文章信息
- miRNA-101通过靶向CDK8调控结肠癌细胞侵袭和Wnt/β-catenin通路活化
- miRNA-101 Regulates Colon Cancer Cell Invasion and Wnt/β-catenin Pathway Activation Through Targeting CDK8
- 肿瘤防治研究, 2018, 45(9): 666-671
- Cancer Research on Prevention and Treatment, 2018, 45(9): 666-671
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.17.1558
- 收稿日期: 2017-12-06
- 修回日期: 2018-05-18
2. 650021 昆明,云南省第一人民医院普外一科;
3. 230001 合肥,中国人民解放军第一零五医院护理部
2. Depatrment of General Surgery, The First People's Hospital of Yunnan Province, Kunming 650021, China;
3. Department of Nursing, The Chinese People's Liberation Army 105 Hospital, Hefei 230001, China
结肠癌是世界范围内高发的消化道肿瘤之一,近20年来发病率呈持续上升趋势,位居我国恶性肿瘤病死率的第三位[1]。miRNA-101(miR-101)在多种肿瘤中发挥肿瘤抑制作用,miR-101可能参与了多种肿瘤的发生、转移和侵袭等过程[2-4]。多项研究表明miR-101在结肠癌组织和细胞系中表达降低[5-6],且与患者病理分期[7]、癌细胞增殖、黏附和侵袭相关[8]。Strillacci等报道miR-101过表达可抑制β-catenin核内聚集[7],但其调控机制尚不清楚。
结肠癌的发病是有多种分子机制参与的复杂过程,Wnt信号异常是结肠癌最常见的发病因素。由于APC或β-catenin的突变过度激活了Wnt信号,使细胞生长不受调控。CDK8是结肠癌的一个致癌基因,降低CDK8表达可抑制结肠癌细胞增殖,诱导细胞凋亡,抑制肺转移并提高存活时间[9-11]。Firestein等发现CDK8表达量和活性都会影响β-catenin的转录活性[10]。He等发现CDK8敲除也会抑制β-catenin的表达[11]。此研究通过miR-101 mimic、pBabe-CDK8转染调控CDK8表达,并研究其对结肠癌细胞侵袭和Wnt/β-catenin激活的影响,为进一步研究直肠癌的发生、发展以及临床治疗提供重要的理论依据。
1 资料与方法 1.1 研究对象随机选择2014年1月—2017年2月在保山市人民医院就诊的结肠癌患者81例,其中男49例,女32例,年龄42~69岁,平均年龄(58.69±6.31)岁。手术后取结肠癌组织作为观察组,取切缘20 mm处癌旁正常组织(阴性切缘)作为对照组。所有患者均经组织病理学确诊,术前均未行放、化疗,并排除其他恶性肿瘤。
1.2 细胞与主要试剂结肠癌细胞株CCD841 CoN、HCT116、HT29、LoVo、SW620和COLO205均购自武汉普诺赛生命科技有限公司;CDK8、β-actin、β-catenin和Lamin B1抗体均购自上海瑞齐生物科技有限公司;DMEM培养基和胎牛血清购自Hyclone(Logan, UT, USA);LipofectamineTM 2000、Opti-MEM、质粒中提试剂盒购自Invitrogen(Grand Island, NY, USA);双荧光素报告监测系统购自Promega(Madison, WI, USA)公司。
1.3 脂质体法转染miR-101 mimicHCT116和LoVo细胞经消化后接种至12孔板,待细胞密度增长为70%左右时进行转染,利用Lipofectaminne 2000进行转染,荧光定量PCR检测过表达后miR-101的蛋白表达。
1.4 qRT-PCR检测按照常规方法提取CCD841 CoN、HCT116、HT29、LoVo、SW620和COLO205细胞总RNA并用反转录试剂盒将之反转录为cDNA。以cDNA为模板,按照All-in-one miRNA Q-PCR Detection Kit说明书(GeneCopoeia)进行反转录后,按SYBR-Green Master Mix进行qRT-PCR实验,并按95℃ 2 min1个循环;95℃ 15 s、复性温度20 s、60℃ 40 s进行扩增,45个循环,并于60℃ 40 s时采集荧光。以β-actin为内参,并用2-ΔΔCt公式计算相对表达量。为了消除操作带来的误差,我们对同一批样品进行了三次扩增。
1.5 Western blot检测将对数生长期的HCT116细胞和LoVo细胞制成适当浓度的细胞悬浮液,接种于24孔板中,培养48 h后收集细胞,提取各组细胞总蛋白,采用BCA法测定各组总蛋白浓度。用8%的SDS聚丙烯酰胺凝胶电泳分离蛋白,然后转致NC膜上。5%脱脂奶粉室温封闭后,分别加入一抗(1:1 000)、二抗(1:2 000)孵育各1 h,暗室中滴加超敏发光液孵育5 min,凝胶成像系统拍照,并用软件Quantity one分析各组蛋白表达量。
1.6 双荧光素酶活性应用在线生物信息学预测软件TargetScan、MICRORNA.ORG、PicTar对miR-101与CDK8间相互作用进行预测,发现以上软件都预测它们能够相互作用。CDK8 3'UTR-wt和CDK 3'UTR-mut由上海GenePharma公司设计合成。将HCT116、LoVo细胞接种于24孔板,当细胞生长至底板70%时,转染构建的荧光素酶报告质粒CDK8 3'UTR-wt和CDK 3’UTR-mut,转染48 h后收取细胞。细胞样品按照Promega双荧光素报告检测系统试剂盒进行检测,检测miR-101对CDK8基因转录激活的影响,以Rellina酶活性值为内参进行比较,所得数据进行t检验统计学分析。
1.7 Transwell细胞迁移及侵袭实验HCT116和LoVo细胞侵袭实验方法按照以前报道的文献进行[12]。HCT116和LoVo细胞被添加到上室后在37℃、5%CO2培养箱中孵化16 h(迁移实验)或18 h(侵袭实验)。取出带有滤膜的小室,将黏附于膜上的细胞用结晶紫染色,每组分别统计200倍视野下细胞穿过Transwell小室的细胞数目。
1.8 TOP-Flash/Fop-flash荧光素酶实验将处于对数生长期的HCT116和LoVo细胞经常规消化后接种于12孔板中,待细胞增长至70%时,随机分为3组,分别转染miR-101 mimic对照(NC组), miR-101 mimic(mimic组)和miR-101 mimic+CDK8(mimic+CDK8组)过表达载体。24 h后转染TOP-Flash/Fop-flash荧光素酶报告质粒。48 h后使用双荧光素酶报告系统(Promega, Madison, WI, USA)测定荧光素酶活性。实验重复三次,每组设3个复孔。
1.9 统计学方法采用SPSS18.0统计学软件和GraphPad Prism软件进行统计分析。所有实验数据以均数±标准差(x±s)表示。组间均数比较采用单因素方差分析,以P < 0.05为差异有统计学意义。
2 结果 2.1 结肠癌组织和细胞系中miR-101与CDK8表达的相关性为探讨miR-101与CDK8的关系,我们测定了3组结肠癌组织及周围正常组织中的miR-101和CDK8的蛋白表达情况。结果显示miR-101在结肠正常组织中的表达(2.114±0.1203)显著高于结肠癌组织(0.7033±0.02316)(P=5.321E-5)。但CDK8 mRNA和CDK8蛋白在结肠癌组织中的表达(3.626±0.1547和0.6957±0.1294)均高于结肠正常组织(1.261±0.04207和0.2811±0.0651),差异均有统计学意义(P=2.246E-5和P=0.0077),见图 1A~D。miR-101在正常CCD841 CoN细胞系(2.205±0.349)中的表达高于结肠癌HCT116、HT29、LoVo、SW620和COLO205细胞系(0.145±0.082、0.940±0.221、0.155±0.155、0.337±0.112和0.245±0.109),可见miR-101在肿瘤细胞系中表达降低,见图 1E。以正常CCD841 CoN细胞为对照,CDK8 mRNA在结肠癌HCT116、HT29、LoVo、SW620和COLO205细胞系中的表达量依次为5.479±0.377、3.155±0.353、6.240±0.434、4.337±0.507和4.245±0.307,与CCD841 CoN比较,差异有统计学意义(P=0.0001, 0.0012, 0.0001, 0.0006和0.0002),可见CDK8转录在肿瘤细胞系中增加,见图 1F。CDK8蛋白在正常CCD841 CoN细胞系及结肠癌HCT116、HT29、LoVo、SW620和COLO205细胞系中的表达依次为0.349±0.117、1.414±0.166、0.963±0.120、1.586±0.143、1.119±0.130和1.040±0.216,可见CDK8蛋白合成在肿瘤细胞系中较多(P=0.0008, 0.0032, 0.0003, 0.0016和0.0083),见图 1G~H。表明在结肠癌和结肠正常组织中miR-101与CDK8表达呈负相关关系,其中以HCT116细胞和LoVo细胞最为显著,所以最终选用这两种细胞进行后续研究。
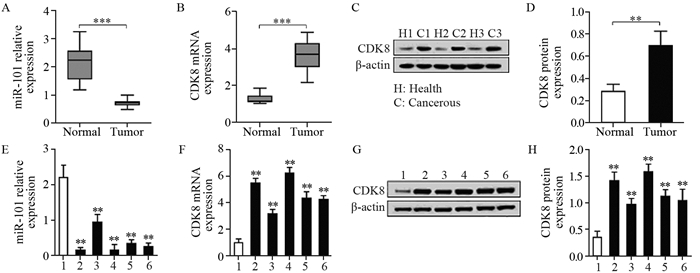
|
| 1: CCD841 CoN; 2: HCT116; 3: HT29; 4: LoVo; 5: SW620; 6: COLO205; A: expression of miR-101 in cancer and adjacent normal tissues; B: mRNA expression of CDK8 in normal tissues and colon cancer tissues; C-D: three groups of adjacent normal tissues and cancer tissues were randomly selected (H1 and C1, H2 and C2, H3 and C3), and Western blot was used to measure the expression level of CDK8; E: relative miR-101 expression in colon cancer cell lines; F-H: CDK8 mRNA and protein expressions in colon cancer cell lines. **: P < 0.01, ***: P < 0.001, compared with normal or CCD841 CoN group 图 1 miR-101和CDK8在结肠癌组织和细胞系中的表达情况 Figure 1 Relative expression of miR-101 and CDK8 in colon cancer tissues and cell lines |
分别对HCT116和LoVo细胞转染相关miR-101 mimic载体后,用qRT-PCR测定miR-101和CDK8的表达情况。结果显示,在HCT116和LoVo细胞株中,mimic组的miR-101含量分别为(4.220±0.497)和(4.440±0.459),显著高于NC组(P=0.0004和P=0.0003),表明脂质体法转染成功,见图 2A。miR-101过表达可以显著降低HCT116和LoVo细胞株中CDK8 mRNA的含量,分别为(0.323±0.089)和(0.298±0.069),与NC组比较差异有统计学意义(P=0.0019和P=0.0017)。此外,miR-101过表达也可导致HCT116和LoVo细胞株中CDK8蛋白合成的降低,分别为(0.420±0.123)和(0.322±0.092),与NC组(1.119±0.191和1.126±0.144)比较差异有统计学意义(P=0.0059和P=0.0012),见图 2B~D。进一步证实了上面的研究结果,miR-101负反馈调节CDK8的转录和翻译过程。
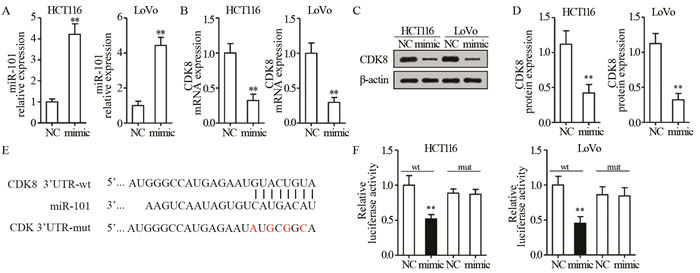
|
| A: miR-101 levels in HCT116 and LoVo cells were detected using qRT-PCR after transfection of miR-101 mimic and mimic control (NC); B: qRT-PCR was used to detect the mRNA expression level of CDK8 mRNA in cells; C-D: Western blot was used to detect the expression level of CDK8 protein in cells; E-F: Dual luciferase reporter assay was used to detect the direct binding of miR-101 to CDK8; **: P < 0.01, compared with NC group; wt: wild-type; mut: mutant-type 图 2 双荧光素酶报告实验检测miR-101与CDK8相互作用关系 Figure 2 Interaction between miR-101 and CDK8 expression detected by dual luciferase reporter assay |
在HCT116和LoVo细胞株中转染miR-101 mimic/miR-101 NC和CDK8 3'UTR-wt/CDK 3'UTR-mut,双荧光素酶报告实验结果表明,在HCT116和LoVo细胞中,miR-101 mimic可以显著降低CDK8 3'UTR荧光酶活性,分别为(0.520±0.061)和(0.453±0.095),与NC组比较差异有统计学意义(P=0.0053和0.0038),见图 2E、F。而当对miR-101与CDK8 3'UTR进行定点突变后,在两种细胞中,miR-101 mimic和miR-101 NC对CDK 3'UTR-mut的荧光素酶活性均无影响,即miR-101失去了抑制荧光素酶活性的作用。说明miR-101可直接与CDK8 3'UTR的结合位点作用来调节荧光素酶的活性。
2.3 miR-101和CDK8在结肠癌细胞侵袭中的作用由上述实验得知,在结肠癌组织中miR-101可直接靶向CDK8 3'UTR调控CDK8的表达。从实验结果可知miR-101过表达导致的CDK8表达降低可以通过转染CDK8过表达载体抑制,见图 3A、B,进一步验证了前面Western blot检测结果,即miR-101负调控CDK8的表达。细胞侵袭实验显示,miR-101过表达能显著减少HCT1116(29.667±3.055)和LoVo细胞(26.667±5.686)侵袭的数量,与NC组(82.667±7.506和81.667±7.638)比较差异有统计学意义(P=0.0003和P=0.0006),而在mimic+CDK8组,CDK8过表达抑制了miR-101转染对癌细胞侵袭的抑制作用,侵袭细胞数量分别增加至(79.000±3.606)和(74.000±8.544)(P=0.0001和P=0.0013),见图 3C、D。在HCT116和LoVo两组细胞中,miR-101过表达可明显下调结肠癌细胞中MMP13、MMP10、MMP3的mRNA表达和上调TIMP3的mRNA表达,CDK8过表达能减弱这种趋势,见图 3E、F。提示miR-101可能通过调控CDK8调控结肠癌细胞侵袭。
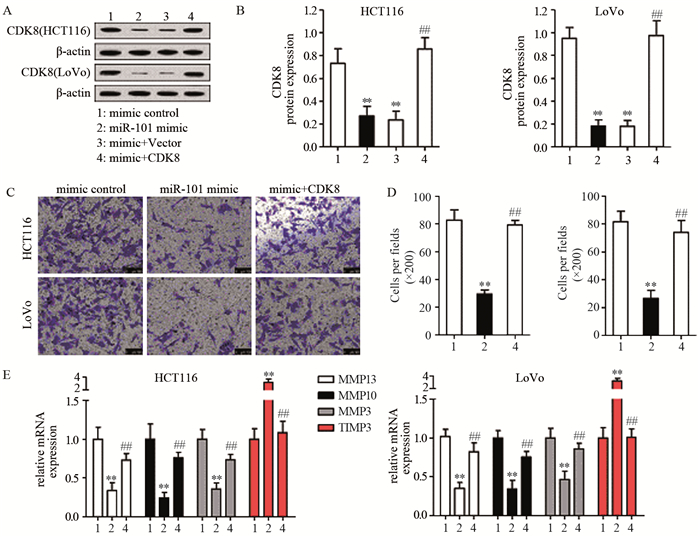
|
| A-B: after transfected with mimic control, miR-101 mimic, miR-101 mimic+vector or miR-101 mimic+CDK8 in HCT116 and LoVo cells, the expression of CDK8 protein in cells was detected by Western blot; C-D: Transwell tests were used to detect cell invasions; E: qRT-PCR was used to detect the mRNA expression of cell invasion related genes; **: P < 0.01, compared with mimic control group; ##: P < 0.01, compared with miR-101 mimic group 图 3 Transwell侵袭实验检测miR101和CDK8对癌细胞的侵袭能力的作用 Figure 3 Effect of miR-101 and CDK8 expression on invasion of colon cancer cells detected by Transwell assay |
本实验中采用TOP-Flash评估Wnt/β-catenin通路的激活/抑制状态,并用Western blot进一步验证细胞核内积累的β-catenin。我们发现与转染对照mimic CON相比(荧光强度分别为2.131±0.085和2.107±0.257),转染miR-101 mimic(荧光强度分别为0.555±0.085和0.407±0.042)能显著降低荧光素酶报告质粒应该强度(P=0.0001和0.0003)而CDK8过表达能显著增加miR-101 mimic转染导致的荧光强度降低(荧光强度分别为1.360±0.110和1.657±0.158)(P=0.0006和0.0002),见图 4A。Western blot分析结果显示,与对照组相比(0.931±0.111和0.991±0.120),miR-101过表达导致细胞核内集聚的β-catenin减少(0.462±0.057和0.530±0.087)(P=0.0029和0.0057)。与之相反CDK8过表达削弱了miR-101 mimic对细胞内β-catenin的降低作用(0.865±0.066和0.802±0.035)(P=0.0013和0.0074),见图 4B~C。研究结果说明miR-101可能是通过调控CDK8激活Wnt/β-catenin通路。
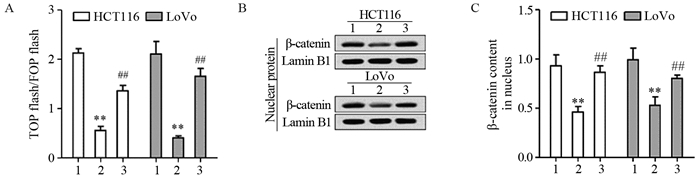
|
| 1: mimic control; 2: miR-101 mimic; 3: mimic+CDK8; A: after transfected with mimic control, miR-101 mimic, miR-101 mimic+vector or miR-101 mimic+CDK8 in HCT116 and LoVo cells, the activation of Wnt/β-catenin pathway was determined using dual luciferase reporter assay; B: Western blot was used to determine the content of β-catenin in nucleus; **: P < 0.01, compared with mimic control group; ##: P < 0.01, compared with miR-101 mimic group 图 4 miR-101靶向CDK8对Wnt/β-catenin信号通路活性影响 Figure 4 Effect of miR-101 and CDK8 on activation of Wnt/β-catenin signaling pathway |
相关文献报道在结肠癌细胞中miR-101表达的下调能促进Wnt/β-catenin通路的活化[7],提示miR-101作为抑癌基因参与肿瘤发生的分子机制,且CDK8对β-catenin有正向调节作用[10, 13-14]。但是,国内关于miR-101和CDK8的关系,以及其在结肠癌发生发展中的作用和机制的相关报道较少,因此本实验应用qRT-PCR和Western blot法检测结肠癌患者癌变组织、癌旁正常组织以及几种结肠癌细胞株中miR-101和CDK8的表达水平。研究结果显示miR-101在癌组织中表达下调,CDK8在癌组织中表达上调,在不同的结肠癌细胞株中出现相似结果。本研究依据CDK8在结肠癌中的表达明显高于癌旁正常组织,提示CDK8对于结肠癌病理进程的重要作用。同时发现在结肠癌组织和细胞系中miR-101与CDK8表达呈负相关关系。因此推测miR-101可靶向作用于CDK8调控结肠癌细胞侵袭。本研究利用双荧光素酶报告实验证实miR-101通过与靶基因CDK8 3'UTR种子区结合而抑制靶基因的表达,表明在结直肠癌中,miR-101可能是通过靶向结合调控CDK8的表达。
为了探讨miR-101对结肠癌细胞系生物学行为能力的影响,本研究向结肠癌细胞系(HCT116、LoVo)中导入miR-101的mimic和mimic+CDK8,发现增加结肠癌细胞株中miR-101表达后,肿瘤细胞的侵袭能力明显下降,CDK8能翻转miR-101过表达引起的抑制癌细胞侵袭。表明在结直肠癌中,miR-101可能是通过靶向作用于CDK8调节结直肠癌细胞的侵袭。CDK8被证实是一些重要的转录程序的共同激活因子,其中包括Wnt/β-catenin通路[15-16]。CDK8是调节活动β-catenin的结肠癌基因,并为癌细胞的增殖活动所必需[15]。Firestein等发现CDK8过表达会影响β-catenin的转录活性[13]。本实验采用TOP-Flash/Fop-flash评估Wnt/β-catenin通路的激活/抑制状态,并用免疫荧光染色法进一步验证细胞核内积累的β-catenin。结果发现miR-101过表达可以显著下调Wnt信号报告基因TOP-Flash荧光素酶活性和细胞核内β-catenin的蛋白表达,CDK8能削弱过表达载体的下调作用。说明miR-101过表达促进β-catenin蛋白从细胞核内向细胞核外转移,提示miR-101通过调控CDK8调控结肠癌细胞Wnt/β-catenin通路活化。
综上所述,本研究证实miR-101在结肠癌组织和细胞株中表达降低。在结肠癌和正常组织中miR-101与CDK8表达呈负相关关系。同时miR-101可通过直接靶向CDK8抑制结肠癌细胞的侵袭,调控Wnt/β-catenin通路活化。因此,miR-101或许能够成为诊断与治疗结肠癌的有用指标,为进一步指导直肠癌的临床治疗提供重要的理论依据。
| [1] | 巫翟, 夏忠胜. 结肠癌多药耐药机制的研究进展[J]. 新医学, 2015, 46(1): 1–6. [ Wu D, Xia ZS. Reseach progress in the mechanism of multidrug resistance in colon cancer[J]. Xin Yi Xue, 2015, 46(1): 1–6. ] |
| [2] | Su H, Yang JR, Xu T, et al. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity[J]. Cancer Res, 2009, 69(3): 1135–42. DOI:10.1158/0008-5472.CAN-08-2886 |
| [3] | Mattie MD, Benz CC, Bowers J, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies[J]. Mol Cancer, 2006, 5(1): 24. DOI:10.1186/1476-4598-5-24 |
| [4] | Bottoni A, Zatelli MC, Ferracin M, et al. Identification of differentially expressed microRNAs by microarray: a possible role for microRNA genes in pituitary adenomas[J]. J Cell Physiol, 2007, 210(2): 370–7. DOI:10.1002/jcp.v210:2 |
| [5] | Strillacci A, Griffoni C, Sansone P, et al. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells[J]. Exp Cell Res, 2009, 315(8): 1439–47. DOI:10.1016/j.yexcr.2008.12.010 |
| [6] | Schee K, Boye K, Abrahamsen TW, et al. Clinical relevance of microRNA miR-21, miR-31, miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer[J]. BMC cancer, 2012, 12(1): 505–12. DOI:10.1186/1471-2407-12-505 |
| [7] | Strillacci A, Valerii MC, Sansone P, et al. Loss of miR-101 expression promotes Wnt/β-catenin signalling pathway activation and malignancy in colon cancer cells[J]. J Pathol, 2013, 229(3): 379–89. DOI:10.1002/path.4097 |
| [8] | Chandramouli A, Onyeagucha BC, Mercado-Pimentel ME, et al. MicroRNA-101 (miR-101) post-transcriptionally regulates the expression of EP4 receptor in colon cancers[J]. Cancer Biol Ther, 2012, 13(3): 175–83. DOI:10.4161/cbt.13.3.18874 |
| [9] | Friedman JM, Jones PA, Liang G. The tumor suppressor microRNA-101 becomes an epigenetic player by targeting the polycomb group protein EZH2 in cancer[J]. Cell Cycle, 2009, 8(15): 2313–4. DOI:10.4161/cc.8.15.9168 |
| [10] | Firestein R, Bass AJ, Kim SY, et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity[J]. Nature, 2008, 455(7212): 547–51. DOI:10.1038/nature07179 |
| [11] | He SB, Yuan Y, Wang L, et al. Effects of cyclin-dependent kinase 8 specific siRNA on the proliferation and apoptosis of colon cancer cells[J]. J Exp Clin Cancer Res, 2011, 30: 109. DOI:10.1186/1756-9966-30-109 |
| [12] | 张涛, 汤为学, 蔡辉, 等. 不同转移潜能乳腺癌细胞亚系的建立与生物学特性的分析[J]. 第三军医大学学报, 2008, 30(18): 1726–9. [ Zhang T, Tang WX, Cai H, et al. Establishment of breast cancer cell sublines with different potential of metastasis and their biological characterstics[J]. Di San Jun Yi Da Xue Xue Bao, 2008, 30(18): 1726–9. DOI:10.3321/j.issn:1000-5404.2008.18.012 ] |
| [13] | Firestein R, Shima K, Nosho K, et al. CDK8 expression in 470 colorectal cancers in relation to β-catenin activation, other molecular alterations and patient survival[J]. Int J Cancer, 2010, 126(12): 2863–73. |
| [14] | Kim MY, Han SI, Lim SC. Roles of cyclin-dependent kinase 8 and β-catenin in the oncogenesis and progression of gastric adenocarcinoma[J]. Int J Oncol, 2011, 38(5): 1375–83. |
| [15] | Cai WS, Shen F, Feng Z, et al. Downregulation of CDK-8 inhibits colon cancer hepatic metastasis by regulating Wnt/β-catenin pathway[J]. Biomed Pharmacother, 2015, 74: 153–7. DOI:10.1016/j.biopha.2015.08.015 |
| [16] | Morris EJ, Ji JY, Yang F, et al. E2F1 represses β-catenin transcription and is antagonized by both pRB and CDK8[J]. Nature, 2008, 455(7212): 552–6. DOI:10.1038/nature07310 |
 2018, Vol. 45
2018, Vol. 45


