文章信息
- PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤中的研究进展
- Research Progress on PI3K/AKT/mTOR Signaling Pathway in Burkitt Lymphoma
- 肿瘤防治研究, 2019, 46(2): 169-173
- Cancer Research on Prevention and Treatment, 2019, 46(2): 169-173
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2019.18.0818
- 收稿日期: 2018-06-19
- 修回日期: 2018-10-25
Burkitt淋巴瘤是一种与Epstein Barr病毒(EBV)、人类免疫缺陷病毒(HIV)的感染有关的高度侵袭性非霍奇金淋巴瘤,是人类生长最快的肿瘤[1]。分为地方型、散发型与免疫缺陷相关型三种类型,在流行病学及miRNA上有各自特点[2]。该病主要发生在儿童及青少年,少数发生于成年人,经短期、强化治疗,如CODOX-M/IVAC方案治疗,疗效显著[3],但感染并发症、中枢受累等因素可能导致患者预后差[4-5]。短期、强效的化疗方案,不良反应强,故对于晚期、耐药、体质较差的患者,寻找精准、高效、低毒的靶向药物显得尤为重要。PI3K/AKT/mTOR信号通路在细胞的生长、分化、代谢、生存以及增殖等方面发挥重要作用[6],其在Burkitt淋巴瘤中激活,通过对该通路的深入研究,为Burkitt淋巴瘤的靶向治疗提供理论基础。近几年,很多针对该信号通路的新药已进入实验、临床研究,以期为淋巴瘤患者提供更精准的治疗。现就PI3K/AKT/mTOR信号通路为基础的Burkitt淋巴瘤研究进展作一总结。
1 PI3K/AKT/mTOR信号通路的作用机制磷脂酰肌醇-3激酶(phosphatidylinositol 3-kinase,PI3K)根据结构和功能分为Ⅰ、Ⅱ和Ⅲ型,其中Ⅰ型在肿瘤细胞中广泛突变。Ⅰ型PI3K是由调节亚基P53和催化亚基P110组成的异源二聚体,又分为ⅠA、ⅠB两型,ⅠA型催化亚基由P110α、P110β和P110δ组成,可被细胞表面酪氨酸激酶激活,ⅠB型催化亚基为P110γ,可被G-蛋白偶联受体激活。被激活的PI3K使其底物3, 4二磷酸磷脂酰肌醇(PIP2)磷酸化为3, 4, 5三磷酸磷脂酰肌醇(PIP3),进而使信号通路活化。PI3K的下游效应分子:蛋白激酶B,又名AKT,可通过磷酸化作用,促进细胞生长,抑制细胞凋亡。而AKT可直接活化哺乳动物雷帕霉素靶蛋白(mammal target of rapamycin, mTOR),mTOR包括两种不同的多分子复合物,mTORC1和mTORC2,其在肿瘤细胞中常过度活化。mTORC1被AKT激活后使真核细胞启动因子4E结合蛋白1(4EBP1)和核糖体蛋白p70S6K磷酸化[7]。磷酸化的4EBP1与真核细胞翻译抑制因子(eIF-4E)分离失活,分离的eIF-4E与翻译分子结合,启动蛋白质的翻译;同样,磷酸化的p70S6K也可以促进蛋白质的合成,见图 1。PI3K/AKT/mTOR信号通路参与蛋白质的合成,在细胞的生长、分化、代谢、生存以及增殖等方面发挥重要作用[8]。目前,一些关于此通路抑制剂的研究,如AKT抑制剂MK2206的Ⅱ期临床试验,结果显示患者获益:8例中,2例获得完全缓解,6例获得部分缓解,中位缓解时间为5~8月[9]。所以,这就要求我们根据实验、临床研究,探讨PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤中的活化机制,应用抑制剂抑制该通路活化,为治疗复发难治的Burkitt淋巴瘤患者寻找新的方法。
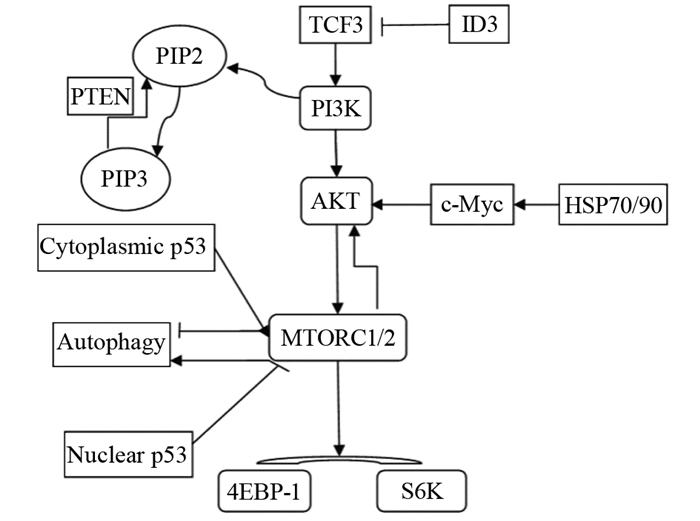
|
| ID3 negatively regulates TCF3, while TCF3 mutation can result in the activation of PI3K. PI3K removes a phosphate from the donor ATP and binds to the acceptor PIP2 to generate PIP3. This leads to the phosphorylation of AKT and its subsequent activation. This action is opposed by PTEN. AKT activates mTOR directly, then mTORC1 will phosphorylate 4EBP1 and p70S6K, while mTORC2 phosphorylates AKT by negative feedback. HSP70/90 is involved in the regulation of c-Myc stability. And accordingly, c-Myc can also affect PI3K/AKT/mTOR. Cytoplasmic p53 inhibits the autophagy by activating mTOR. On the other hand, nuclear p53 promotes autophagy by inhibiting mTOR. TCF3: transcription factor 3; ID3: inhibitor of DNA binding 3; PI3K: phosphatidylinositol 3-kinase; AKT/PKB: Protein Kinase B; mTOR: mammal target of rapamycin; PTEN: phosphatase and tension homology deleted on chromosome ten; PIP2: phosphatidylinositol-4, 5-bisphosphate: PIP3: phosphatidylinositol-3, 4, 5-triphosphate; HSP70/90: heat shock protein70/90; c-Myc: cellular myelocytomatosis oncogene; 4EBP-1: 4E binding protein; S6K: S6 kinase 图 1 PI3K/AKT/mTOR信号通路与其他信号在Burkitt淋巴瘤相互作用图解 Figure 1 Schematic of interaction between PI3K/AKT/mTOR signal pathway and other signals in Burkitt lymphoma |
研究发现,PI3K在肿瘤细胞中主要通过扩增及增加复制导致翻译增加、通路活化,而基因突变少见[8]。在Burkitt淋巴瘤中,PI3K/AKT/mTOR信号通路中任何分子异常都可能导致通路的活化。Sander等[10]通过免疫印迹实验(Western blot)发现AKT第437位上丝氨酸和p70S6K第389位上苏氨酸磷酸化,并且应用PI3K抑制剂LY-294002可逆转磷酸化的激酶,结果证实了PI3K通路在Burkitt淋巴瘤中活化。Schmitz等[11]应用高通量的RNA测序和RNA干涉筛选发现在散发型Burkitt淋巴瘤中,70%存在转录因子TCF3或其负调节因子ID3突变,而TCF3的突变可引起PI3K通路的活化。第10号染色体缺失性磷酸酶-张力蛋白同源性基因(phosphatase and tension homology deleted on chromosome ten,PTEN)是一种肿瘤抑制基因,通过对PIP3去磷酸化,对AKT起到负调节作用[8]。PTEN的缺失,可使PI3K/AKT/mTOR信号通路活化,进而导致肿瘤的发生,常常提示疾病预后不良[12]。Pfeifer等[13]的研究中,PTEN的缺失与PI3K/AKT/mTOR和c-Myc信号通路活化有关,用全PI3K抑制剂LY294002处理弥漫大B细胞淋巴瘤患者样本及淋巴瘤细胞系,发现细胞活性降低,样本分析显示55%的生发中心来源的弥漫大B细胞淋巴瘤存在PTEN缺失,这为生发中心来源的Burkitt淋巴瘤的发病机制和治疗方法提供了新的研究方向。
研究已证实PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤中活化,故可通过应用PI3K/AKT/mTOR信号通路抑制剂靶向治疗Burkitt淋巴瘤。PI3K抑制剂主要分三种类型:全Ⅰ型PI3K抑制剂,选择性PI3K亚型抑制剂,全Ⅰ型PI3K和mTOR双元抑制剂[14]。早期的全Ⅰ型PI3K抑制剂,如LY294002或渥曼青霉素,因脱靶作用等,现临床较少应用。新型全Ⅰ型PI3K抑制剂,例如:BKM120(buparlisib)、SAR245408和BAY 80-6946显示出较少的脱靶作用及较好的耐受性。目前,多种选择性PI3K亚型抑制剂已进入临床研究,例如:p110δ抑制剂idelalisib、PI3K-γδ抑制剂IPI-145和PI3K-αβ抑制剂Rigosertib等,对各种类型淋巴瘤显示出较好的疗效[15]。第一代AKT抑制剂哌立福辛,因临床效果一般,且存在细胞毒性,现已没有针对此药物的临床试验。第二代AKT抑制剂MK-2206,不论是临床前淋巴瘤细胞系研究,还是对患者的临床研究,都有显著效果。mTORC1抑制剂包括依维莫司、替西罗莫司等,然而,mTORC1抑制可通过mTORC2的负反馈作用使PI3K通路活化,进而使AKT磷酸化[8],故单一的mTORC1抑制剂可能因AKT的磷酸化而使患者复发或耐药。李纯团[16]发现,NVP-BEZ235可通过同时抑制PI3K、mTOR的活性,引起下游信号分子AKT、PRS6磷酸化水平下降,PI3K/AKT/mTOR信号通路传导减弱,进而起到抗Burkitt淋巴瘤的作用。我们还发现其他药物通过抑制PI3K/AKT/mTOR信号通路,起到治疗Burkitt淋巴瘤、其他类型淋巴瘤及实体瘤的作用,如PI3K-αγδ抑制剂GDC-0332、PI3K-αδ抑制剂Pictilisib[17]、PI3K-α抑制剂BYL719[18]以及PI3K/mTOR抑制剂GDC-0084[19]、Apitolisib[20]等。
细胞中存在多种信号通路,各通路间可通过正负调节相互联系,一条通路被抑制,可能引起另一通路的激活,故单一的PI3K/AKT/mTOR信号通路的靶向治疗易导致患者复发、耐药,这就需要我们寻找针对不同靶点的多重抑制剂。Huang等[21]发现在Burkitt淋巴瘤CA46细胞中,P-AKT高表达,而黄岑苷可以使P-AKT和P-mTOR下调并促进Burkitt淋巴瘤细胞凋亡。Lim等通过体外实验得出,漆黄素通过靶向PI3K和mTOR通路诱导Burkitt淋巴瘤Raji细胞凋亡[22]。目前,应用PI3K/AKT/mTOR信号通路抑制剂对各种类型淋巴瘤进行靶向治疗已进入实验、临床研究,但少有针对Burkitt淋巴瘤的临床试验,期待未来有更多的临床研究投入,为成年、晚期、耐药的Burkitt淋巴瘤患者带来治疗希望。近几年,研究发现PI3K/AKT/mTOR通路受多种因素影响,除了PTEN外,PI3K/AKT/mTOR通路与c-Myc基因、自噬等在Burkitt淋巴瘤中相互作用受到越来越多研究者的重视,通过实验及临床研究,探讨相互关系及作用机制,为患者提供了新的治疗思路。
3 c-Myc表达和PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤中的关系在所有类型的Burkitt淋巴瘤中,c-Myc通过与免疫球蛋白基因座易位,导致自身过度表达,其表达的蛋白分子是参与细胞分化与凋亡等活动的细胞转录因子。c-Myc一方面通过促进细胞增殖,抑制细胞分化的方式,促进肿瘤发生,但同时也促进细胞凋亡[10]。在Burkitt淋巴瘤细胞中,PI3K/AKT/mTOR信号通路可促进热休克蛋白70和90(HSP70/90)的表达,这些分子伴侣表达上调,参与调节c-Myc的稳定性[23],相应地,c-Myc也可调节mTOR的活性[24],见图 1。Park等利用大鼠,将c-Myc插入免疫球蛋白基因促使其过度表达,但未诱导形成Burkitt淋巴瘤模型[25]。Sander等指出单一的c-Myc过度表达并不足以导致肿瘤的发生,并通过MYC过度表达和PI3K通路的激活,在转基因大鼠上诱导出类似人类Burkitt淋巴瘤的生发中心B细胞来源的淋巴瘤,并且在来源于生发中心B细胞肿瘤中发现c-Myc和P110共表达[26]。虽然c-Myc具有促进细胞增殖,抑制细胞分化的作用,但因其也可促进细胞凋亡,所以,c-Myc过度表达联合PI3K/AKT/mTOR信号通路激活才可导致肿瘤的发生。Granato等[24]通过研究槲皮素对Burkitt淋巴瘤的作用,发现可以通过减少c-Myc表达和PI3K/AKT/mTOR通路活化抑制Burkitt淋巴瘤。上述实验证实了c-Myc与PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤的发生上相互作用,促进肿瘤的生成,成为Burkitt淋巴瘤治疗的又一新靶点。
4 自噬和PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤治疗中的作用与c-Myc作用机制不同,自噬是由自噬相关基因调控的一种细胞死亡过程,与PI3K/AKT/mTOR信号通路、p53(抑癌基因)等有关。p53对细胞自噬具有双重调控作用,一方面,胞质中的p53通过激活mTOR/AMP激酶通路抑制自噬,另一方面,胞核内的p53则通过抑制mTOR和使Beclin与Bcl-2解离,结合Ⅲ型PI3K,促进自噬。当胰岛素或生长因子与其受体结合后,激活mTORC1,促进自噬。PI3K/AKT/mTOR信号通路与细胞自噬过程密切相关,故可通过一些药物作用于该信号通路,促进细胞自噬性死亡,杀死肿瘤细胞,从而治疗Burkitt淋巴瘤。Dong等将替西罗莫司与丙戊酸共同作用于Burkitt淋巴瘤细胞系,发现通过抑制mTOR通路和MYC癌基因蛋白可促进细胞自噬死亡[27]。Granato等[24]发现,槲皮素可通过抑制mTOR通路,引发Burkitt淋巴瘤细胞自噬。而雷帕霉素和地塞米松联合应用,可通过mTOR/P70S6K通路抑制Raji细胞(Burkitt淋巴瘤细胞)的活性[28-29]。有研究报道,肿瘤细胞在缺氧等微环境中可启动保护性自噬,提高生存能力,而放化疗可能导致肿瘤细胞通过增强自噬来减少伤害,与肿瘤的耐药机制有关。Maclean等将自噬抑制剂氯喹作用与Burkitt淋巴瘤小鼠模型,使p53依赖的细胞凋亡[30]。故我们可以通过应用自噬抑制剂,增强肿瘤细胞对放化疗的敏感度,提高治疗效果。
综上可知,自噬对Burkitt淋巴瘤治疗具有双重作用,一方面,通过PI3K/AKT/mTOR信号通路,激活自噬,促进肿瘤细胞的自噬性死亡,另一方面,又能通过自噬抑制剂,抑制自噬,增强肿瘤细胞对放化疗的敏感度。故我们要充分研究不同药物的作用机制,利用PI3K/AKT/mTOR信号通路与自噬的关系,提高成年、晚期、耐药的Burkitt淋巴瘤患者治疗效果,减少不良反应,减轻患者痛苦。
5 展望研究已证实PI3K/AKT/mTOR信号通路在Burkitt淋巴瘤中呈激活状态,该通路的抑制剂对Burkitt淋巴瘤有抑制作用。但Burkitt淋巴瘤发病率较低,目前针对该疾病的PI3K/AKT/mTOR通路靶向治疗的临床试验较少,期待未来有更多的临床研究投入,同时加强对PTEN、c-Myc、自噬与PI3K/AKT/mTOR信号通路的多重抑制的研究,设计与单克隆抗体或其他通路抑制剂的联合用药。目前正在研究中的其他基因如:ID3、TCF3、CCND3、GNA13和SMARCA4等,在Burkitt淋巴瘤中突变率高,这些突变基因将成为新的治疗靶点[31-33]。此外,我们更需要增加新技术,如二代测序技术在此领域的应用,使更多的成年、晚期、耐药的Burkitt淋巴瘤患者从中获益。
作者贡献
孙媛媛:对相关素材进行归纳整理、综合分析,论文撰写与修改; 张明智:论文的审核与修改
| [1] | Rowe M, Fitzsimmons L, Bell Al. Epstein-Barr virus and Burkitt lymphoma[J]. Chin J Cancer, 2014, 33(12): 609–19. |
| [2] | Said J, Lones M, Yea S. Burkitt Lymphoma and MYC: What else is new[J]. Adv Anat Pathol, 2014, 21(3): 160–5. DOI:10.1097/PAP.0b013e3182a92cde |
| [3] | Casulo C, Friedberg J. Treating Burkitt Lymphoma in Adults[J]. Curr Hematol Malig Rep, 2015, 10(3): 266–71. DOI:10.1007/s11899-015-0263-4 |
| [4] | Jacobson C, LaCasce A. How Ⅰ treat Burkitt lymphoma in adults[J]. Blood, 2014, 124(19): 2913–20. DOI:10.1182/blood-2014-06-538504 |
| [5] | Molyneux EM, Rochford R, Griffin B, et al. Burkitt's lymphoma[J]. Lancet, 2012, 379(9822): 1234–44. DOI:10.1016/S0140-6736(11)61177-X |
| [6] | 刘慧, 李鑫, 胡腾鹏, 等. PI3K/AKT/mTOR信号通路抑制剂在淋巴瘤中的研究进展[J]. 中国肿瘤临床, 2016, 43(5): 211–5. [ Liu H, Li X, Hu TP, et al. Research progress on PI3K/AKT/mTOR signaling pathway in lymphoma[J]. Zhongguo Zhong Liu Lin Chuang, 2016, 43(5): 211–5. DOI:10.3969/j.issn.1000-8179.2016.05.388 ] |
| [7] | Spender LC, Inman GJ. Developments in Burkitt's lymphoma novel cooperations in oncogenic MYC signaling[J]. Cancer Manag Res, 2014, 6: 27–38. |
| [8] | Westin JR. Status of PI3K/Akt/mTOR pathway inhibitors in lymphoma[J]. Clin Lymphoma Myeloma Leuk, 2014, 14(5): 335–42. DOI:10.1016/j.clml.2014.01.007 |
| [9] | Oki Y, Fanale M, Romaguera J, et al. Phase Ⅱ study of an AKT inhibitor MK2206 in patients with relapsed or refractory lymphoma[J]. Br J Haematol, 2015, 171(4): 463–70. DOI:10.1111/bjh.2015.171.issue-4 |
| [10] | Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis[J]. Cancer Cell, 2012, 22(2): 167–79. DOI:10.1016/j.ccr.2012.06.012 |
| [11] | Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics[J]. Nature, 2012, 490(7418): 116–20. DOI:10.1038/nature11378 |
| [12] | Zou ZJ, Zhang R, Fan L, et al. Low expression level of phosphatase and tensin homolog deleted on chromosome ten predicts poor prognosis in chronic lymphocytic leukemia[J]. Leuk Lymphoma, 2013, 54(6): 1159–64. DOI:10.3109/10428194.2012.733880 |
| [13] | Pfeifer M, Grau M, Lenze D, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma[J]. Proc Natl Acad U S A, 2013, 110(30): 12420–5. DOI:10.1073/pnas.1305656110 |
| [14] | Yap TA, Bjerke L, Clarke PA, et al. Drugging PI3K in cancer: refining targets and therapeutic strategies[J]. Curr Opin Pharmacol, 2015, 23: 98–107. DOI:10.1016/j.coph.2015.05.016 |
| [15] | Yap TA, Garrett MD, Walton MI, et al. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises[J]. Curr Opin Pharmacol, 2008, 8(4): 393–412. DOI:10.1016/j.coph.2008.08.004 |
| [16] | 李纯团.PI3K/AKT/mTOR信号通路抑制剂对伯基特淋巴瘤作用机制的研究[D].福建: 福建医科大学, 2015. [ Li CT. The effect of PI3K/AKT/mTOR signal pathway inhibitors on Burkitt Lymphoma[D]. Fujian: Fujian Medical University, 2015. ] http://cdmd.cnki.com.cn/Article/CDMD-10392-1016747810.htm |
| [17] | Yamamoto N, Fujiwara Y, Tamura K, et al. Phase Ia/Ib study of the pan-class Ⅰ PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer[J]. Invest New Drugs, 2017, 35(1): 37–46. |
| [18] | Pongas GN, Annunziata CM, Staudt LM. PI3Kδ inhibition causes feedback activation of PI3Kα in the ABC subtype of diffuse large B-cell lymphoma[J]. Oncotarget, 2017, 8(47): 81794–802. |
| [19] | Stumpf A, McClory A, Yajima H, et al. Development of an Efficient, Safe, and Environmentally Friendly Process for the Manufacture of GDC-0084[J]. Org Process Res Dev, 2016, 20(4): 751–9. DOI:10.1021/acs.oprd.6b00011 |
| [20] | Dolly SO, Wagner AJ, Bendell JC, et al. Phase Ⅰ Study of Apitolisib (GDC-0980), Dual Phosphatidylinositol-3-Kinase and Mammalian Target of Rapamycin Kinase Inhibitor, in Patients with Advanced Solid Tumors[J]. Clin Cancer Res, 2016, 22(12): 2874–84. DOI:10.1158/1078-0432.CCR-15-2225 |
| [21] | Huang Y, Hu J, Zheng J, et al. Down-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin[J]. J Exp Clin Cancer Res, 2012, 31: 48. DOI:10.1186/1756-9966-31-48 |
| [22] | Lim JY, Lee JY, Byun BJ, et al. Fisetin targets phosphatidylinositol-3-kinase and induces apoptosis of human B lymphoma Raji cells[J]. Toxicol Rep, 2015, 2: 984–9. DOI:10.1016/j.toxrep.2015.07.004 |
| [23] | Giulino-Roth L, van Besien HJ, Dalton T, et al. Inhibition of Hsp90 Suppresses PI3K/AKT/mTOR Signaling and Has Antitumor Activity in Burkitt Lymphoma[J]. Mol Cancer Ther, 2017, 16(9): 1779–90. DOI:10.1158/1535-7163.MCT-16-0848 |
| [24] | Granato M, Rizzello C, Romeo MA, et al. Concomitant reduction of c-Myc expression and PI3K/AKT/mTOR signaling by quercetin induces a strong cytotoxic effect against Burkitt's lymphoma[J]. Int J Biochem Cell Biol, 2016, 79: 393–400. DOI:10.1016/j.biocel.2016.09.006 |
| [25] | Park SS, Kim JS, Tessarollo L, et al. Insertion of c-Myc into Igh Induces B Cell and Plasma Cell Neoplasms in Mice[J]. Cancer Res, 2005, 65(4): 1306–15. DOI:10.1158/0008-5472.CAN-04-0268 |
| [26] | Sander S, Rajewsky K. Burkitt lymphomagenesis linked to MYC plus PI3K in germinal center B cells[J]. Oncotarget, 2012, 3(10): 1066–7. |
| [27] | Dong LH, Cheng S, Zheng Z, et al. Histone deacetylase inhibitor potentiated the ability of MTOR inhibitor to induce autophagic cell death in Burkitt leukemia/lymphoma[J]. J Hematol Oncol, 2013, 6: 53. DOI:10.1186/1756-8722-6-53 |
| [28] | 谭化姣, 毛峥嵘. 自噬与淋巴瘤治疗新进展[J]. 中华病理学杂志, 2017, 46(5): 365–8. [ Tan HJ, Mao ZR. Autophagy and progress of lymphoma treatment[J]. Zhonghua Bing Li Xue Za Zhi, 2017, 46(5): 365–8. ] |
| [29] | 王炜, 王志彬, 高玉环. 国产雷帕霉素对人淋巴瘤细胞Raji增殖的影响[J]. 肿瘤防治研究, 2012, 39(2): 157–60. [ Wang W, Wang ZB, Gao YH. Effect of rapamycin in proliferation of human burkitt lymphoma cells[J]. Zhong Liu Fang Zhi Yan Jiu, 2012, 39(2): 157–60. DOI:10.3971/j.issn.1000-8578.2012.02.010 ] |
| [30] | Maclean KH, Dorsey FC, Cleveland JL, et al. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis[J]. J Clin Invest, 2008, 118(1): 79–88. DOI:10.1172/JCI33700 |
| [31] | Dunleavy K, Little RF, Wilson WH. Update on Burkitt Lymphoma[J]. Hematol Oncol Clin North Am, 2016, 30(6): 1333–43. DOI:10.1016/j.hoc.2016.07.009 |
| [32] | Greenough A, Dave SS. New clues to the molecular pathogenesis of Burkitt lymphoma revealed through next-generation sequencing[J]. Curr Opin Hematol, 2014, 21(4): 326–32. DOI:10.1097/MOH.0000000000000059 |
| [33] | Schmitz R, Ceribelli M, Pittaluga S, et al. Oncogenic mechanisms in Burkitt lymphoma[J]. Cold Spring Harb Perspect Med, 2014, 4(2): pii: a014282. DOI:10.1101/cshperspect.a014282 |
 2019, Vol. 46
2019, Vol. 46


