文章信息
- 肿瘤心脏病学——一门超越心血管毒性管理的学科
- Onco-Cardiology: A Specialty that Goes Beyond Managing Cardiovascular Toxicity
- 肿瘤防治研究, 2018, 45(11): 821-830
- Cancer Research on Prevention and Treatment, 2018, 45(11): 821-830
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.18.0828
- 收稿日期: 2018-06-19
- 修回日期: 2018-07-25
2. 518101 深圳,南方医科大学深圳医院肿瘤科
Onco-cardiology (also known as Cardio-oncology) is defined as the medical specialty focused on the management of cardiovascular diseases in cancer patients. Significant progress has been achieved in the last several decades in the prevention, early detection, and treatment of both cardiac and oncologic diseases. Despite these advances, these two medical conditions continue to constitute the most common causes of deaths in the developed countries and although cancer remains a leading cause of morbidity and mortality worldwide, the survival rate of patients with malignancy has steadily increased over the last 4 decades. The 5-year relative survival rate of these patients increased from around 50% between 1975 and 1977 to around 68% between 1999 and 2005[1]. In the USA alone, around 14.5 million cancer survivors were alive in 2014 and it is estimated that by 2024, the population of cancer survivors will be close to 19 million[2].
As the armamentarium of cancer therapies grew, several treatment modalities were found to be associated with acute and also long-term cardiovascular side effects impacting these patients outcome. Since the late 1970’s and over the last several decades, multiple research programs and protocols were focused on studying and identifying the molecular and cellular mechanism of cancer therapy related cardiovascular toxicities. In the 1970s- 1990s, the focus was aimed at the toxicities related to traditional chemotherapy drugs like anthracyclines and the vascular and pericardial diseases secondary to radiation therapy. Between late 1990s and 2010, the interest shifted towards toxicities related to certain targeted cancer therapies like trastuzumab and the oral tyrosine kinase inhibitors (TKIs). In the last 3-5 years, we started dealing with the emerging concern related to the rare but life-threatening events caused by cancer immunotherapy and specifically the immune checkpoint inhibitors (ICIs). These chemotherapies related cardiovascular toxicities are becoming particularly relevant since the improvement in the life expectancies of cancer patients has dramatically extended the use and duration of such therapies. Furthermore, the advances in medical oncology with secondary improvement in cancer survival has allowed the latency of delayed cardiovascular side effects of key cancer therapies to manifest years after the cancer therapy was completed[3]. Pediatric cancer survivors for example were reported to have a 15-fold increase in relative risk of heart failure, especially after anthracycline and radiation therapy[3]. It has also been reported that cardiovascular causes are responsible for the death of 33% of cancer survivors compared to a 51% mortality rate directly linked to cancer recurrence and progression[4].
The management of the debilitating symptoms associated with cancer and heart diseases presents a major challenge to patients, families, and health care providers throughout the entire duration of cancer care. In order to effectively contribute to the effective management of these complex patients, onco-cardiology specialty uses two essential programs that complement each other: One is aimed at clinical care and the other one is focused on research. The main aim and objectives of clinical practice in onco-cardiology is to help tackle these challenges by optimizing cardiovascular conditions BEFORE (for patients with known heart diseases), DURING (for patients experiencing acute toxicities) and AFTER (cancer survivors) cancer treatment in order to reach optimal patient outcome. On the other hand, a main goal of the research program in onco-cardiology is to investigate mechanisms of cardiotoxicities and use such knowledge to help manage side effects and also to help advance cardiovascular discoveries by offering the opportunities for a better understanding of cardiovascular physiology and pathology. In fact, the continued and evolving discoveries of mechanisms of cardiac toxicity of many traditional and also targeted cancer therapies fueled the interest of scientists and cardiologists in using novel cardiovascular biomarkers and imaging in order to help predict, monitor and prevent cardiovascular toxicities and also develop safer and better tolerated drugs.
1 The wide spectrum of cardiovascular syndromes in Onco-cardiologyCardiovascular diseases in cancer patients can precede the diagnosis of cancer or can be related to the malignancy itself or its therapy. The direct cancer effects on the heart and the cardiotoxicity of chemotherapeutic agents can lead to a wide range of cardiovascular syndromes as shown in figure1.
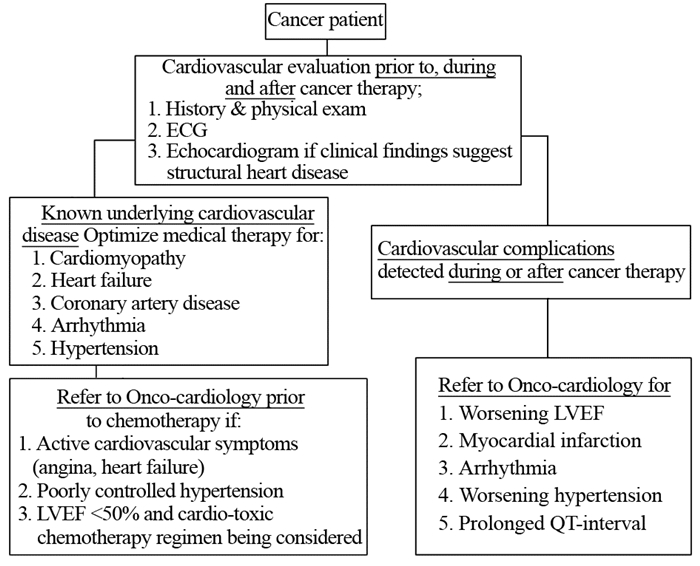
|
| Figure 1 The workflow of patients with cancer and heart disease |
Onco-cardiologists are often asked to assess cancer patients with a wide variety of acute and chronic cardiovascular illnesses. Contrary to what is widely believed, the vast majority of these patients have their cardiovascular events triggered by mechanisms other than pure chemotherapy related cardiotoxicity. These events are often related to previously non-diagnosed or already established but stable underlying structural heart disease like stable angina, asymptomatic coronary artery disease, asymptomatic valvular heart disease, cardiomyopathy or well controlled chronic arrhythmia. In fact, these patients are typically more susceptible to the cardio-toxic side effects of cancer therapies and they also face the challenge and risks of altering chronic stable cardiac medications. One example of these typical challenges include management of dual anti-platelets therapy in patients with prior coronary stents and facing risks of chemotherapy-induced thrombocytopenia[5-7].
Another commonly encountered clinical challenge is related to the evaluation and management of new onset cardiomyopathy and acute heart failure syndromes in patients receiving chemotherapy. While the most feared etiology in this setting is the progressive, permanent and irreversible myocardial damage related to toxicities, the majority of these patients have their symptoms in fact triggered by mechanisms other than drug toxicity[8-9]. Despite some overlap, a clinically useful approach at evaluating and managing these patients is to divide them into two groups based on their initial clinical presentation:
a: The first group includes patients presenting with sudden and new onset acute heart failure with associated systolic dysfunction (Low ejection fraction below 50%). These include cardiomyopathies related to sepsis, stress cardiomyopathy, and myocarditis (toxic or infectious).
b: The second group includes patients presenting with subacute or chronic heart failure symptoms and those with underlying structural heart disease (i.e. hypertensive or valvular heart disease), those with chemotherapy-induced cardiomyopathy and those with infiltrative myocardial condition related to their underlying malignancies or its therapy (i.e. amyloidosis, iron overload).
This typical example related to heart failure also applies to many other cardiovascular syndromes like coronary and arterial ischemia (TKIs, cisplatin)[10-11], arrhythmia (Ibrutinib)[12-13], QT prolonging agents, pericardial diseases (ICIs, radiation, infection) and hypertension (TKIs)[14]. A good understanding of the differences between these etiologies and an accurate clinical diagnosis are at the core of an onco-cardiology program. An accurate and correct diagnosis is essential for the appropriate care of these patients in order to adequately select those who can resume the same cancer therapies versus who need alternative cancer therapeutic approach. Since cardiovascular complications of cancer therapy can have a profound and lasting effect on the health of patients with active cancer or cancer survivors, it is becoming obvious that clinical care of these patients require adequate understanding of both medical fields.
2 Mechanism of cardiotoxicity as a platform for cardiovascular discoveriesThe discovery of new biologic pathways responsible for certain cellular function and its role in certain cardiovascular diseases and cancer, has provided important explanations of the mechanisms of cardiac toxicity. This strategy has the potential for facilitating the discovery of novel biomarkers in addition to identifying the exact target of intervention in order to help manage cardiovascular toxicity and also help develop safer and better tolerated cancer drugs. A typical example is the breast cancer targeted therapy drug trastuzumab: this agent in fact targets ErbB2 receptors in breast cancer cells and has been shown to improve outcome and prolong survival in these HER2+/ErbB2 breast cancer patients[15]. These same receptors within the cardiac myocyte are, however, responsible for normal cell function and specifically in the cardiac myocyte repair. When affected by ErbB2 inhibitors like trastuzumab, the natural mechanism of myocyte repair is impacted and secondary mitochondrial dysfunction can be observed[16]. In addition to the benefit of improving strategies to prevent drug toxicities, the better understanding of these biologic pathways targeted by cancer therapy may also provide a better understanding of certain heart diseases and its management. For example, the discovery of the key role of ERB2 in normal cardiac function and its relation to trastuzumab toxicity, was followed by the discovery of the benefit of an ERB2 agonist called neuregulin-1 in the animal models of heart failure[17]. Neuregulin-1 is currently being studied for the treatment of heart failure in humans.
Another biologic example is the observation that Bruton tyrosine kinase receptor is expressed in the atria of human heart tissue. This tyrosine kinase is in fact the target of the novel drug therapy with ibrutinib used in certain hematologic malignancies including lymphoma and chronic lymphocytic leukemia. This drug has been associated with significant incidents of atrial arrhythmia, potentially mediated by its on-target effect and more specifically by inhibiting the PI3K-Akt signaling pathway[13]. Similarly, another group of targeted cancer therapy drugs include several oral tyrosine kinase inhibitors (TKIs) that belong to the group of vascular signaling pathway inhibitors (VSP) and include sunitib, sorafenib, ponatinib, axitinib and others. These drugs are commonly used in many cancers due to their proven oncologic efficacy related anti-angiogenesis effect that results from inhibiting the physiologic function of the several vascular endothelial growth factors (VEGF) receptors[18-19]. Their un-intended effects on the normal vasculature however has been the source of many of their side effects including new-onset and severe hypertension and also thromboembolic arterial event[20].
Other mechanisms of toxicities have also been extensively studied and reported with older, conventional chemotherapeutic drugs like the anthracyclines: cardiovascular toxicity of this drug has been recently linked to inhibition of TopoisomeraseⅡβ[21]. This enzyme is responsible for normal myocyte function and its inhibition would partially explain cardiovascular toxicity of Adriamycin. Preventive measures aiming at upholding the function of Topoisomerase Ⅱβ during anthracyclines therapy are currently being studied as potential cardioprotective drugs.
More recently, breakthrough discoveries of several therapeutic modalities permitted the utilization of the immune system in the fight against cancer. This allowed the development of the immune checkpoint inhibitors or ICIs and more recently the approval of engineered T cells in the treatment of many cancers. ICIs are now recognized for being responsible for serious cardiac side effects. Cardiovascular toxicities are rare, but suspected to be underestimated[22] and include a wide spectrum of cardiovascular syndromes from atrial fibrillation and pericarditis to pericardial effusion and subclinical or smoldering myocarditis to full-blown fulminant myocarditis leading to ventricular arrhythmia and even death[23]. Over the last few years, clinicians and scientist tried to better understand the pathophysiology of these immune system mediated cardiovascular syndromes. These efforts are pointing towards the likelihood of certain T cell lineage selection in infiltrating the myocardium and triggering different type of myocarditis (Lymphocytic and Giant cell myocarditis)[24-25]. Current research efforts are focusing on a better understanding of these T cell response and behavior[26] that hopefully will help minimize chances of drug induced myocarditis, and also improve options for its therapy (steroids versus immunomodulatory therapy). These discoveries may ultimately shed more light on the pathophysiology and improve management of myocarditis in general.
3 The role of cardiac monitoring in the early detection of cardiotoxicityDiscoveries in cardiotoxicity research has played a major role in our knowledge of mechanism of toxicity and also established our current understanding of the biologic differences between each group of cancer therapy drugs. These findings were translated into a better understanding of the needed tools to monitor and treat these cardiotoxicies. Over the last decade, various guidelines emerged in an attempt to help clinicians monitor and treat these patients[5, 27-28]. Many of these guidelines are however based on limited and controversial clinical data. Most are difficult to implement in daily clinical practice and are often met with skepticism. The main source for these controversies are the fact that cardiovascular outcomes are typically not included as main aims in oncologic trials and most of the cardiovascular toxicity prevention and monitoring studies are structurally flawed and usually include a small cohort of patients.
Below is a brief review of our practical and simplified approach at evaluating patients with suspected cardiotoxicity in our daily practice and is based on our current understanding of mechanism of toxicity and how it is different between cancer drugs. This is by no means a perfect approach that fits every patient but it does give the reader a general understanding of our thinking process as we face these clinical challenges on daily basis. (Figure2)
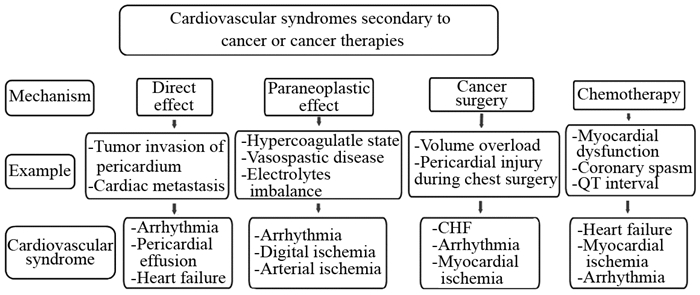
|
| CHF: congestive heart failure Figure 2 Tumor and anti-cancer therapy associated cardiovascular toxicities |
a-Cardiotoxicity related to traditional chemotherapies like anthracyclines, include myocardial cell damage that is initially well tolerated in patients with normal cardiac function at baseline. Very rarely, patients would experience myocardial dysfunction during therapy and this is due to the excellent myocardial reserve and ability of the heart to compensate for limited number of myocytes injured or lost. Symptoms hence tend to manifest years after therapy when the limited myocardial reserve is overwhelmed following a second insult (myocardial ischemia, hypertension, infection, etc.). Monitoring for potential initial myocardial injury during anthracycline therapy using cardiac biomarker has been proposed to guide timing of initiation of cardio protective drugs. The complexity of this monitoring process in a busy practice makes it a difficult to implement for every patient. We use cardiac biomarkers for the purpose of myocardial toxicity detection only on case-by-case basis when certain clinical scenario are suggestive of possible cardiotoxicity. Serial cardiac imaging using echocardiography techniques on routine basis is also impractical and is typically reserved for baseline cardiac function assessment or after certain cumulative dose of anthracyclines (> 350mg/m2) has been used. Universal use of pharmacologic interventions to prevent cardiotoxicities (dexrazoxane, beta blocker or ACE inhibitor use) continue to be controversial.
b- Cardiovascular toxicities related to targeted cancer therapies is much more heterogeneous with predominantly cellular dysfunction and alteration of biologic pathways compared to the more serious myocardial injury observed with anthracyclines: In the case of toxicities related to targeted therapy with trastuzumab, left ventricular systolic dysfunction is typically transient and resolve after drug cessation. Patients can hence be safely monitored by serial echocardiography[28]. Cardiac imaging on the other hand plays very limited role in managing toxicities related to other targeted therapy drugs like the VSP inhibitors mentioned above. Patients using these drugs should simply be followed with clinical monitoring for hypertension or vascular ischemia[20]. Serial cardiac imaging does not appear to play any significant role in the absence of symptoms suggestive of myocardial dysfunction. Another group of TKIs (vemurafenib, Ibrutinib, vandetanib, nilotinib) are associated with arrhythmia or QT prolongation. Serial ECGs should suffice in monitoring these patients[20].
c-The third group of cardiotoxic cancer drugs include the new immunotherapy medications ICIs. Patients receiving these therapies do not appear to benefit from serial imaging. This is due to the rare and acute nature of their cardiovascular toxicities. Serial cardiac biomarkers have been suggested by many oncologic societies[27] to monitor for subclinical or smoldering myocarditis but these recommendations are mainly driven by expert opinions and not based on any objective evidence.
4 Management and outcomeThe management of the cardiovascular syndromes related to cancer or cancer therapy typically follows the standard of care established for non-cancer patients with cardiac diseases. Such approach however may not be associated with the expected clinical response or cardiovascular outcome as shown for example in the analysis of patients undergoing implantation of ventricular assisted device for heart failure: Compared to patients with cardiomyopathy related to other etiologies, those with chemotherapy induced cardiomyopathy required more right ventricular assist device support[29]. A possible explanation to such finding is the scattered and patchy myocardial damage in both ventricles caused by chemotherapy with secondary higher incidence of right ventricular failure. This observation is an example of how clinical course, treatment and outcome of certain cardiovascular syndromes can differ between cancer patients and the non-cancer population and also highlights the importance and the necessity for dedicated building strong clinical relationships and collaborative efforts between oncologists and cardiologists.
5 SummaryThis is an exciting time in Onco-cardiology as we witness the interest in this field growing exponentially and the number of medical centers adding dedicated onco-cardiology services continues to grow. Heart disease and cancer are the two leading causes of morbidity and mortality worldwide. Patients typically experience cardiovascular events related to underlying asymptomatic structural heart disease that become unmasked during the stress of cancer therapies. Other cancer patients experience cardiovascular toxicity caused by certain cancer therapies which remains a major cause of concern for oncologists and cardiologists. This issue is becoming particularly relevant since the improvement in the life expectancies of cancer patients has dramatically extended the use and duration of such therapies. The recent discoveries of new mechanisms of cardiac toxicity fueled the interest of researchers in using novel cardiovascular biomarkers in order to help predict, monitor and prevent cardiovascular toxicities and also develop safer and better tolerated drugs. Clinicians on the other hand, continue to better define and use models for therapy selection and monitoring during and after cancer therapy. Onco-cardiology continues to evolve in order to address the cardiovascular needs of these patients whose optimal outcome mandates close and collaborative efforts between cardiologists and oncologists in a multidisciplinary approach. Future research in this field would definitely benefit from adding cardiovascular outcomes as a common measurable outcome in cancer trials using accurate and standardized assessment tools of cardiotoxicity. Involvement of cardiologists in cancer patients’ care has changed from focusing on managing cardiovascular complications of therapy to an overall assistance in the care of these patients from the initial cancer diagnosis to survivorship.
·译文· 0 引言肿瘤心脏病学是一门研究肿瘤患者心血管疾病的医学学科。近几十年来,尽管在恶性肿瘤性疾病及心脏病的预防、早期诊断和治疗水平取得了显著的进展,这两类疾病仍然构成了发展中国家最常见的死亡原因。虽然在世界范围内恶性肿瘤仍是高发病率、高死亡率疾病,但是肿瘤患者生存率在近40年都在稳步上升。在1975—1977年间恶性肿瘤患者的5年生存率约50%,1999—2005年间已上升至约68%。仅仅在美国,2014年约有1 450万的肿瘤幸存者,而据估计,至2024年该数值将上升至1 900万。
治疗癌症的方法越来越多,但一些方法被证实因存在急性或长期的心血管毒性而影响到患者的生存。从20世纪70年代末到最近几十年,许多研究和课题专注于肿瘤治疗相关的心血管毒性的分子生物学及细胞生物学机制的探讨。70年代至90年代间,研究的重点是与传统化疗药物如蒽环类药物有关的心脏毒性,以及放射治疗后的血管和心包疾病。20世纪末至21世纪初,研究方向转为靶向治疗药如曲妥珠单抗、小分子酪氨酸酶抑制剂等所致的心脏毒性。近3~5年间,我们开始关注免疫治疗药物罕见的、但可能危及生命的心血管相关事件,尤其是免疫检查点抑制剂药物。由于有了这些药物的治疗,使得肿瘤患者预期生存寿命延长,此时心血管毒性变得尤为重要。此外,肿瘤治疗的进展和癌症生存率的提高使得治疗相关的心血管副作用延迟至癌症治疗完成后的数年才显现出来,例如儿童肿瘤幸存者发生心力衰竭的相对风险增加了15倍,尤其是在使用过阿霉素类药物和胸腔放疗之后。另外,据报道,尽管51%的肿瘤患者直接死因为肿瘤复发或转移,但仍有33%的肿瘤幸存者死于心血管相关疾病。
恶性肿瘤及心血管病导致的衰竭症状成为了患者、家属及医疗机构在诊治癌症过程中最大的挑战。为了更好地将有效的治疗手段应用于诊治该类病情复杂的患者,肿瘤心脏病学的专家采用了临床和科研相结合的方式进行研究。临床实践最主要的目的和意义在于在肿瘤治疗前(患者有基础心血管疾病)、治疗中(患者发生了急性心脏毒性)及治疗后(肿瘤幸存者)及时改善心血管功能、提高患者预后。而实验研究的目标则在于探索心血管毒性的机制,更好地理解心血管系统疾病的病理生理过程,并利用这些知识来帮助控制副作用。事实上,正是在对传统化疗及靶向药物对心血管毒性的作用机制的持续探索中,科学家及心血管病专家们燃起了对发现新的心血管相关标志物或影像表现的兴趣,从而来预测、监测和预防心血管毒性、发现更安全有效的治疗药物。
1 肿瘤心脏病学中各种各样的心血管病综合征肿瘤患者心血管病的发生可以早于恶性肿瘤的诊断,或者由于恶性肿瘤本身或治疗的副作用而发生。肿瘤本身或者化疗药物的心血管毒性所致的心血管综合征见图 1。
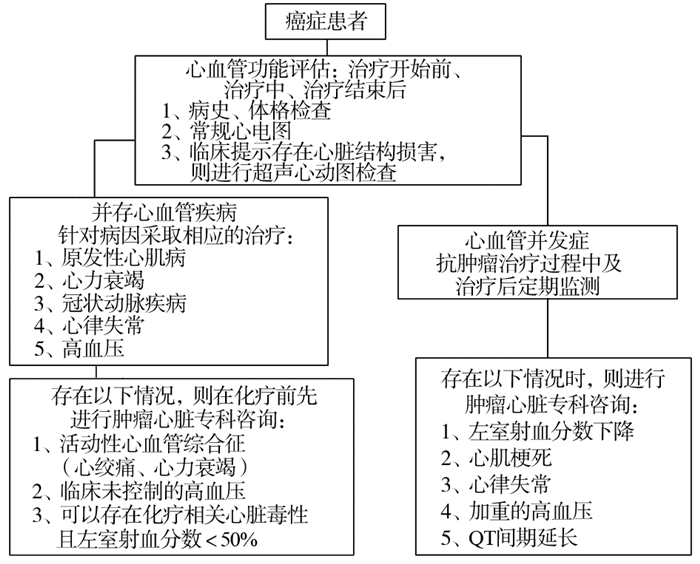
|
| 图 1 肿瘤合并心脏病患者的诊治流程 |
肿瘤心脏病学的专家经常被要求评估各种急、慢性心血管疾病。然而,与众所周知相反的是,大多数的肿瘤患者发生的心血管疾病并不仅仅是因为化疗药物的毒性作用,这些患者可能已经存在还没被诊断出来的心血管病,或者心脏结构已有改变但尚处于疾病的代偿期,例如:稳定性心绞痛、无症状性冠状动脉疾病、无症状的心脏瓣膜病、心肌病或控制良好的慢性心律不齐。事实上,这些患者通常更容易受到癌症治疗的心脏毒性副作用的影响,也面临着更换长期稳定的心脏药物的挑战和风险。典型的例子包括对有冠状动脉支架的患者进行双重抗血小板治疗,同时还要面临化疗引起血小板减少症的风险。
临床上经常遇到的另一个难题是如何鉴别和治疗新发现的心肌病及化疗所致的急性心力衰竭。虽然最令人担心的病因是与毒性有关的渐进性、永久性和不可逆转的心肌损害,但大多数患者的症状实际上是由药物毒性以外的机制引起的。尽管会有一些重叠,但是,临床评估和管理这些患者的一个有效的方法是,根据他们最初的临床表现分成两组:组一:突发的急性心力衰竭合并收缩功能不全(左室射血分数EF < 50%),包括脓毒症性心肌炎、应激性心肌病、化学毒性或感染相关性心肌炎。组二:亚急性或慢性心力衰竭,常见为存在潜在结构性心脏病(如高血压、瓣膜病)的患者,他们因化疗药引起心肌病或其他抗肿瘤治疗方式引起心肌缺血(如心肌淀粉样变、铁沉积)。
除了心力衰竭外,还有其他心血管疾病如小分子酪氨酸酶抑制剂和顺铂引起的冠状动脉缺血、依鲁替尼引起的心律失常、免疫检查点抑制剂、放疗、感染引起的QT间期延长和心包疾病,以及小分子酪氨酸激酶抑制剂相关的高血压等。对引起这些疾病相关的病因的理解和准确的临床诊断就是肿瘤心脏病学的主要研究内容。肿瘤心脏病学有助于对这些心脏疾病的准确诊断,能够及时地对患者进行诊治,判断能否继续采用该方案治疗的合适患者,而对有禁忌的患者选择其他治疗方案。心血管相关并发症的发生对肿瘤患者或肿瘤幸存者的健康会造成深刻而持续的影响,这就要求该领域的专家应对肿瘤学和心血管病学都有足够的了解。
2 心脏毒性机制的研究可作为心血管疾病发现的平台通过对心脏毒性相关机制的研究,我们发现新的分子生物学通路的改变造成了特定的细胞功能损伤,从而导致了肿瘤和心血管疾病。这些生物标志物的发现可以使科学家通过特定的阻滞剂来抑制相关心血管毒性,同时也能筛选出更安全、耐受性更好的肿瘤治疗药物。乳腺癌的靶向治疗药物“曲妥珠单抗”就是一个典型的例子:它特定性作用于乳腺癌细胞ErbB2位点,从而延长HER2/ErbB2阳性的乳腺癌患者的生存。然而由于正常心肌细胞上也有相同的分子靶点,该药抑制了心肌细胞功能,尤其是对心肌损伤后自然修复机制影响,继发了线粒体功能障碍。通过理解这些分子损伤机制,可以更好地管理肿瘤治疗引起的相关心脏副作用,理解相关的心脏疾病表现及给予有效的治疗。例如,通过发现ERB2通路在心肌细胞的作用能理解曲妥珠单抗与心脏毒性的关系,同时还发现了一种名为neuregulin-1的ERB2激动剂在心脏衰竭的动物模型中的作用。现如今Neuregulin-1已经用于治疗人类心脏衰竭疾病的研究。
Bruton小分子酪氨酸激酶位点表达于健康者心脏组织,同时该位点也是治疗恶性肿瘤性疾病如淋巴瘤、慢性淋巴细胞白血病的重要药物依鲁替尼的作用靶点。该药引起的房性心律失常,就与其靶点抑制效应,尤其对PI3K-Akt通路的抑制有关。另外一组血管生成信号通路抑制剂,包括舒尼替尼、索拉菲尼、帕纳替尼、阿西替尼等,作用于抑制血管内皮生长因子的受体从而抑制肿瘤血管的生成,被广泛用于治疗不同的癌种。而他们对正常血管的抑制作用则导致了高血压、血栓栓塞症等药物不良反应的发生。
传统化疗药物——蒽环类药的心脏毒性作用也被广泛报道和研究,近期研究发现它的心脏毒性是由于抑制了拓扑异构酶Ⅱβ。这种酶负责正常的肌细胞功能,其抑制作用可以部分解释阿霉素(其中一种蒽环类药物)的心血管毒性。目前能维护拓扑异构酶Ⅱβ活动的药物正在研究中,作为潜在的心血管药物来预防蒽环类药的心脏毒性。最近,通过激活人体免疫系统对抗肿瘤的治疗方式获得了突破性的进展,从而使得免疫检查点抑制剂或免疫T细胞激活剂作为抗肿瘤药物而获批用于多种恶性肿瘤的治疗。免疫检查点抑制剂现在也被认为有严重的心脏毒副作用。虽然该类药的心脏毒性事件发生率低,但表现多样,可引起心房纤颤、心包炎、心包积液,可能导致亚临床期或潜伏期心肌炎发展为全面爆发的心肌炎,从而导致恶性室性心律失常、甚至死亡,其相关的心脏毒性可能被低估了。在过去的几年里,临床医生和科学家试图更好地去理解这些免疫系统介导的心血管综合征的病理生理学。他们发现T细胞浸润心肌细胞从而诱发了不同类型的心肌炎(淋巴细胞性和巨细胞性心肌炎)。目前的研究致力于更好地理解这些T细胞反应和行为,希望能帮助减少药物诱发心肌炎的可能性,并改善其治疗方案(类固醇与免疫调节疗法)。这些发现最终可能会对病理生理学有更深入的了解,并改善心肌炎的整体治疗。
3 心脏监测对早期发现心血管毒性的重要性心脏毒性相关研究中的发现对我们理解毒性作用机制方面有相当重要的作用,同时也建立起我们对不同癌种治疗药物之间生物学差异的理解。这些发现变成一种必需的工具,使我们能更好地监测和治疗心血管不良反应。近几十年间,为了帮助临床医师监测和治疗这些患者,出现了各种各样的指南。然而,大多数指南是基于有限的甚至是有争议的临床数据,以至于很难运用于临床实际,经常遭受质疑。这些争议产生的原因主要在于:(1)很多心血管毒性的结果并不是作为肿瘤临床试验的主要研究目标而得出的;(2)基于心血管毒性的监测和预防的临床试验大多有缺陷或者病例数较少。
基于目前对心脏毒性机制的理解以及其在不同抗癌药物中的不同表现,我们作了简短的综述,展示了在临床实践中以较为简单的方式评估患者出现的可疑的心脏毒性。虽然这不是适用于每例患者的完美方法,但基本可以给予读者在面对这些并发症的挑战时有一些常规的诊疗思路,见图 2。
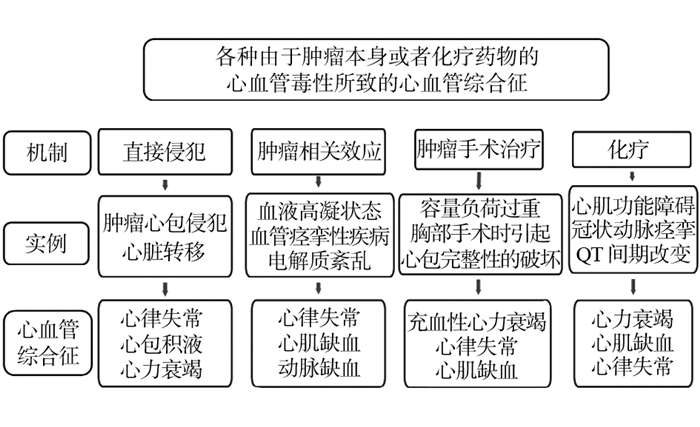
|
| 图 2 肿瘤或抗肿瘤治疗相关性心脏病 |
(1)传统化疗药如蒽环类造成的心脏损伤,包括心肌细胞的损害,在基线心功能正常的患者早期是可以耐受的,很少在治疗期间造成心肌功能障碍。这是因为心肌细胞强大的修复能力,以及在仅有少数心肌受损时其余心肌细胞可以进行代偿。然而当数年后发生心肌的二次损伤(比如心肌缺血缺氧、高血压、感染等),心肌细胞有限的修复能力无法代偿时才会显现出相关症状。在蒽环类药物治疗期间可以定期监测心脏生物标志物从而监测可能的心脏损伤,指导临床及时使用心脏保护药物。然而,在繁忙的实践中,这些监测过程的复杂性使得它很难运用于每一例患者。我们仅在临床症状提示可能有心脏损害时才进行心脏标志物的检测以鉴别心脏毒性。常规进行心脏影像学检查如超声心动图检查也是不现实的,它们一般用于基线治疗前心功能的评估,或者蒽环类用药达到一定的累积剂量(如 > 350 mg/m2)时进行。常规使用药物干预(如右丙亚胺、β受体阻滞剂或血管紧张素转化酶抑制剂)来避免心脏毒性也是存在争议的。
(2)与蒽环类药物引起的严重的心肌损伤相比,靶向治疗相关的心血管不良反应则更有异质性,其主要造成细胞功能障碍及改变信号通路的转导:如曲妥珠单抗造成的左室收缩功能不全通常在停药治疗后可以发生逆转。临床中可以反复进行超声心动图来监测心脏毒性。其他靶向药物相关的心脏毒性则很少用到心脏影像检查技术,用药过程中仅需要对患者可能出现的临床症状,定期地随访监测,如高血压、缺血性心肌病。在没有出现提示心肌功能障碍症状的情况下,一系列的心脏成像并不会发挥任何重要作用。其他靶向药物,像维洛替尼、依鲁替尼、凡德他尼、尼罗替尼等,可能造成心律失常、QT间期延长,连续的心电图检查应该足够对这些患者进行监测。
(3)第三大类心血管毒性药物主要是免疫治疗新药如免疫检查点抑制剂等。患者在使用这些药物过程中定期心脏影像检查似乎并没有多大的临床价值,因为源于这种药物造成心血管毒性主要表现为急性的、罕见的特性。定期的心脏标志物的监测被推荐用于这类药物可能造成的亚临床或者隐匿性心肌炎,但相关推荐主要来自于专家推荐而非客观的临床证据。
4 治疗和预后肿瘤或肿瘤治疗相关的心血管并发症的治疗主要遵循非肿瘤患者心脏疾病的标准治疗方法。然而,这种方法可能得不到预期的临床疗效,例如在对植入心室辅助设备的心脏衰竭患者的分析中显示:与其他病因的患者相比,化疗相关的心肌炎患者需要更多的右心辅助支持装置。可能是因为化疗所致的心肌炎患者的右心室更易遭受二次损伤,心肌受损更加严重。因此临床上肿瘤患者在不同的疾病进程、治疗方案中所产生的心血管毒性反应不同于非肿瘤患者,所以更体现出肿瘤科专家与心血管疾病专家间建立良好的临床联系的重要性和必要性。
5 总结当前,我们见证了肿瘤心脏病学领域中临床医学中心以指数式的增长,使得该领域的诊疗服务得以继续发展,这是一件令人振奋的事情。在世界范围内,心血管疾病及癌症都是高发病率、高死亡率的疾病。患者往往会经历与潜在的无症状结构性心脏疾病相关的心血管事件,这些疾病在癌症治疗的压力下逐渐显露出来。另外一些患者在特定的抗肿瘤治疗的过程中发生了至今仍让癌症及心脏专家担忧的心血管毒性。随着肿瘤患者预期寿命的延长,这些不良反应的管理变得相当重要。新的心脏不良反应机制的发现,燃起了研究者们利用新型的心脏标志物检测的兴趣,来帮助预测、监测和预防心脏毒性,并开发更为安全和可耐受的药物。另一方面,临床医师能更好地运用研究结果来筛选能耐受治疗的患者,在治疗的同时监测不良反应的发生。肿瘤心脏病学继续发展,以满足肿瘤患者在诊治肿瘤的同时也需密切关注心血管不良反应的需求。心脏病学家和肿瘤学家以多学科的方式密切合作才能使患者的治疗取得最佳效果。未来,在癌症试验中使用精确和标准化的心脏毒性评估工具,将心血管结果作为一种常见的可测量结果一定会使这一领域的研究受益。心脏病学家对癌症患者治疗的参与已经从专注于治疗心血管并发症,转变为从癌症的最初诊断到治疗后幸存时的全程管理。
| [1] | Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012[J]. CA Cancer J Clin, 2012, 62(4): 220–41. DOI:10.3322/caac.v62:4 |
| [2] | American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2014-2015[J]. Atlanta: American Cancer Society, 2014. |
| [3] | Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer[J]. N Engl J Med, 2006, 355(15): 1572–82. DOI:10.1056/NEJMsa060185 |
| [4] | Ning Y, Shen Q, Herrick K, et al. Cause of death in cancer survivors [Abstract][J]. Cancer Res, 2012, 72: LB–339. |
| [5] | Iliescu CA, Grines CL, Herrmann J, et al. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of India, and sociedad Latino Americana de Cardiologia intervencionista)[J]. Catheter Cardiovasc Interv, 2016, 87(5): E202–23. DOI:10.1002/ccd.26379 |
| [6] | Iliescu CA, Cilingiroglu M, Giza DE, et al. "Bringing on the light" in a complex clinical scenario: Optical coherence tomography-guided discontinuation of antiplatelet therapy in cancer patients with coronary artery disease (PROTECT-OCT registry)[J]. Am Heart J, 2017, 194: 83–91. DOI:10.1016/j.ahj.2017.08.015 |
| [7] | Iliescu C, Durand JB, Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients[J]. Tex Heart Inst J, 2011, 38(3): 259–60. |
| [8] | Giza DE, Lopez-Mattei J, Vejpongsa P, et al. Stress-Induced Cardiomyopathy in Cancer Patients[J]. Am J Cardiol, 2017, 120(12): 2284–8. DOI:10.1016/j.amjcard.2017.09.009 |
| [9] | Oliveira GH, Mukerji S, Hernandez AV, et al. Incidence, predictors, and impact on survival of left ventricular systolic dysfunction and recovery in advanced cancer patients[J]. Am J Cardiol, 2014, 113(11): 1893–8. DOI:10.1016/j.amjcard.2014.03.018 |
| [10] | Hassan SA, Palaskas N, Kim P, et al. Chemotherapeutic Agents and the Risk of Ischemia and Arterial Thrombosis[J]. Curr Atheroscler Rep, 2018, 20(2): 10. DOI:10.1007/s11883-018-0702-5 |
| [11] | Sanon S, Lenihan DJ, Mouhayar E. Peripheral arterial ischemic events in cancer patients[J]. Vasc Med, 2011, 16(2): 119–30. DOI:10.1177/1358863X10388346 |
| [12] | Lee HJ, Chihara D, Wang M, et al. Ibrutinib-related atrial fibrillation in patients with mantle cell lymphoma[J]. Leuk Lymphoma, 2016, 57(12): 2914–6. DOI:10.3109/10428194.2016.1169408 |
| [13] | McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling[J]. Blood, 2014, 124(25): 3829–30. DOI:10.1182/blood-2014-10-604272 |
| [14] | Mouhayar E, Salahudeen A. Hypertension in cancer patients[J]. Tex Heart Inst J, 2011, 38(3): 263–5. |
| [15] | Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J]. N Engl J Med, 2001, 344(11): 783–92. DOI:10.1056/NEJM200103153441101 |
| [16] | Sawyer DB, Zuppinger C, Miller TA, et al. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity[J]. Circulation, 2002, 105(13): 1551–4. DOI:10.1161/01.CIR.0000013839.41224.1C |
| [17] | Gao R, Zhang J, Cheng L, et al. A Phase Ⅱ, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure[J]. J Am Coll Cardiol, 2010, 55(18): 1907–14. DOI:10.1016/j.jacc.2009.12.044 |
| [18] | Groarke JD, Cheng S, Moslehi J. Cancer-drug discovery and cardiovascular surveillance[J]. N Engl J Med, 2013, 369(19): 1779–81. DOI:10.1056/NEJMp1313140 |
| [19] | Rees ML, Khakoo AY. Molecular mechanisms of hypertension and heart failure due to antiangiogenic cancer therapies[J]. Heart Fail Clin, 2011, 7(3): 299–311. DOI:10.1016/j.hfc.2011.03.004 |
| [20] | Mouhayar E, Durand JB, Cortes J. Cardiovascular toxicity of tyrosine kinase inhibitors[J]. Expert Opin Drug Saf, 2013, 12(5): 687–96. DOI:10.1517/14740338.2013.788642 |
| [21] | Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity[J]. Nat Med, 2012, 18(11): 1639–42. DOI:10.1038/nm.2919 |
| [22] | Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade[J]. N Engl J Med, 2016, 375(18): 1749–55. DOI:10.1056/NEJMoa1609214 |
| [23] | Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity[J]. Circulation, 2017, 136(21): 2085–7. DOI:10.1161/CIRCULATIONAHA.117.030571 |
| [24] | Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy[J]. J Immunother Cancer, 2016, 4: 50. DOI:10.1186/s40425-016-0152-y |
| [25] | Reuben A, Petaccia de Macedo M, McQuade J, et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab[J]. Oncoimmunology, 2017, 6(12): e1361097. DOI:10.1080/2162402X.2017.1361097 |
| [26] | Wei SC, Levine JH, Cogdill AP, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade[J]. Cell, 2017, 170(6): 1120–33. DOI:10.1016/j.cell.2017.07.024 |
| [27] | Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group[J]. J Immunother Cancer, 2017, 5(1): 95. DOI:10.1186/s40425-017-0300-z |
| [28] | Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography[J]. J Am Soc Echocardiogr, 2013, 26(9): 1013–32. DOI:10.1016/j.echo.2013.07.005 |
| [29] | Oliveira GH, Dupont M, Naftel D, et al. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support)[J]. J Am Coll Cardiol, 2014, 63(3): 240–8. DOI:10.1016/j.jacc.2013.09.040 |
 2018, Vol. 45
2018, Vol. 45
