛选后,挑选生长状态良好的单菌落,接种于5 ml LB液体培养基(50 μg/ml氨芐西林)中,37℃振荡,培养过夜。次日,取对数生长期菌液转接于大瓶培养液中,进行扩大培养;加入诱导剂IPTG(0、0.2、0.4、0.6、0.8、1.0、1.2、1.4 mmol/L),30℃诱导表达3 h。10 000 r/min离心10 min,弃上清液,收集菌体,直接煮沸,上样,SDS-PAGE电泳分析;以未诱导的菌体(0 mmol/L IPTG)作阴性对照,以未插入外源基因的空质粒菌体为空白对照。
1.2.3 融合基因合成及pTT5表达载体构建以anti-CEA scFv/pET32a(+)为模板,以P1和P2为引物,使用PCR扩增法删除anti-CEA scFv基因末端终止子,并在基因两端引入酶切位点,获得anti-CEA scFv(-t);循环参数:94℃预变性5 min,94℃变性1 min,55℃退火1 min,72℃延伸1 min,循环30次后72℃延伸10 min。利用同样方法,以P3和P4为引物,以anti-CA19-9 scFv/pET32a(+)为模板,获得anti-CA19-9 scFv(-t)。为进一步探索提高抗体产量可能,使用重叠区基因扩增法,在anti-CEA scFv(-t)基因5’端加入hIL-2信号肽基因;以P5和P2为引物,以IL-2信号肽(含重复区)和anti-CEA scFv(-t)为模板进行PCR扩增,构建IL-2信号肽与anti-CEA scFv(-t)融合基因—IL2s-anti-CEA scFv(-t);循环参数:94℃预变性5 min,94℃变性1 min,55℃退火1 min,72℃延伸90 s,循环30次后72℃延伸10 min。anti-CEA scFv(-t)、anti-CA19-9 scFv(-t)和IL2s-anti-CEA scFv(-t)三种基因扩增产物使用EcoRⅠ和NcoⅠ双酶切,1%琼脂糖凝胶电泳检测。利用胶回收试剂盒纯化抗体基因片段;并与同样双酶切的Fc/pTT5载体片段进行连接(反应体系T4 Ligase和T4 Ligase Buffer),16℃过夜。次日,将连接有抗体基因的载体分别导入感受态DH5α中,并将菌液涂布于LB抗性平板上(含有50 μg/ml氨芐西林),37℃培养过夜。16~24 h后,挑取阳性单菌落,扩大培养,进行酶切和测序鉴定。
1.2.4 不同scFv-Fc/pTT5在HEK293细胞中表达在DMEM培养基(含10%FBS)中接种HEK293细胞,37℃、5%CO2培养箱中培养。测G418筛选浓度和维持浓度。待细胞融合度达到80%时,将HEK293细胞以4×105个/孔密度接种于6孔培养板中;细胞再生长至约70%~80%时,按说明书方法进行脂质体转染,并以未转染的HEK293细胞作为阴性对照。载体和脂质体混合后,室温孵育20 min,加入HEK293细胞中,37℃、5%CO2培养箱中培养。转染24 h后,G418(1 μg/ml)加压筛选;48 h后,每天取5 ml培养物,离心收集细胞。超声法破碎细胞,镜检破碎率,提取上清液和沉淀中的蛋白,SDS-PAGE电泳分析。
1.2.5 rProtein-A FF亲和层析纯化抗体融合蛋白A液:20 mmol/L PBS(pH7.0),B液:0.1 mol/L甘氨酸(pH3.0),C液:1 mol/L Tris(pH8.0)。A液平衡rProtein-A FF层析柱,将含有anti-CEA scFv-Fc/pTT5和anti-CA19-9 scFv-Fc/pTT5的两种细胞总蛋白粗提液分别上样,用A液以相同流速洗脱至读数稳定,再用B液缓慢洗脱;同时,用C液以1/10接样(以内含0.5 ml C液的离心管接收流穿液);最后,用甘氨酸(pH2.5)和C液交替洗脱层析柱,NaN3封柱。
1.2.6 Western blot法检测抗体融合蛋白表达取三个单链抗体融合基因转染HEK293细胞后的培养液浓缩液、细胞破碎后上清液和沉淀中蛋白提取液、纯化产物等各20 μl上样,10%十二烷基硫酸钠聚丙烯酰胺凝胶(SDS-PAGE)电泳,100 mA电流半干转2 h;2% BSA室温封闭2 h,弃掉封闭液,将硝酸纤维素膜置于干净培养皿中,加入5 μg/ml鼠抗人IgG Fc单克隆抗体(anti-Human IgG Fc mAb),4℃保湿过夜;TBST洗3次,每次5~10 min,加入1:2 500稀释的HRP标记山羊抗鼠IgG H & L多克隆抗体,室温放置1.5 h;TBST洗涤3次,每次5~10 min;DAB显色。
1.2.7 基于细胞的竞争性ELISA检测scFv亲和力收获CEA阳性细胞(SW480)和CEA/CA19-9双阳性细胞(CFPAC-1),计数;铺装于96孔板中(50 000个/孔),培养过夜。次日,移除培养基;用150 μl PBS和2% FBS洗涤。弃上清液,每孔加入150 μl 1nmol/L鼠抗人CA19-9 mAb稀释液和鼠抗人CEA mAb稀释液,室温孵育1 h。弃上清液,洗涤(方法同前);每孔加入150 μl不同浓度(浓度梯度:1~100 nmol/L)的anti-CEA scFv-Fc和anti-CA19-9 scFv-Fc(待测抗体),室温孵育1 h。弃上清液,洗涤;每孔加150 μl HRP标记山羊抗鼠IgG H & L多克隆抗体(1:10 000倍稀释),室温孵育1 h。弃上清液,洗涤;每孔加150 μl DAB显色液,15 min后终止反应,利用酶标仪测定450 nm处吸光度值。每个样品重复3孔。根据吸光率制作饱和结合图;评估解离常数(KD),确定能够抵消50% CEA和CA19-9亲本抗体的anti-CEA scFv-Fc和anti-CA19-9 scFv-Fc用量。
2 结果 2.1 表达载体构建及鉴定抗CEA scFv和抗CA19-9 scFv两段基因经编辑和基因合成后,被克隆到pET32a(+),并转入DH5α中。在抗性平板中,随机挑取5个单菌落,提取质粒并进行双酶切(BamHⅠ和XhoⅠ)鉴定;鉴定结果符合两个单链抗体基因大小(anti-CEA scFv,708 bp;anti-CA19-9 scFv,735 bp)。将鉴定后的单链抗体-pET32a(+)重组质粒送上海生工测序鉴定,测序结果与设计序列完全吻合(图略)。
2.2 抗体在原核细胞中的表达测序验证后,将anti-CEA scFv/pET32a(+)和anti-CA19-9 scFv/pET32a(+)表达载体导入感受态E.coli BL21(DE3),IPTG诱导表达3 h后进行SDS-PAGE分析。结果见图 1~2,在40 kDa上方都有过表达条带,与anti-CEA scFv(42.55 kDa)和anti-CA19-9 scFv(43.97 kDa)预测蛋白质量相近;其中,anti-CEA scFv/pET32a(+)在1.0 mmol/L IPTG浓度下表达量最高。而anti-CA19-9 scFv/pET32a(+)则在0.4~0.6 mmol/L IPTG浓度下有较高表达。相反,阴性对照(0 mmol/L IPTG)在40 kDa上方并没有过表达;而空白对照(pET32a)不仅在该处没有过表达,18 kDa处还形成过表达(空载体表达蛋白)。据此判断,在E.coli BL21(DE3)中,anti-CEA scFv和anti-CA19-9 scFv都存在过表达可能。
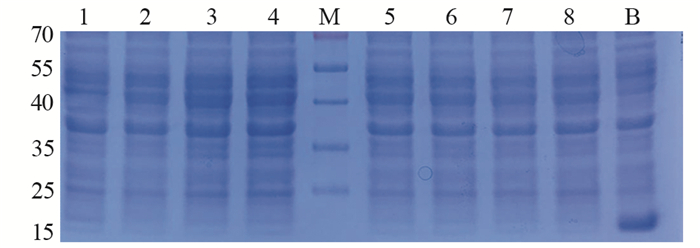
|
| M: protein marker; 1-8: IPTG (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4mmol/L); B: blank, pET32a (+) (no scFv gene) 图 1 Anti-CEA scFv/pET32a(+)在E.coli BL21(DE3)中IPTG诱导表达 Figure 1 Anti-CEA scFv/pET32a (+) expressed in E.coli BL21 (DE3) with IPTG |
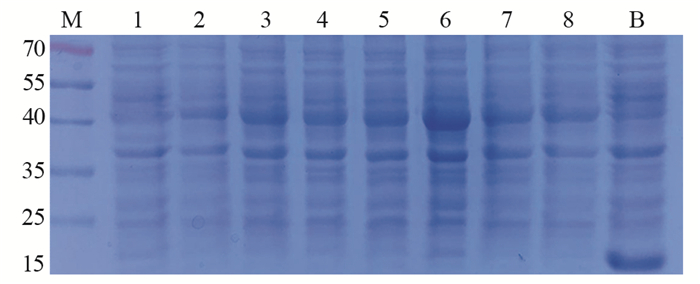
|
| 1-4 and 5-8: IPTG (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4mmol/L); M: protein marker; B: blank, pET32a (+) (no scFv gene) 图 2 Anti-CA19-9 scFv/pET32a(+)在E.coli BL21(DE3)中IPTG诱导表达 Figure 2 Anti-CA19-9 scFv/pET32a (+) expressed in E.coli BL21 (DE3) with IPTG |
anti-CEA scFv(-t)、anti-CA19-9 scFv(-t)和IL2s-anti-CEA scFv(-t)三种基因扩增产物分别与同样经EcoRⅠ和NcoⅠ双酶切的Fc/pTT5质粒连接后,分别导入DH5α中;经酶切鉴定和测序鉴定,证明三个单链抗体基因与Fc/pTT5正确连接,见图 3~5。
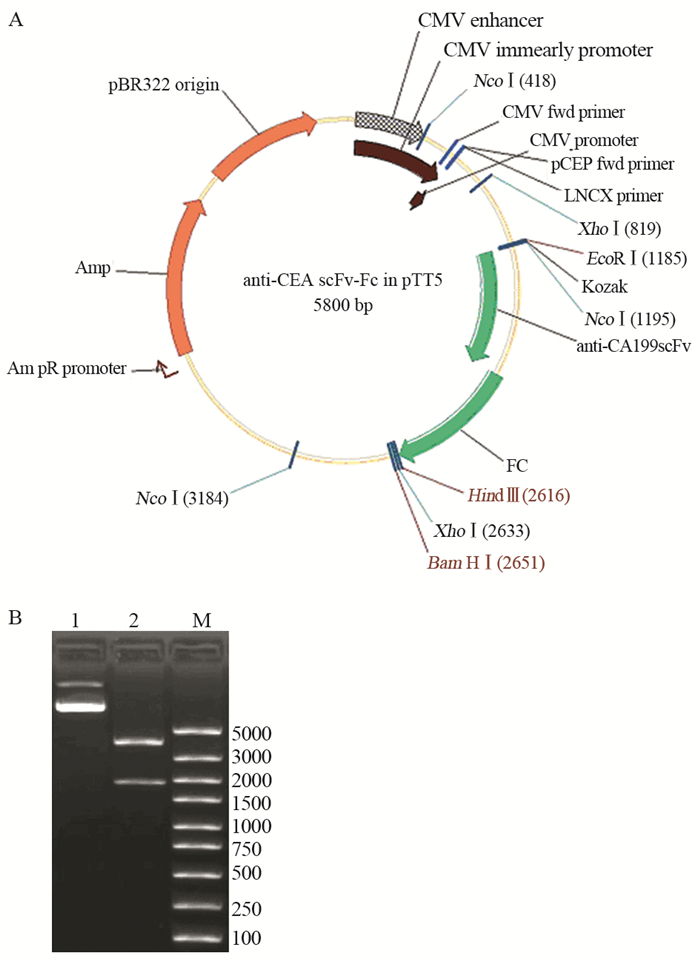
|
| A: the diagrammatic sketch of anti-CA19-9 scFv-Fc/pTT5; B: anti-CA19-9 scFv-Fc/pTT5 digested by double enzymes; 1: the plasmid of anti-CA19-9 scFv-Fc/pTT5; 2: anti-CA19-9 scFv-Fc/pTT5 digested by EcoRⅠ and NcoⅠ; M: DNA marker 图 3 Anti-CA19-9 scFv-Fc/pTT5表达载体构建及酶切鉴定 Figure 3 Construction of anti-CA19-9 scFv-Fc/pTT5 and identification of restriction enzyme |
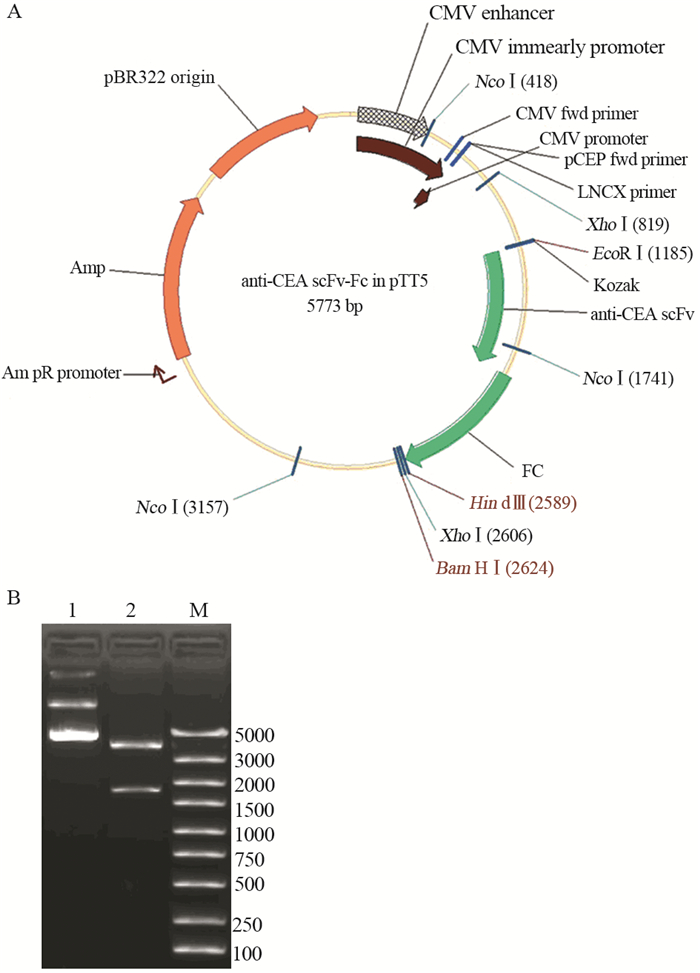
|
| A: the diagrammatic sketch of anti-CEA scFv-Fc/pTT5; B: anti-CEA scFv-Fc/pTT5 digested by double enzymes; 1: the plasmid of anti-CEA scFv-Fc/pTT5; 2: anti-CEA scFv-Fc/pTT5 digested by EcoRⅠand NcoⅠ; M: DNA marker 图 4 Anti-CEA scFv-Fc/pTT5表达载体构建及酶切鉴定 Figure 4 Construction of anti-CEA scFv-Fc/pTT5 and identification of restriction enzyme |
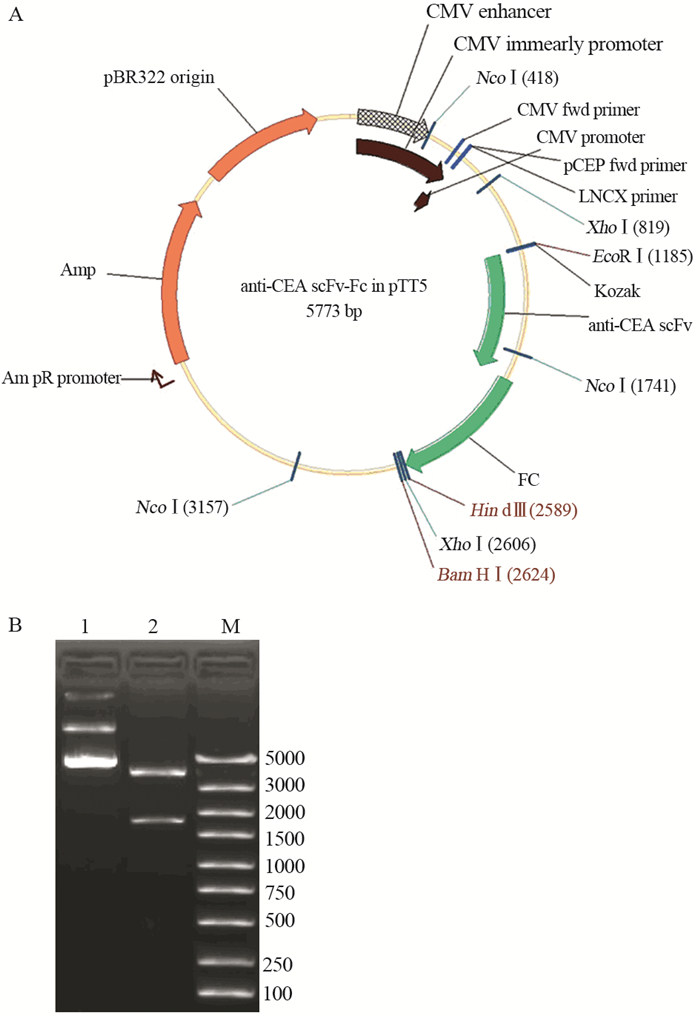
|
| A: the diagrammatic sketch of IL2s-anti-CEA scFv-Fc/pTT5; B: IL2s-anti-CEA scFv-Fc/pTT5 digested by double enzymes; 1: the plasmid of IL2s-anti-CEA scFv-Fc/pTT5; 2: IL2s-anti-CEA scFv-Fc/pTT5 digested by EcoRⅠ and NcoⅠ; M: DNA marker 图 5 IL2s-anti-CEA scFv-Fc/pTT5表达载体构建及酶切鉴定 Figure 5 Construction of IL2s-anti-CEA scFv-Fc/pTT5 and identification of restriction enzyme |
anti-CEA scFv(-t)-Fc/pTT5、anti-CA19-9 scFv(-t)-Fc/pTT5和IL2s-anti-CEA scFv(-t)-Fc/pTT5转染HEK293细胞,G418加压筛选。转染48 h后,取细胞培养物上清液、细胞破碎后上清液和沉淀、纯化和鉴定。Western blot检测结果显示,anti-CEA scFv-Fc和anti-CA19-9 scFv-Fc在细胞沉淀中有表达条带,在细胞上清液中没有表达条带;无论是培养液中还是细胞上清液和沉淀中都未见带有IL-2信号肽基因的IL2s-anti-CEA scFv-Fc表达条带。在转染72 h后,除能看到anti-CEA scFv-Fc和anti-CA19-9 scFv-Fc在细胞沉淀中的表达条带,IL2s-anti-CEA scFv-Fc在细胞上清液和沉淀中也出现表达条带;其中,anti-CEA scFv-Fc经rProtein-A FF柱子纯化后能在Western blot检测中显示清晰条带。所有条带大小与三种抗体融合蛋白预测分子质量相当(anti-CEA scFv-Fc,50.5 kDa;anti-CA19-9 scFv-Fc,51.9 kDa;IL2s-anti-CEA scFv-Fc,52.6 kDa),见图 6~7。
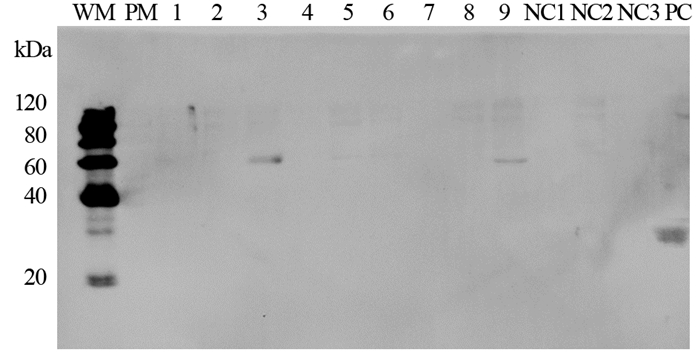
|
| WM:Western marker; PM:Pre-stained marker; 1-3: medium supernatant, cell supernatant and pellet of HEK293 that were transfected with anti-CEA scFv-Fc/pTT5 for 48h; 4-6: medium supernatant, cell supernatant and pellet of HEK293 that were transfected with IL2s-anti-CEA scFv-Fc/pTT5 for 48h; 7-9: medium supernatant, cell supernatant and pellet of HEK293 that were transfected with anti-CA19-9 scFv-Fc/pTT5 for 48h; NC: negative control, medium supernatant, cell supernatant and pellet of HEK293; PC: positive control, recombinant human IgG 图 6 Western blot法检测转染scFv-Fc/pTT5 48h后HEK293细胞 Figure 6 HEK293 cells detected by Western blot after transfection of scFv-Fc/pTT5 for 48h |
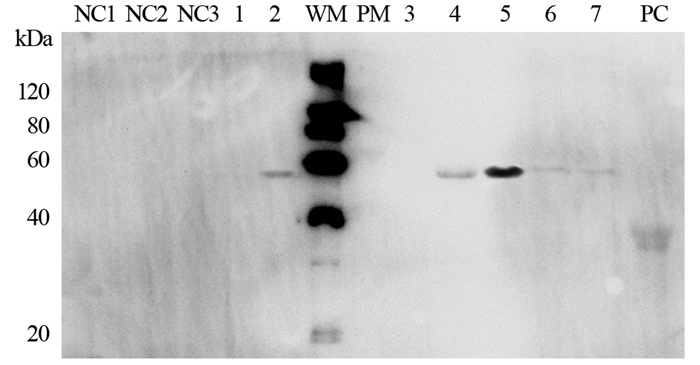
|
| NC: negative control, medium supernatant, cell supernatant and pellet of HEK293; 1-2: cell supernatant and pellet of HEK293 that were transfected with anti-CA19-9 scFv-Fc/pTT5 for 72h; 3-5: cell supernatant and pellet of HEK293 that were transfected with anti-CEA scFv-Fc/pTT5 for 72h; 6-7: cell supernatant and pellet of HEK293 that were transfected with IL2s-anti-CEA scFv-Fc/pTT5 for 72h; PC: positive control, recombinant human IgG 图 7 Western blot法检测转染scFv-Fc/pTT5 72h后的HEK293细胞 Figure 7 HEK293 cells detected by Western blot after transfection of scFv-Fc/pTT5 for 72h |
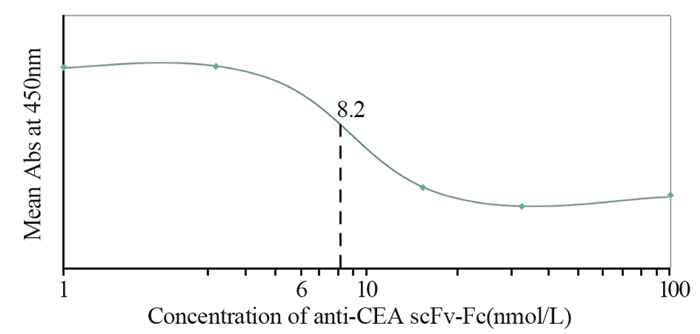
通过竞争结合实验,与完整单克隆抗体相比较,确定两种单链抗体anti-CEA scFv-Fc和anti-CA19-9 scFv-Fc相对亲和力。对每个样品吸光度值进行平均,并绘制饱和结合图。经过ELISA测定和饱和结合曲线图分析,anti-CA19-9 scFv-Fc相对亲和力约为6 nmol/L,anti-CEA scFv-Fc相对亲和力约为8.2 nmol/L,见图 8~9。
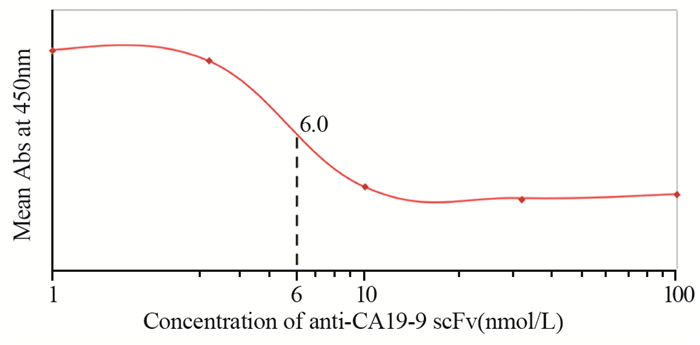
|
| 图 8 Anti-CA19-9 scFv-Fc相对亲和力(与鼠抗人CA19-9单克隆抗体对比) Figure 8 Relative binding affinity of anti-CA19-9 scFv-Fc, compared with anti-human CA19-9 mAb |
|
| 图 9 Anti-CEA scFv-Fc相对亲和力(与鼠抗人CEA单克隆抗体对比) Figure 9 Relative binding affinity of the anti-CEA scFv-Fc, compared with anti-human CEA mAb |
虽然CAR-T在急性淋巴细胞白血病(AML)等癌症治疗方面取得巨大突破,但治疗实体瘤(如胃肠癌、胰腺癌等)还面临诸多挑战[7-8]。主要困难之一就是实体瘤没有像B细胞血液肿瘤CD19那样的特异性标志物[9]。CEA是目前研究最深入的肿瘤标志物之一;在多种内胚层组织、上皮性组织起源细胞及相关疾病中均有表达,在肿瘤细胞免疫逃避及扩散/转移中都具有重要作用;在结直肠癌中表达最高(60%~90%)[10],已被广泛应用于结直肠癌的诊断及靶向治疗。人CEA基因家族位于19号染色体q13.2区,共29个基因,其中CEACAM5(CEA, CD66e)通过糖基磷脂酰肌醇(glycosyl phosphatidylinositol, GPI)连接于细胞膜[11]。CA19-9是一种类黏膜蛋白形式的糖蛋白,也是多种癌症检测的重要参考指标之一。CA19-9对于胰腺癌的特异性为90%,敏感度为81%;被认为是胰腺癌中最具价值的肿瘤标志物[12]。CEA和CA19-9都是位于肿瘤细胞表面的抗原物质,在肿瘤细胞中高表达,可与单克隆抗体结合,非常适合作为肿瘤免疫治疗靶标[13-15]。通过基因工程技术,我们构建了靶向CEACAM5和CA19-9的单链抗体融合蛋白(scFv-Fc)真核表达载体。为提高抗体表达效率和便于检测其表达,通过重叠区基因扩增法[16-18]和亚克隆技术向抗CEA scFv和抗CA19-9 scFv下游引入hIgG1 Fc基因,并在抗CEA scFv上游增加人IL-2信号肽,获得3个单链抗体融合蛋白的表达载体anti-CEA scFv-Fc、IL2s-anti-CEA scFv-Fc和anti-CA19-9 scFv-Fc;将表达载体转入HEK293细胞中进行表达。通过Western blot等证明,三个抗体均可以通过Fc段抗体融合技术在HEK293细胞内进行表达;但培养基中未检测到IL2s-anti-CEA scFv-Fc,表明没有产生分泌型anti-CEA scFv。考虑到IL2s-anti-CEA scFv-Fc在细胞上清和沉淀中均有表达,可以确定IL2信号肽没有发挥作用,具体原因有待下一步研究确定。从结果分析来看,三个抗体在细胞中表达效率仍然不高。为明确抗CEA scFv和抗CA19-9 scFv的亲和力及识别肿瘤细胞的阳性结合率,通过竞争性细胞ELISA检测两种抗体对CEA阳性细胞(SW480)和CEA/CA19-9双阳性细胞(CFPAC-1)进行竞争性结合实验,证实两种抗体可以与肿瘤细胞表面抗原CEA和CA19-9特异性结合。通过抗CEA单链抗体和抗CA19-9单链抗体在HEK293细胞中表达,我们获取了抗体基因的优化序列,并对抗体识别抗原的能力进行了研究,证实两种单链抗体能够较好的识别结直肠癌等消化道肿瘤细胞表面抗原,为后续研究肿瘤特异性抗体药物和改造T细胞进行过继性免疫治疗奠定了基础。
作者贡献
陆文斌:具体实验实施、论文撰写;宫雪超:负责细胞培养,并参与论文撰写;金建华:通信作者、课题设计;胡文蔚:参与部分实验设计;王月:负责Elisa检测;张华:提供IgG1 Fc-pTT5载体,并给予对实验设计具有重要价值的建议
| [1] | Salmikangas P, Kinsella N, Chamberlain P. Chimeric antigen receptor T-cells (CAR T-Cells) for cancer immunotherapy - moving target for industry?[J]. Pharm Res, 2018, 35(8): 152. DOI:10.1007/s11095-018-2436-z |
| [2] | 陆文斌, 宫雪超, 金建华, 等. TCR/CAR修饰的T细胞在肿瘤免疫治疗中的研究进展和优化策略[J]. 免疫学杂志, 2018, 34(2): 163–73. [ Lu WB, Gong XC, Jin JH, et al. Research progress and therapeutic design of TCR/CAR modified T cells in tumor immunotherapy[J]. Mian Yi Xue Za Zhi, 2018, 34(2): 163–73. ] |
| [3] | Zhang E, Yang P, Gu J, et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy[J]. J Hematol Oncol, 2018, 11(1): 102. DOI:10.1186/s13045-018-0646-9 |
| [4] | Shiozawa M, Chang CH, Huang YC, et al. Pharmacologically upregulated carcinoembryonic antigen-expression enhances the cytolytic activity of genetically-modified chimeric antigen receptor NK-92MI against colorectal cancer cells[J]. BMC Immunol, 2018, 19(1): 27. |
| [5] | 莫然, 王东进. 以糖蛋白类肿瘤标志物为靶向的嵌合抗原受体修饰T细胞研究进展[J]. 临床与病理杂志, 2015, 35(1): 132–6. [ Mo R, Wang DJ. Research progress in chimeric antigen receptor modified T cells targeted glycoprotein tumor marker[J]. Lin Chuang Yu Bing Li Za Zhi, 2015, 35(1): 132–6. ] |
| [6] | Houghton JL, Abdel-Atti D, Scholz WW, et al. Preloading with unlabeled CA19.9 targeted human monoclonal antibody leads to improved PET imaging with 89Zr-5B1[J]. Mol Pharm, 2017, 14(3): 908–15. DOI:10.1021/acs.molpharmaceut.6b01130 |
| [7] | Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment[J]. Sci Rep, 2017, 7(1): 10541. DOI:10.1038/s41598-017-10940-8 |
| [8] | Lichtenegger FS, Krupka C, Haubner S, et al. Recent developments in immunotherapy of acute myeloid leukemia[J]. J Hematol Oncol, 2017, 10(1): 142. DOI:10.1186/s13045-017-0505-0 |
| [9] | Riaz IB, Zahid U, Kamal MU, et al. Anti-CD 19 and anti-CD 20 CAR-modified T cells for B-cell malignancies: a systematic review and meta-analysis[J]. Immunotherapy, 2017, 9(12): 979–93. DOI:10.2217/imt-2017-0062 |
| [10] | 赵素花. 癌胚抗原在结直肠癌进展及免疫治疗中的作用[J]. 中国实用医药, 2013, 8(33): 44–5. [ Zhao SH. The Role of Carcinoembryonic Antigen in The Progression and Immunotherapy of Colorectal Cancer[J]. Zhongguo Shi Yong Yi Yao, 2013, 8(33): 44–5. ] |
| [11] | 侯志波, 郑杰. 癌胚抗原基因家族与肿瘤[J]. 生化与医学, 2005, 25(2): 137–9. [ Hou ZB, Zheng J. The Family of Carcinoembryonic Antigen and Tumors[J]. Sheng Hua Yu Yi Xue, 2005, 25(2): 137–9. ] |
| [12] | 党军强, 王梓年. 胰腺癌诊断的生物标记物研究进展[J]. 现代肿瘤医学, 2013, 21(10): 2358–61. [ Dang JQ, Wang ZN. Advances in Biomarker Research for Pancreatic Cancer Diagnosis[J]. Xian Dai Zhong Liu Yi Xue, 2013, 21(10): 2358–61. DOI:10.3969/j.issn.1672-4992.2013.10.73 ] |
| [13] | Burga RA, Thorn M, Point GR, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T[J]. Cancer Immunol Immunother, 2015, 64(7): 817–29. DOI:10.1007/s00262-015-1692-6 |
| [14] | Girgis MD, Federman N, Rochefort MM, et al. An engineered anti-CA19-9 cys-diabody for positron emission tomography imaging of pancreatic cancer and targeting of polymerized liposomal nanoparticles[J]. J Surg Res, 2013, 185(1): 45–55. |
| [15] | Zhang C, Wang Z, Yang Z, et al. Phase Ⅰ Escalating-Dose Trial of CAR-T Therapy Targeting CEA+ Metastatic Colorectal Cancers[J]. Mol Ther, 2017, 25(5): 1248–58. DOI:10.1016/j.ymthe.2017.03.010 |
| [16] | 段桂华, 沈珊珊, 诸葛宇征. 改良重叠区扩增法构建Rac1相关质粒[J]. 生物技术通报, 2013, 6: 177–82. [ Duan GH, Shen SS, Zhuge YZ. Construction of Rac1 related plasmids by improved overlap extension[J]. Sheng Wu Ji Shu Tong Bao, 2013, 6: 177–82. ] |
| [17] | Xue QJ, Li YQ, Yang CQ, et al. Anti-tumour research of recombinant BCG using BZLF1 and hGM-CSF fusion genes[J]. Vaccine, 2017, 35(12): 1599–607. DOI:10.1016/j.vaccine.2017.02.024 |
| [18] | Wong N, Major P, Kapoor A, et al. Amplification of MUC1 in prostate cancer metastasis and CRPC development[J]. Oncotarget, 2016, 7(50): 83115–133. |
 2019, Vol. 46
2019, Vol. 46


