文章信息
- 沉默YAP通过Wnt/β-catenin信号通路影响胃癌BGC823细胞的凋亡
- Effect of Yes-associated Protein Knockdown on Apoptosis of Gastric Cancer BGC823 Cells Through Wnt/β-catenin Signaling Pathway
- 肿瘤防治研究, 2018, 45(11): 864-869
- Cancer Research on Prevention and Treatment, 2018, 45(11): 864-869
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.18.0495
- 收稿日期: 2018-04-13
- 修回日期: 2018-07-18
Yes相关蛋白(Yes-associated protein, YAP)参与调控细胞的生长、器官的发育,与肿瘤的发生有关[1]。研究显示,YAP在乳腺癌、卵巢癌等肿瘤中表达异常,并且其表达水平的高低与肿瘤患者分期及预后等有关[2-3]。在胃癌中发现YAP过度表达,并且与胃癌患者的病理特征等有关[4]。YAP敲低可以抑制多种肿瘤细胞的增殖,如胰腺癌、乳腺癌等,对于肿瘤细胞的凋亡也具有调控作用[5-6]。Wnt/β-catenin在真核生物体细胞内广泛存在,在正常的成熟细胞中激活水平极低,而在肿瘤细胞中过度激活,与肿瘤细胞的增殖、凋亡等有关[7]。研究表明,YAP可以通过作用于Wnt/β-catenin信号通路影响细胞的生长,提示两者之间存在潜在联系[8]。本实验以BGC823细胞为体外研究对象,通过小RNA干扰技术降低细胞中YAP的表达,明确YAP对胃癌BGC823细胞凋亡的影响及对Wnt/β-catenin信号通路的调控作用。
1 材料与方法 1.1 材料BGC823细胞购自中国医学科学院基础医学研究所基础医学细胞中心;含半胱氨酸的天冬氨酸蛋白水解酶3(cysteinyl aspartate specific proteinase 3, Caspase-3)活性测定试剂盒和含半胱氨酸的天冬氨酸蛋白水解酶9(cysteinyl aspartate specific proteinase 9, Caspase-9)活性测定试剂盒为武汉艾美捷公司产品;Lipofectamine2000为美国Invitrogen公司产品;MMLV反转录试剂盒为美国Promega公司产品;qRT-PCR为大连宝生物公司产品;β-连环蛋白(β-catenin)抗体、c-myc抗体、细胞周期蛋白D1(CyclinD1)抗体、辣根过氧化物标记的二抗均为美国Abcam公司产品;YAP、β-actin引物由上海生工公司合成;YAP siRNA1、YAP siRNA2、siRNA control为美国Santa Cruz Biotech公司产品。YAP siRNA1:5’-GCAUCUUCGACAGUCUUCUTT-3’,5’-AGAAGACUGUCGAAGAUGCTT-3’。YAP siRNA2:5’-GGUGAUACUAUCAACCAAATT-3’,5’-UUUGGUUGAUAGUAUCACCTT-3’。
1.2 细胞分组BGC823细胞中转染YAP siRNA1和YAP siRNA2,同时转染siRNA control,依次命名为si-YAP1、si-YAP2和si-NC,以不作任何处理的BGC823细胞记为Control。细胞转染用Lipofectamine2000,具体步骤参照转染试剂说明书。BGC823细胞培养参数为:饱和湿度、37℃、5%CO2培养箱。细胞为90%时,用0.25%的胰蛋白酶消化传代细胞。细胞培养于含有10%胎牛血清的RPMI1640中。
1.3 qRT-PCR测定转染后细胞YAP mRNA水平Control、si-NC、si-YAP1、si-YAP2细胞培养48 h以后,提取总RNA。用MMLV反转录试剂盒进行反转录,程序为42℃ 65 min;70℃ 5 min;合成的cDNA保存于-80℃。以cDNA为模板,用qRT-PCR扩增。引物序列如下:β-actin F5’-CGTCTTCCCCTCCATCGT-3’,β-actin R5’-GAAGGTGTGGTGCCAGATTT-3’。YAP F5’-ACCCACAGCTCAGCATCTTCG-3’,YAP R5’-TGGCTTGTTCCCATCCATCAG-3’。结果以2-ΔΔCt法计算。
1.4 蛋白质印迹法测定转染后细胞YAP蛋白水平Control、si-NC、si-YAP1、si-YAP2细胞培养48 h以后,提取细胞中的总蛋白,蛋白保存在-80℃。取蛋白样品,用BCA法对蛋白进行定量。以每个泳道添加40 μg蛋白进行电泳,电泳前将蛋白样品和上样缓冲液以等体积混合煮沸5 min。在浓缩胶中以90 V的低压进行电泳,在分离胶中以120 V的电压电泳,观察染料进入到分离胶的底部之后关闭电源。把蛋白凝胶上的蛋白转移到PVDF膜上,以100 mA电流进行转膜,转膜在4℃进行。转膜结束后把膜放在用TBST稀释的牛血清白蛋白中封闭2 h。取出膜,放入含有YAP一抗(1:200稀释)的平皿中,4℃反应过夜后,再把膜放入含有HRP标记的二抗(1:3 000稀释)中,室温孵育2 h。取出膜,用ECL显色试剂盒显色,凝胶成像仪拍照,分析灰度值,以β-actin为内参。
1.5 MTT法测定细胞增殖Control、si-NC、si-YAP1细胞种植到96孔板中,分别培养24、48、72、96 h以后,用MTT法测定各组胃癌细胞增殖情况。步骤如下:在每孔中添加20 μl的MTT,把细胞放在37℃培养箱内继续培养约4 h,取出培养板,在每个孔中依次添加150 μl的DMSO溶液,置于振荡仪上10 min,放在酶标仪上测定每孔492 nm的A值。每个组设置5个重复孔,测定前以空白孔调零,空白孔中不加细胞。
1.6 平板克隆检测细胞克隆形成能力Control、si-NC、si-YAP1细胞以每个培养皿200个细胞接种到细胞培养皿中,每个培养皿中含有10 ml的培养液。放在37℃、5%CO2培养箱中孵育14 d后,肉眼可以观察到形成的细胞克隆。把培养液吸弃后,用PBS洗涤2次,加入4%的多聚甲醛固定各组细胞15 min。用吉姆萨染色液染约20 min后,用水把染液冲洗掉,放在空气中干燥。计数细胞克隆数目,用(细胞克隆数目÷细胞总数)×100%表示细胞克隆形成率。
1.7 流式细胞术测定细胞凋亡Control、si-NC、si-YAP1细胞分别培养48 h后,用胰蛋白酶消化,收集细胞,用PBS把细胞沉淀洗涤2次。在细胞中添加195 μl的结合缓冲液、5 μl的Annexin V-FITC,混合后再加入10 μl的PI,充分混合,流式细胞仪测定细胞凋亡。
1.8 分光光度法检测Caspase-3、Caspase-9活性Control、si-NC、si-YAP1细胞分别培养48 h后,收集各组细胞,分别用Caspase-3、Caspase-9活性检测试剂盒测定细胞中Caspase-3、Caspase-9活性。用酶标仪测定各组样品在405 nm的A值,用实验组A值÷对照组A值表示Caspase-3、Caspase-9活性。同时用蛋白质印迹法测定细胞中β-catenin、c-myc、cyclinD1、Cleaved Caspase-3、Cleaved Caspase-9蛋白表达,步骤同上,β-catenin、c-myc、cyclinD1、Cleaved Caspase-3、Cleaved Caspase-9一抗分别以1:600稀释。
1.9 Wnt/β-catenin激活剂对敲低YAP后的胃癌细胞增殖和凋亡的影响以20 mmol/L的Wnt/β-catenin激活剂LiCl处理在0 h加入转染YAP siRNA1后的细胞,记为si-YAP1+LiCl。用MTT实验测定si-YAP1+LiCl、si-YAP1细胞48 h的增殖情况,步骤同1.5。平板克隆实验测定克隆形成能力,步骤同1.6。流式细胞术测定48 h细胞凋亡,步骤同1.7。分光光度法检测48 h细胞中Caspase-3、Caspase-9活性,步骤同1.8。蛋白质印迹法测定48 h细胞中β-catenin、c-myc、cyclinD1、Cleaved Caspase-3、Cleaved Caspase-9蛋白水平,步骤同1.4。
1.10 统计学方法实验数据用SPSS21.0软件统计分析,计量资料以(x±s)表示,多组差异比较用单因素方差,组间比较用LSD-t检验,以P < 0.05为差异有统计学意义。
2 结果 2.1 转染后胃癌细胞中YAP的表达Control、si-NC、si-YAP1、si-YAP2细胞中YAP mRNA水平分别为1.00、(1.01±0.12)、(0.36±0.05)、(0.47±0.03),蛋白水平分别为(0.38±0.04)、(0.39±0.06)、(0.14±0.02)、(0.21±0.04)。si-NC胃癌细胞中的YAP mRNA和蛋白水平与Control比较没有变化。si-YAP1、si-YAP2胃癌细胞中的YAP mRNA和蛋白水平明显低于Control,差异有统计学意义(t1=22.170, P=0.000; t2=30.599, P=0.000; t3=9.295, P=0.000; t4=5.205, P=0.000)。si-YAP1胃癌细胞中的YAP mRNA和蛋白水平明显低于si-YAP2,差异有统计学意义(t1=3.268, P=0.001; t2=2.711, P=0.007),见图 1。YAP siRNA1对胃癌细胞中YAP的转录和表达抑制作用更强,后续选用si-YAP1继续研究。
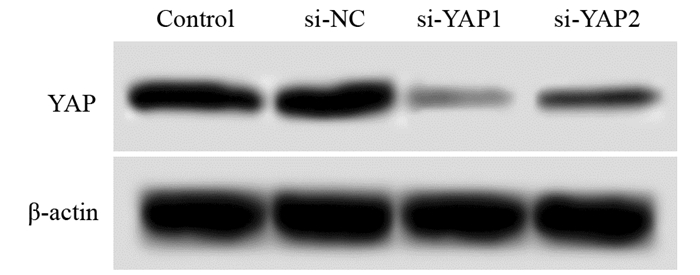
|
| 图 1 蛋白质印迹法测定转染后的胃癌细胞中YAP蛋白水平 Figure 1 YAP protein level in gastric cancer cells after transfection detected by Western blot |
Control、si-NC、si-YAP1细胞24 h的A值分别为0.35±0.04、0.36±0.03、0.24±0.01;48 h的A值分别为0.56±0.06、0.54±0.05、0.28±0.03;72 h的A值分别为0.86±0.09、0.87±0.09、0.31±0.02;96 h的A值分别为0.98±0.07、0.99±0.12、0.35±0.04。克隆形成率分别为(39.62±3.24)%、(38.49±4.51)%、(25.14±2.36)%。si-NC胃癌细胞A值和克隆形成率与Control比较没有变化。si-YAP1胃癌细胞A值和克隆形成率明显低于Control,差异有统计学意义(t1=4.621, P=0.000; t2=7.230, P=0.000; t3=10.333, P=0.000; t4=13.535, P=0.000; t5=6.257, P=0.000)。YAP敲低可以降低胃癌细胞增殖和克隆形成能力,见图 2。

|
| *:P < 0.05 图 2 YAP表达下调对细胞生长影响 Figure 2 Effect of down regulation of YAP expression on growth of gastric cancer cells |
Control、si-NC、si-YAP1细胞Caspase-3活性分别为1.00、1.03±0.14、2.61±0.22,Caspase-9活性分别为1.00、0.99±0.11、3.25±0.30,凋亡率分别为(6.32±0.58)%、(6.53±0.71)%、(32.71±2.64)%,Cleaved Caspase-3蛋白水平依次为0.15±0.06、0.16±0.03、0.62±0.05,Cleaved Caspase-9蛋白水平依次为0.52±0.07、0.54±0.09、0.97±0.12。si-NC胃癌细胞凋亡率和Caspase-3、Caspase-9活性与Control比较没有变化。si-YAP1胃癌细胞凋亡率、Caspase-3活性、Caspase-9活性和Cleaved Caspase-3、Cleaved Caspase-9蛋白水平明显高于Control,差异有统计学意义(t1=12.676, P=0.000; t2=12.990, P=0.000; t3=16.911, P=0.000; t4=10.423, P=0.000; t5=5.610, P=0.000),见图 3。YAP敲低可以诱导胃癌细胞凋亡,增加Caspase-3、Caspase-9活性。
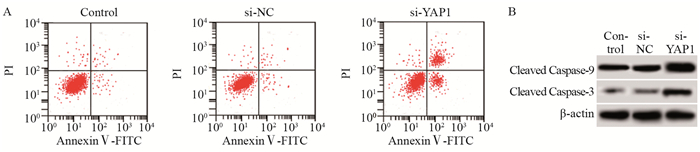
|
| A: the apoptosis of Control, si-NC, si-YAP1 groups; B: the protein expression of Cleaved Caspase-3 and Cleaved Caspase-9 in control, si-NC and si-YAP1 groups 图 3 YAP下调对胃癌细胞凋亡的影响 Figure 3 Effect of down regulation of YAP expression on apoptosis of gastric cancer cells |
Control、si-NC、si-YAP1细胞β-catenin水平分别为(0.32±0.03)、(0.33±0.02)、(0.10±0.01),c-myc水平分别为(0.48±0.05)、(0.49±0.08)、(0.05±0.02),cyclinD1水平分别为(0.66±0.06)、(0.67±0.05)、(0.14±0.06)。si-NC胃癌细胞中β-catenin、c-myc、cyclinD1蛋白水平与Control比较没有变化。si-YAP1胃癌细胞中β-catenin、c-myc、cyclinD1蛋白水平明显低于Control,差异有统计学意义(t1=12.050, P=0.000; t2=13.830, P=0.000; t3=10.615, P=0.000)。YAP敲低可以抑制胃癌细胞中β-catenin、c-myc、cyclinD1蛋白表达,抑制Wnt/β-catenin信号通路激活,见图 4。
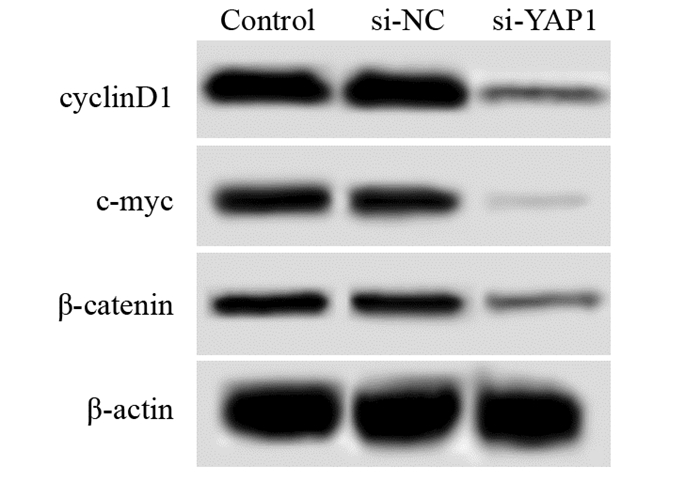
|
| 图 4 蛋白质印迹法检测敲低YAP对胃癌细胞中β-catenin、c-myc、cyclinD1蛋白的影响 Figure 4 Effect of YAP knockdown on expression of betacatenin, c-myc and cyclinD1 proteins in gastric cancer cells detected by Western blot |
si-YAP1、si-YAP1+LiCl细胞中β-catenin水平分别为(0.12±0.03)、(0.29±0.05),c-myc水平分别为(0.16±0.03)、(0.54±0.06),cyclinD1水平分别为(0.15±0.04)、(0.71±0.07)。si-YAP1+LiCl胃癌细胞中β-catenin、c-myc、cyclinD1蛋白水平明显高于si-YAP1,差异有统计学意义(t1=5.450; P=0.000, t2=9.812, P=0.000; t3=12.031, P=0.000)。Wnt/β-catenin信号通路激活剂可以促进YAP敲低后的胃癌细胞中β-catenin、c-myc、cyclinD1蛋白表达,激活Wnt/β-catenin信号通路,见图 5。
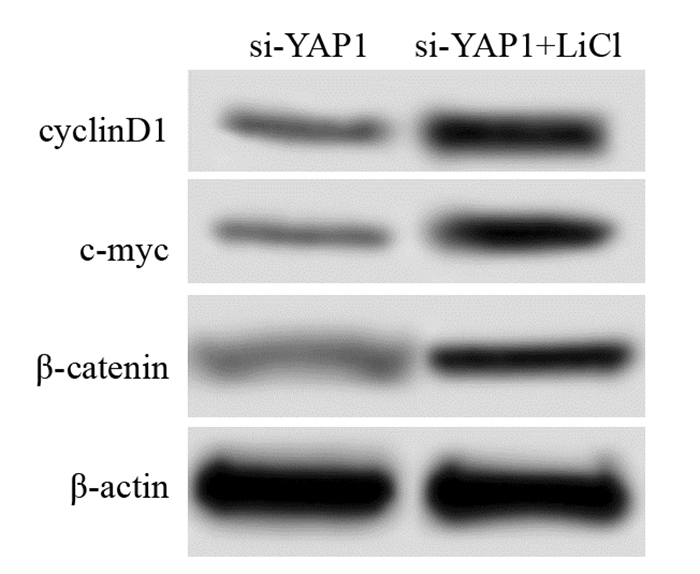
|
| 图 5 蛋白质印迹法检测Wnt/β-catenin信号通路激活剂对敲低YAP的胃癌细胞中β-catenin、c-myc、cyclinD1蛋白的影响 Figure 5 Effects of Wnt/β-catenin signaling pathway activator on expression of β-catenin, c-myc and cyclinD1 proteins in gastric cancer cells with YAP knockdown detected by Western blot |
si-YAP1、si-YAP1+LiCl细胞A值分别为0.26±0.04、0.34±0.03;克隆形成率分别为(23.51±2.30)%、(31.14±2.69)%;凋亡率分别为(34.26±3.28)%、(15.69±1.27)%;Caspase-3活性分别为1.00、(0.62±0.06),Caspase-9活性分别为1.00、(0.76±0.05),Cleaved Caspase-3水平为(0.53±0.04)、(0.32±0.06);Cleaved Caspase-9水平为(0.94±0.11)、(0.43±0.05)。si-YAP1+LiCl胃癌细胞A值和克隆形成率明显高于si-YAP1,而细胞凋亡率和Caspase-3、Caspase-9活性、Cleaved Caspase-3水平、Cleaved Caspase-9水平明显低于si-YAP1,差异有统计学意义(t1=2.771, P=0.006, t2=3.734, P=0.000, t3=9.145, P=0.000, t4=10.970, P=0.000, t5=8.314, P=0.000, t6=5.044, P=0.000, t7=7.311, P=0.000),见图 6。Wnt/β-catenin信号通路激活剂可以降低YAP敲低诱导的胃癌细胞凋亡和增殖抑制作用。

|
| A: the apoptosis of si-YAP1 and si-YAP1+LiCl; B: the protein expression of cleaved Caspase-3 and cleaved Caspase-9 in si-YAP1 and si-YAP1+LiCl groups 图 6 Wnt/β-catenin信号通路激活剂对敲低YAP的胃癌细胞凋亡影响 Figure 6 Effect of Wnt/ β-catenin signaling pathway activator on apoptosis of gastric cancer cells with YAP knockdown |
人YAP基因定位在11q13染色体上,其编码的蛋白质与转录激活有关,其本身并不能同DNA特异性结合,可以通过与存在于细胞核内的相关因子结合从而影响基因的表达[9]。YAP是Hippo的下游效应分子,其参与调控细胞的生长过程,是一个潜在的癌因子[10]。多西环素可以调控YAP的表达影响小鼠肺癌的发生和发展,而抑制YAP以后,肺癌小鼠肿瘤恶性程度降低[11]。在乳腺癌、胰腺癌、肺癌等患者的癌组织中均检测到YAP的过度表达,并且与患者的预后有关[12-14]。YAP可以调控肿瘤细胞的生长,在视网膜母细胞瘤、卵巢癌等肿瘤细胞中均得到验证[15-16]。YAP在胃癌组织中高表达,并且与胃癌裸鼠成瘤能力有关[17]。本实验结果显示,YAP敲低后的胃癌细胞增殖能力下降,并且克隆形成能力也降低,说明YAP下调后可以发挥抑制胃癌的作用,这与上述研究报道相符合。
本实验结果还显示,YAP下调后的胃癌细胞凋亡增多,并且细胞中Caspase-3、Caspase-9活化水平也升高,YAP敲低诱导Caspase-3、Caspase-9介导的胃癌细胞凋亡。很多研究报道称,YAP不仅参与肿瘤细胞的生长,还与肿瘤细胞凋亡有关[18]。研究显示,在肝癌细胞SMMC-7721中转染YAP shRNA后,细胞的生长速度明显减慢,并且细胞凋亡增多[19]。Caspase级联反应参与肿瘤细胞的凋亡,Caspase-3是该级联反应的执行因子,以酶原的形式存在于正常细胞中,其氮端含有一个前区,可以与Caspase-9等起始因子结合,在受到激活后可以诱导细胞凋亡的发生,细胞凋亡主要分为线粒体途径、死亡受体途径,其中Caspase-9参与线粒体凋亡途径,受到线粒体内相关信号刺激以后,可以活化形成Cleaved Caspase-9,从而激活Caspase级联反应,诱导凋亡发生[20]。
Wnt/β-catenin是经典的Wnt信号通路的分支,在正常情况下,β-catenin可以由多个蛋白酶形成的泛素化蛋白酶体介导而处于降解状态,使得细胞内维持低水平的β-catenin,当细胞受到相关信号刺激以后,导致细胞内的β-catenin降解受阻,β-catenin大量聚集以后转移至细胞核内,影响下游基因的转录,调控细胞的生长[21-22]。Wnt/β-catenin与多种疾病的发生有关,在糖尿病心肌病、缺血再灌注、哮喘、肺炎等疾病中均有重要作用[23-24]。研究显示,Wnt/β-catenin在肿瘤中过度激活,其下游靶基因c-myc、cyclinD1转录和表达水平升高,是肿瘤细胞过度增殖和凋亡减少的重要原因[25]。YAP与Wnt/β-catenin共同作用影响胚胎的发育,组织器官的形成,在胶质瘤中发现,YAP可以影响胶质瘤细胞中Wnt/β-catenin的激活影响肿瘤细胞的生长[26-27]。本研究表明,YAP下调可以降低Wnt/β-catenin信号通路的激活水平,减少下游靶基因c-myc、cyclinD1的表达,而用Wnt/β-catenin信号通路激活剂处理后,YAP下调对胃癌细胞增殖凋亡的影响减弱,说明YAP可以通过抑制Wnt/β-catenin信号通路的激活再通过线粒体途径调控胃癌细胞的凋亡。
综上,YAP下调后可以诱导胃癌细胞的生长并促进胃癌细胞凋亡,并且其作用机制与抑制Wnt/β-catenin信号通路有关。YAP是潜在的治疗胃癌的基因靶点,沉默其表达后可以发挥抗肿瘤的作用,这为以后靶向YAP治疗胃癌提供了有力依据。本实验只在一株胃癌细胞系中进行了初步探讨,以后会在多株胃癌细胞和动物实验中进行验证,为明确YAP在胃癌中的作用提供可靠依据。
| [1] | Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer[J]. Nat Rev Cancer, 2015, 15(2): 73–9. DOI:10.1038/nrc3876 |
| [2] | Oku Y, Nishiya N, Shito T, et al. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers[J]. FEBS Open Bio, 2015, 5(1): 542–9. DOI:10.1016/j.fob.2015.06.007 |
| [3] | Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells[J]. Cell Commun Signal, 2013, 11(1): 31. DOI:10.1186/1478-811X-11-31 |
| [4] | 张正良, 车向明, 白郑海, 等. FOXA1和YAP在胃癌中的表达及临床意义[J]. 西安交通大学学报(医学版), 2015, 36(5): 667–71. [ Zhang ZL, Che XM, Bai ZH, et al. The expressions and clinical significance of FOXA1 and YAP in gastric carcinoma[J]. Xi'an Jiaotong Da Xue Xue Bao (Yi Xue Ban), 2015, 36(5): 667–71. ] |
| [5] | 李映璇, 高绥之, 苏松, 等. Hippo-YAP信号通路活化参与二甲双胍抑制胰腺癌细胞生长的作用[J]. 中华胰腺病杂志, 2016, 16(2): 82–6. [ Li YX, Gao SZ, Su S, et al. Hippo-YAP signaling pathway activation was involved in the inhibitory effect of metformin on the growth of pancreatic cancer PANC1 cells[J]. Zhonghua Yi Xian Bing Za Zhi, 2016, 16(2): 82–6. DOI:10.3760/cma.j.issn.1674-1935.2016.02.003 ] |
| [6] | Hua K, Yang W, Song H, et al. Up-regulation of miR-506 inhibits cell growth and disrupt the cell cycle by targeting YAP in breast cancer cells[J]. Int J Clin Exp Med, 2015, 8(8): 12018–27. |
| [7] | Bellei B, Pitisci A, Catricalà C, et al. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation[J]. Pigment Cell Melanoma Res, 2011, 24(2): 309–25. DOI:10.1111/j.1755-148X.2010.00800.x |
| [8] | Konsavage WM, Kyler SL, Rennoll SA, et al. Wnt/β-Catenin Signaling Regulates Yes-associated Protein (YAP) Gene Expression in Colorectal Carcinoma Cells[J]. J Biol Chem, 2012, 287(15): 11730–9. DOI:10.1074/jbc.M111.327767 |
| [9] | Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation[J]. J Hepatol, 2015, 63(3): 679–88. DOI:10.1016/j.jhep.2015.04.011 |
| [10] | Li C, Wang S, Zhen X, et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis[J]. Nat Cell Biol, 2017, 19(2): 106–19. DOI:10.1038/ncb3464 |
| [11] | Lau AN, Curtis SJ, Fillmore CM, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis[J]. EMBO J, 2014, 33(5): 468–81. DOI:10.1002/embj.201386082 |
| [12] | Di Benedetto A, Mottolese M, Sperati F, et al. The Hippo transducers TAZ/YAP and their target CTGF in male breast cancer[J]. Oncotarget, 2016, 7(28): 43188–98. |
| [13] | Zhou X, Su J, Feng S, et al. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells[J]. Oncotarget, 2016, 7(48): 79076–88. |
| [14] | Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells[J]. Oncotarget, 2016, 7(32): 51922–33. |
| [15] | Brodowska K, AI-Moujahed A, Marmalidou A, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation[J]. Exp Eye Res, 2014, 124: 67–73. DOI:10.1016/j.exer.2014.04.011 |
| [16] | Yagi H, Asanoma K, Ohgami T, et al. GEP oncogene promotes cell proliferation through YAP activation in ovarian cancer[J]. Oncogene, 2016, 35(34): 4471–80. DOI:10.1038/onc.2015.505 |
| [17] | Zhou Z, Zhu JS, Xu ZP, et al. Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits growth and induces apoptosis in the SGC7901 gastric cancer cell line[J]. Mol Med Rep, 2011, 4(6): 1075–82. |
| [18] | Xie X, Hu Y, Xu L, et al. The role of miR-125b-mitochondria-caspase-3 pathway in doxorubicin resistance and therapy in human breast cancer[J]. Tumour Biol, 2015, 36(9): 7185–94. DOI:10.1007/s13277-015-3438-7 |
| [19] | 王琪, 邓志华. 短发夹RNA沉默YAP表达对肝癌细胞SMMC-7721增殖和凋亡的影响[J]. 中国药物与临床, 2013, 13(9): 1117–20. [ Wang Q, Deng ZH. Effects of shRNA on human hepatocellular carcinoma cell line SMMC-7721 apoptosis and proliferation via Yes-associated protein expression silencing[J]. Zhongguo Yao Wu Yu Lin Chuang, 2013, 13(9): 1117–20. ] |
| [20] | Checinska A, Hoogeland BS, Rodriguez JA, et al. Role of XIAP in inhibiting cisplatin-induced caspase activation in non-small cell lung cancer cells[J]. Exp Cell Res, 2016, 313(6): 1215–24. |
| [21] | Fan D, Ren B, Yang X, et al. Upregulation of miR-501-5p activates the wnt/β-catenin signaling pathway and enhances stem cell-like phenotype in gastric cancer[J]. J Exp Clin Cancer Res, 2016, 35(1): 177. DOI:10.1186/s13046-016-0432-x |
| [22] | Chiacchiera F, Rossi A, Jammula S, et al. Polycomb Complex PRC1 Preserves Intestinal Stem Cell Identity by Sustaining Wnt/β-Catenin Transcriptional Activity[J]. Cell Stem Cell, 2016, 18(1): 91–103. DOI:10.1016/j.stem.2015.09.019 |
| [23] | Yang X, Lv JN, Li H, et al. Curcumin reduces lung inflammation via Wnt/β-catenin signaling in mouse model of asthma[J]. J Asthma, 2017, 54(4): 335–40. DOI:10.1080/02770903.2016.1218018 |
| [24] | 王凯, 李建远. Wnt/β-catenin信号通路与发育和疾病研究进展[J]. 中国医学创新, 2012, 9(11): 160–1. [ Wang K, Li JY. Advance of Wnt/β-catenin Pathway in Development and Disease[J]. Zhongguo Yi Xue Chuang Xin, 2012, 9(11): 160–1. DOI:10.3969/j.issn.1674-4985.2012.11.103 ] |
| [25] | Zhang K, Zhu S, Liu Y, et al. ICAT inhibits glioblastoma cell proliferation by suppressing Wnt/β-catenin activity[J]. Cancer Lett, 2015, 357(1): 404–11. DOI:10.1016/j.canlet.2014.11.047 |
| [26] | Reis M, Czupalla CJ, Ziegler N, et al. Endothelial Wnt/βcatenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression[J]. J Exp Med, 2012, 209(9): 1611–27. DOI:10.1084/jem.20111580 |
| [27] | 许飞, 张进, 马端. Hippo/YAP和Wnt/β-catenin通路的对话[J]. 遗传, 2014, 36(2): 95–102. [ Xu F, Zhang J, Ma D. Crosstalk of Hippo/YAP and Wnt/β-catenin pathways[J]. Yi Chuan, 2014, 36(2): 95–102. ] |
 2018, Vol. 45
2018, Vol. 45
