文章信息
- 全脑照射联合靶向药物对HER2阳性乳腺癌脑转移患者生存的影响
- Effects of Whole-brain Radiotherapy Combined with Targeted Therapy on Improving Survival of HER2-positive Breast Cancer Patients with Brain Metastases
- 肿瘤防治研究, 2018, 45(11): 917-922
- Cancer Research on Prevention and Treatment, 2018, 45(11): 917-922
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.18.0324
- 收稿日期: 2018-03-12
- 修回日期: 2018-09-06
表皮生长因子受体2(HER2)阳性是乳腺癌脑转移(BCBM)的危险因素之一[1-2]。虽然抗HER2靶向药物在提高Ⅳ期HER2阳性乳腺癌颅外肿瘤控制和生存方面疗效明确, 但是它对单纯BCBM灶和生存的独立作用尚有争论[3-5]。主要靶向药物曲妥珠单抗(赫赛汀)分子量大, 难以通过完整血脑屏障进入中枢神经系统(central nervous system, CNS)[6]。但近年来一些研究表明对脑转移患者行全脑照射(whole-brain radiation therapy, WBRT)能够进一步破坏血脑屏障, 增加药物渗透性[7], 因此靶向药物在围WBRT期应用有可能增强WBRT对脑部肿瘤的控制, 从而提高生存。本研究通过回顾性多因素Cox分析, 探讨靶向药物联合WBRT对HER2阳性BCBM患者总体生存期(overall survival, OS)和颅内病灶控制是否有增益作用。
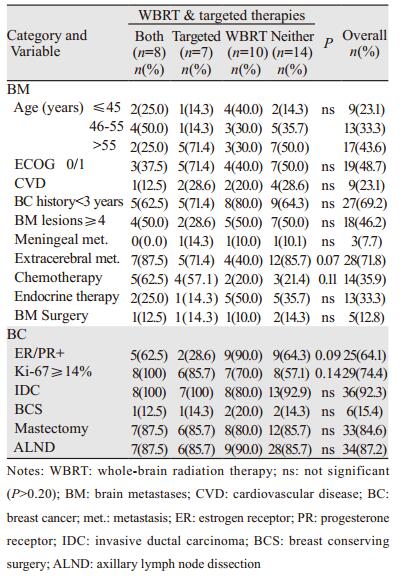
1 资料与方法 1.1 资料和随访收集2013—2015年在河北医科大学第四医院首次诊治的HER2阳性BCBM女性患者39例。入组前排除立体定向放疗(SRT)参与者2例。全部入组病例均有原发灶病理和免疫组织化学(IHC)或荧光原位杂交(FISH)检测HER2受体蛋白过度表达或基因扩增水平证实为HER2阳性浸润性乳腺癌, CT或MRI诊断为BCBM。随访截至2016年12月1日, 计算患者OS和PFS。采用无进展生存期(progression-free survival, PFS)间接衡量病灶控制。基准线资料包括年龄、美国东部肿瘤协作组活动状态评分(ECOG)、心血管疾病(CVD)、乳腺癌病史(年)、脑转移灶数目、脑膜累及状态、有无脑外病灶以及其他脑转移治疗手段(手术、化疗、内分泌治疗), 还包括手术方式(乳房保留vs.切除, 腋下淋巴清扫术)、原发乳腺肿瘤雌激素受体(ER)、孕激素受体(PR)状态以及Ki-67表达水平。
1.2 WBRT和靶向药物联合分组按WBRT和靶向药物应用状态, 将患者分为四组, 即靶向药物与WBRT联合应用组、靶向药物组、WBRT、“均无”(即其他治疗组)。所有WBRT均采用三维适形或调强放疗。靶向药物包括拉帕替尼和曲妥珠单抗, 首次应用均在BCBM诊断之后。靶向药物联合WBRT组的靶向药物应用覆盖WBRT治疗期间。
1.3 统计学方法采用SAS9.20统计软件处理数据。变量描述采用平均值、标准差、中位数、范围和百分数。组间比较采用方差分析、χ2检验或Fisher’s精确检验。利用K-M曲线评估死亡和失败率、OS和PFS中位数(MST)以及其95%CI; 采用Log rank检验评估组间差异。经单因素Cox比例风险模型筛选(P < 0.25)并结合文献复习确定多因素分析协变量。P < 0.05为差异有统计学意义。
2 结果 2.1 整体人群资料脑转移年龄为51.5±9.4(52, 27~65)岁, 乳腺癌病史≤3年者69.2%、脑转移灶≥4个者46.2%、合并脑外病灶71.8%、原发肿瘤ER/PR阳性64.1%、Ki-67高表达(≥14%)74.4%, 见表 1。
行WBRT者占46.2%(18例), WBRT剂量20~45 Gy/2~4.5周, 单次剂量1.8~2.2 Gy; WBRT剂量≥40 Gy者占77.8%(14/18), 4例行WBRT同期或序贯肿瘤局部补量放疗10~15 Gy。靶向药物应用者38.5%(15例), 除2例应用拉帕替尼外, 其余均应用曲妥珠单抗(6 mg/kg, 每3周一次方案); 所有靶向药物首次应用均在BCBM诊断之后且持续应用超过6月; 靶向药物联合WBRT者, 开始靶向药物应用时间均在WBRT前0~10月, 结束时间均在WBRT后3~12月。脑转移灶手术(包括部分切除)者12.8%(5例)。内分泌治疗者33.3%(13例), 药物包括他莫昔芬、氟维司群、卵巢切除或功能抑制剂、芳香化酶抑制剂。化疗者35.9%(14例), 药物包括卡培他滨、铂类、微管抑制剂类、替莫唑胺和甲氨蝶呤。非靶向药物治疗应用均在BCBM诊断之后。所有WBRT病例(包括同期靶向治疗者)放疗期间无2级以上急性胃肠和骨髓不良反应。
联合应用组、靶向药物组、WBRT组和其他治疗组病例百分率(例数)分别为20.5%(8例)、17.9%(7例)、25.7%(10例)和35.9%(14例); 除单纯WBRT组中合并脑外病灶率偏低(40%)和单纯靶向药物组ER/PR阳性率偏低(28.6%)外, 其他组间差异无统计学意义(P > 0.10), 见表 1。化疗者中64.3%(9/14)利用靶向药物, 高于非化疗者中靶向药物应用率24%(6/25)(χ2检验P=0.015), WBRT应用率在化疗和非化疗者两组中分别为50%(7/14)和44%(11/25)(χ2检验P=0.718)。
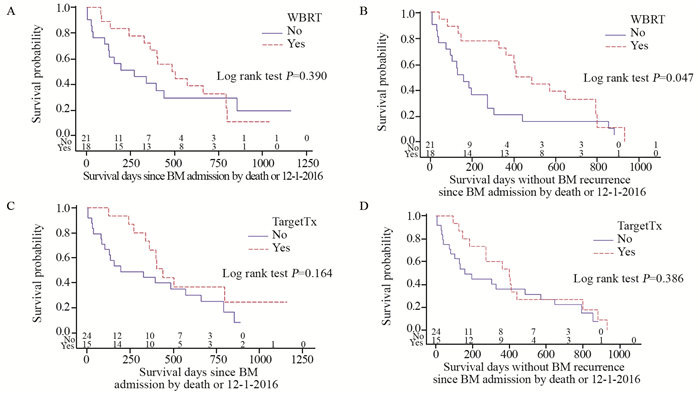
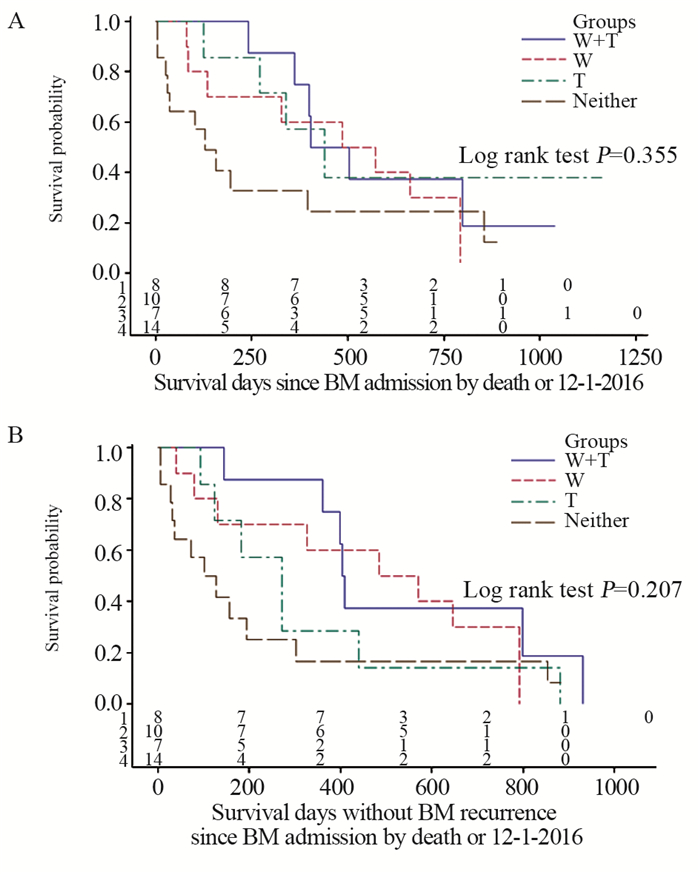
2.2 总体及各组患者生存情况患者OS和PFS中位值(95%CI)分别为13.3(6.5~19.0)和10.1(4.4~13.6)月。截至随访日, 累计总死亡率为74.4%(29例), 治疗失败率(死亡、颅内复发或新灶)为87.2%(34例)。K-M曲线评估1年死亡率47%、失败率58%, WBRT或靶向药物应用者OS和PFS均优于未应用者, 见图 1, 但仅WBRT(有vs.无)组间PFS差异有统计学意义(P=0.047), 可能与病例数偏少有关。K-M曲线评估两者联合、单纯靶向药物、单纯WBRT和其他治疗各亚组的中位生存(OS vs.PFS)分别为8(15.1 vs.13.5)、7(14.7 vs.9.0)、10(17.6 vs.17.6)和14(4.3 vs.3.8)月; Log rank检验P值分别为0.355(OS)和0.207(PFS), 见图 2。需多因素Cox分析评估WBRT和靶向药物对患者生存的影响。
|
| A:OS by WBRT; B:PFS by WBRT; C:OS by targeted therapy; D:PFS by targeted therapy 图 1 全脑照射或靶向治疗分组人群总体生存和颅内无进展生存曲线 Figure 1 Kaplan-Meier OS and intracranial PFS curves of study population stratified by whle-brain radiation therapy(WBRT) or targeted therapy (TargetTx) |
|
| A:OS by WBRT(W) & Targeted therapy (T); B:PFS by WBRT(W) & Targeted therapy (T) 图 2 全脑照射和靶向治疗应用四组人群总体生存和颅内无进展生存曲线 Figure 2 Kaplan-Meier OS and intracranial PFS curves of study population cross-stratified by whole-brain radiation therapy (W) and targeted therapy (T) |
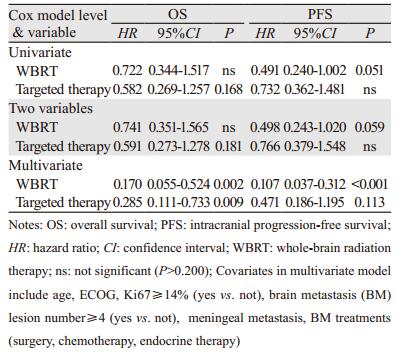
不同于单纯靶向药物应用, WBRT在单独和与靶向药物联合分析中均提示其对PFS影响有或接近有统计学意义, HR值依次为0.491(P=0.051)和0.498(P=0.059), 支持K-M曲线Log rank检验结果。多因素分析提示WBRT对OS和PFS的影响具有非常显著的统计学意义, HR值分别为0.170(P=0.002)和0.107(P < 0.001);靶向药物应用也能够明显提高OS, 可降低死亡率71.5%(HR=0.285, P=0.009), 但对提高PFS差异无统计学意义(P=0.113), 见表 2。
|
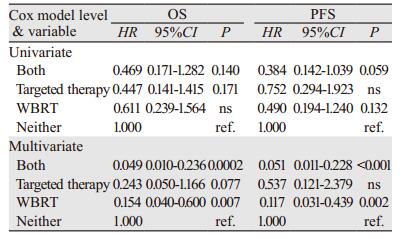
单因素分析结果表明WBRT联合靶向药物应用与其他治疗组对提高PFS上有接近统计学意义(HR=0.384, P=0.059)。与其他治疗组相比, 多因素分析结果显示WBRT联合靶向药物应用和单纯WBRT在提高OS和PFS上差异也有统计学意义, OS的HR值分别为0.049(P < 0.001)和0.154(P=0.007);PFS的HR值分别为0.051(P < 0.001)和0.117(P=0.002);单纯靶向药物也能提高OS, 但差异尚未达到统计学意义(HR=0.243, P=0.077), 见表 3。在提高生存方面, 靶向药物应用不能取代WBRT; 与其他治疗组相比, 无靶向药物应用组WBRT亦能够降低女性HER2阳性BCBM患者总体生存率和治疗失败率, 其值分别降至84.6%和88.3%;WBRT联用靶向药物时, 其值分别为95.1%和94.9%。设单纯WBRT组为对照组, WBRT联合靶向药物组OS和PFS的HR值分别为0.316(P=0.065)和0.434(P=0.167), 提示靶向药物应用对WBRT提高生存有增强作用。设单纯靶向药物组为对照组, WBRT联合靶向药物组OS和PFS的HR值为0.201(P=0.065)和0.095(P=0.003), 表示WBRT也能提高靶向治疗生存疗效。
|
有研究表明, 无论是否联合化疗或内分泌治疗, WBRT和靶向治疗均能提高HER2阳性BCBM患者OS和PFS[8-9]。本研究通过对近期临床数据进行Cox多因素分析, 显示靶向药物不能替代WBRT, 但对WBRT提高HER2阳性BCBM患者的OS有增益作用; 同样, WBRT也能够改善靶向药物的生存疗效。虽然本研究为回顾性分析, 且样本量偏少, 结论尚需临床试验研究证实, 也需要放射生物和靶向药物在HER2阳性BCBM患者CNS代谢方面的理论研究支持, 但该结论对临床和科研有一定指导意义。
靶向药物对HER2阳性BCBM颅内病灶和生存的独立影响是近年来研究热点[10]。和ER/PR受体不同, BCBM灶的HER2表达与原发肿瘤有高度一致性[11]。虽然作为首选靶向药物的曲妥珠单抗应用明显提高了HER2阳性乳腺癌生存期, 间接地促使近50%的晚期肿瘤患者复发于CNS, 但是大分子难以通过血脑屏障的特性限制了其对脑转移患者颅内病灶的疗效[6]。但有研究报道靶向药物能够通过血脑屏障且对HER2阳性BCBM治疗有效[12-14]。Dijkers等通过PET跟踪扫描用89Zr标记的曲妥珠单抗并测量其脑灶内的相对摄取值, 提示靶向药物能够部分渗透破坏了的血脑屏障[12]。Park等[13]研究表明单独应用曲妥珠单抗能明显延长HER2阳性乳腺癌BM后的中位生存期(MST)(14.9月vs.4.0月, P < 0.001)。Jacot等[14]回顾性分析39例应用曲妥珠单抗衍生药T-DM1的HER2阳性BCBM患者, 显示中位PFS为6.1月。拉帕替尼是一种表皮生长因子1(HER1)和HER2双靶点酪氨酸激酶抑制剂, 分子量小且具有亲脂性, 较容易通过血脑屏障[15]。对已接受过其他靶向药物治疗的HER阳性BCBM患者, 单独应用拉帕替尼能观察到6%CNS反应率, 联用卡培他滨时有18%~38%CNS反应率; 对首诊首治HER2阳性BCBM患者有高达66%反应率且疗效稳定[16-18]。这些研究结果使拉帕替尼联合卡培他滨成为目前HER2阳性BCBM首选治疗方法之一[4]。Yap等研究了来自6个亚洲国家的280例HER2阳性BCBM患者, 发现拉帕替尼联合曲妥珠单抗者中位生存期最长, 明显高于无靶向药物治疗者(26月vs.6月, HR=0.37, 95%CI:0.19~0.72)[19]。虽然阿法替尼已被FDA批准用于EGFR基因突变阳性NSCLC脑转移治疗, 但LUX-Breast 1和LUX-Breast 3临床试验均为阴性, 所以还不能确定增加阿法替尼能否给HER2阳性BCBM带来额外益处[20-21]。目前其他多靶点(包括HER2、erbB4等)不可逆酪氨酸激酶抑制剂(如类那替尼、妥卡替尼)等研究也有相关报道[22-23]。总之, 本研究多因素分析显示, 与其他治疗组相比, 单纯靶向药物(曲妥珠单抗、拉帕替尼)能够提高OS且差异有统计学意义(HR=0.243, P=0.077), 但对PFS提升差异无统计学意义(HR=0.537, P=0.244), 推测与样本量小有关; 就整体人群多因素分析结果而言, 靶向药物应用提高了OS(HR=0.285, P=0.009), 但对PFS的提高差异无统计学意义(0.471, P=0.113), 同样推测与样本量小有关。靶向药物提高OS机制尚不明确。有文献报道靶向药物独立提高OS是由于有效地控制了脑外病灶, 使HER2阳性BCBM患者生存有了明显提高[13, 24]。还有研究发现HER2阳性BC患者发生BM以后继续应用曲妥珠单抗仍能提高生存和颅内病灶控制[8, 25]。本研究虽然缺乏直接肿瘤测量数据, 但是靶向药物提高PFS的趋势说明其对颅内病灶控制的有效性。
目前文献缺乏针对BCBM的WBRT随机研究, 仅少数回顾性研究表明WBRT能够提高BCBM(包括HER2阳性)的OS或PFS[26-27]。Kim等回顾性分析400例BCBM, 其中WBRT占85%(339例中, 310例≥20 Gy), 结果显示“足量”照射(≥20 Gy)组中位OS较未接受“足量”照射者长4.3月(7.5 vs.3.2月, P < 0.001)[27], 但未提供HER2阳性患者的百分比。本研究中单纯WBRT患者10例, OS和PFS中位生存分别为14.7月和9.0月, 高于“均无”组(14例)。单独和交叉项多因素分析均提示WBRT能够提高OS和PFS(均P < 0.01)。
本研究结果表明WBRT和靶向治疗在提高HER2阳性BCBM生存方面有协同作用, 但机制无法确定。Stemmler等研究发现WBRT后曲妥珠单抗的脑脊液-血清浓度比值的中位数由1:420提高到了1:76[7]。Chargari等回顾分析31例HER2阳性BCBM患者应用曲妥珠单抗(17例2 mg/kg/周和14例6 mg/kg/3周剂量)和同期WBRT(26例30 Gy/10 f)者, 报道脑灶客观缓解率74%、MST 18月并且无≥2级急性不良反应, 提示两者联合应用安全且有效[28]。目前文献缺乏两者协同作用的前瞻性研究, 作者推测, WBRT和靶向治疗协同作用可能与WBRT能够提高靶向药物血脑屏障通过率、靶向药物能够增强脑转移灶对射线的敏感度以及靶向药物能够更有效地控制脑外病灶有关。
本研究分析时考虑了可能影响HER2阳性BCBM患者生存的其他因素。许多研究发现年龄偏低、体能评分差、多脑转移灶、脑膜受累、Ki-67高表达为BCBM患者生存的危险因素[4, 6, 11]; 在治疗方面, 对寡转移灶(1~3)BM者行手术或SRT(不管是否行WBRT)是一种有效治疗手段[4]。为了避免SRT可能对结果变量的独特影响, 本研究人群排除了SRT参与者2例。尽管到目前为止还没有任何系统药物得到FDA批准治疗BCBM, 但是文献中也有些证据表明化疗药物(蒽环类、铂类、卡培他滨、替莫唑胺)和内分泌治疗(他莫昔芬、芳香化酶抑制剂)应用能够使BCBM(包括HER2阳性者)颅内肿瘤控制或生存上获益[4, 11, 29-31]。因此, 对上述因素的调节分析降低了它们组间差别可能给本研究结果变量带来的差异影响。然而本研究入组病例相对较少, 未能对靶向药物(尤其是WBRT前后持续使用时间)和其他治疗(如内分泌治疗或化疗)量化后作因素调节分析, 有可能影响结论的评估。
总之, 本研究虽然病例数偏少、生存数据可信区间大, 但应用独立和交叉项的多因素分析增加了结论的可靠性。在得到临床随机试验证实之前, 本结论将对HER2阳性BCBM患者联合应用靶向药物和WBRT具有一定的临床指导意义。同时, 该结论也提示开展靶向药物联合SRT治疗寡转移BCBM研究的潜在价值。本研究证实靶向药物应用联合WBRT对提高HER2阳性BCBM患者生存有增益影响。
| [1] | Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases[J]. Breast, 2014, 23(5): 623–8. DOI:10.1016/j.breast.2014.06.009 |
| [2] | Sperduto PW, Kased N, Roberge D, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer[J]. J Neurooncol, 2013, 112(3): 467–72. DOI:10.1007/s11060-013-1083-9 |
| [3] | Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases:circumventing the blood-brain barrier[J]. Cancer Treat Rev, 2013, 39(3): 261–9. DOI:10.1016/j.ctrv.2012.05.006 |
| [4] | Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases:American Society of Clinical Oncology clinical practice guideline[J]. J Clin Oncol, 2014, 32(19): 2100–8. DOI:10.1200/JCO.2013.54.0955 |
| [5] | Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab:a retrospective substudy of the HERA trial (BIG 1-01)[J]. Lancet Oncol, 2013, 14(3): 244–8. DOI:10.1016/S1470-2045(13)70017-2 |
| [6] | Koo T, Kim IA. Brain metastasis in human epidermal growth factor receptor 2-positive breast cancer:from biology to treatment[J]. Radiat Oncol J, 2016, 34(1): 1–9. DOI:10.3857/roj.2016.34.1.1 |
| [7] | Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier[J]. Anticancer Drugs, 2007, 18(1): 23–8. DOI:10.1097/01.cad.0000236313.50833.ee |
| [8] | Morikawa A, Wang R, Patil S, et al. Characteristics and prognostic factors for patients with HER2-overexpressing breast cancer and brain metastases in the era of HER2-targeted therapy:an argument for earlier detection[J]. Clin Breast Cancer, 2017, pii:S1526-8209(17): 30394–4. |
| [9] | Le Scodan R, Jouanneau L, Massard C, et al. Brain metastases from breast cancer:prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death[J]. BMC Cancer, 2011, 11: 395. DOI:10.1186/1471-2407-11-395 |
| [10] | Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer[J]. Cancer Treat Rev, 2018, 67: 71–7. DOI:10.1016/j.ctrv.2018.05.004 |
| [11] | Shen Q, Sahin AA, Hess KR, et al. Breast cancer with brain metastases:clinicopathologic features, survival, and paired biomarker analysis[J]. Oncologist, 2015, 20(5): 466–73. DOI:10.1634/theoncologist.2014-0107 |
| [12] | Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer[J]. Clin Pharmacol Ther, 2010, 87(5): 586–92. DOI:10.1038/clpt.2010.12 |
| [13] | Park YH, Park MJ, Ji SH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients[J]. Br J Cancer, 2009, 100(6): 894–900. DOI:10.1038/sj.bjc.6604941 |
| [14] | Jacot W, Pons E, Frenel JS, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases[J]. Breast Cancer Res Treat, 2016, 157(2): 307–18. DOI:10.1007/s10549-016-3828-6 |
| [15] | Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer[J]. Pharm Res, 2012, 29(3): 770–81. DOI:10.1007/s11095-011-0601-8 |
| [16] | Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE):a single-group phase 2 study[J]. Lancet Oncol, 2013, 14(1): 64–71. DOI:10.1016/S1470-2045(12)70432-1 |
| [17] | Lin NU, Eierman W, Greil R, et al. Randomized phase Ⅱ study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases[J]. J Neurooncol, 2011, 105(3): 613–20. DOI:10.1007/s11060-011-0629-y |
| [18] | Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine[J]. Ann Oncol, 2011, 22(3): 625–30. DOI:10.1093/annonc/mdq434 |
| [19] | Yap YS, Cornelio GH, Devi BC, et al. Brain metastases in Asian HER2-positive breast cancer patients:anti-HER2 treatments and their impact on survival[J]. Br J Cancer, 2012, 107(7): 1075–82. DOI:10.1038/bjc.2012.346 |
| [20] | Cortés J, Dieras V, Ro J, et al. Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3):a randomised, open-label, multicentre, phase 2 trial[J]. Lancet Oncol, 2015, 16(16): 1700–10. DOI:10.1016/S1470-2045(15)00373-3 |
| [21] | Harbeck N, Huang CS, Hurvitz S, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1):an open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2016, 17(3): 357–66. DOI:10.1016/S1470-2045(15)00540-9 |
| [22] | Freedman RA, Gelman RS, Wefel JS, et al. Translational Breast Cancer Research Consortium (TBCRC) 022:a phase Ⅱ trial of Neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases[J]. J Clin Oncol, 2016, 34(9): 945–52. DOI:10.1200/JCO.2015.63.0343 |
| [23] | Murthy R, Borges VF, Conlin A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases:a non-randomised, open-label, phase 1b study[J]. Lancet Oncol, 2018, 19(7): 880–8. DOI:10.1016/S1470-2045(18)30256-0 |
| [24] | Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab:incidence, survival, and risk factors[J]. Oncologist, 2007, 12(7): 766–73. DOI:10.1634/theoncologist.12-7-766 |
| [25] | Park IH, Ro J, Lee KS, et al. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer[J]. Ann Oncol, 2009, 20(1): 56–62. |
| [26] | Tsao MN, Xu W, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases[J]. Cochrane Database Syst Rev, 2018, 1: CD003869. |
| [27] | Kim HJ, Im SA, Keam B, et al. Clinical outcome of central nervous system metastases from breast cancer:differences in survival depending on systemic treatment[J]. J Neurooncol, 2012, 106(2): 303–13. DOI:10.1007/s11060-011-0664-8 |
| [28] | Chargari C, Idrissi HR, Pierga JY, et al. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients[J]. Int J Radiat Oncol Biol Phys, 2011, 81(3): 631–6. DOI:10.1016/j.ijrobp.2010.06.057 |
| [29] | Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases:a retrospective, exploratory analysis in EMILIA[J]. Ann Oncol, 2015, 26(1): 113–9. DOI:10.1093/annonc/mdu486 |
| [30] | Linot B, Campone M, Augereau P, et al. Use of liposomal doxorubicin-cyclophosphamide combination in breast cancer patients with brain metastases:a monocentric retrospective study[J]. J Neurooncol, 2014, 117(2): 253–9. DOI:10.1007/s11060-014-1378-5 |
| [31] | Siena S, Crinò L, Danova M, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery:a multicenter phase Ⅱ study[J]. Ann Oncol, 2010, 21(3): 655–61. DOI:10.1093/annonc/mdp343 |
 2018, Vol. 45
2018, Vol. 45