文章信息
- LSD1调控Foxo3a对卵巢癌细胞增殖和迁移的影响
- Effect of LSD1 on Proliferation and Metastasis of Ovarian Cancer Cells by Regulating Foxo3a
- 肿瘤防治研究, 2018, 45(8): 545-549
- Cancer Research on Prevention and Treatment, 2018, 45(8): 545-549
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.17.1548
- 收稿日期: 2017-12-05
- 修回日期: 2018-02-22
2. 212000 镇江,江苏大学附属医院肿瘤科
2. Department of Oncology, Affiliated Hospital of Jiangsu University, Zhenjiang 212000, China
卵巢癌是女性生殖系统的三大恶性肿瘤之一,在妇科肿瘤中死亡率最高,高达63%[1]。晚期患者的5年生存率仅为20%[2]。因此,如何控制卵巢癌转移是肿瘤临床治疗中迫切需要解决的问题。
赖氨酸特异性去甲基化酶1(lysine specific demethylase 1, LSD1)是一种黄素腺嘌呤二核苷酸依赖的胺氧化酶,可特异性去除组蛋白(histones)H3上第4位(H3K4)和第9位(H3K9)赖氨酸残基上的单甲基和二甲基修饰。LSD1在细胞中发挥多种生物学功能,影响了多种肿瘤的发生和发展。在一些恶性肿瘤,如乳腺癌[3]、胃癌、神经母细胞瘤[4]、肝癌[5]、肺癌[6]、膀胱癌[7]和急性髓系白血病[8]中LSD1均高表达;近期的研究表明,LSD1在卵巢癌组织中高表达[9]。本课题组前期研究[10]表明,稳定敲低LSD1表达能够抑制卵巢癌HO8910细胞侵袭和转移,然而LSD1在卵巢癌细胞侵袭转移中的作用机制,尚不清楚。
Foxo3a是细胞内重要的转录因子,参与细胞的增殖、分化、新陈代谢等多个方面的调控[11-12],对肿瘤细胞分化、侵袭和转移起关键性作用。Foxo3a被证实在多种肿瘤中起到抑癌基因的作用,如在乳腺癌细胞中,Foxo3a能够抑制乳腺癌细胞上皮间质转化(EMT),从而抑制细胞侵袭与迁移。
1 材料与方法 1.1 细胞和试剂诱导型稳定转染LSD1-shRNA的HO8910细胞株(HO8910-LSD1-shRNA)为本实验室保存。兔抗人LSD1购自美国Cell Signaling Technology公司,兔抗人Foxo3a购自Epitomics公司,兔抗人E-cadherin、兔抗人N-cadherin、兔抗人Snail购自Cell Signaling Technology公司,兔抗人α-tubulin单克隆抗体、羊抗兔二抗购自美国Bioworld公司;ECL显影试剂购自美国Millipore公司,聚凝胺(polybrene)、Dox和嘌呤霉素(puromycin)购自美国Sigma-Aldrich公司,LipofectAMINE2000试剂购自美国Invitrogen公司,Foxo3a过表达试剂A6730-5MG购自美国Sigma公司。PVDF膜购自美国Bio-Rad公司。
1.2 细胞培养细胞培养于含10%胎牛血清、青霉素(100 u/ml)和链霉素(100 μg/ml)的DMEM培养液(均购自美国Gibco公司),环境为37℃、5%CO2的饱和湿度条件下进行培养。
1.3 蛋白质印迹法检测LSD1、Foxo3a和EMT相关基因表达将稳定沉默LSD1基因的HO8910细胞(HO8910-LSD1-shRNA)以8 000个/孔的密度接种于6孔板中,12 h细胞贴壁后,加入浓度为1、10和100 ng/ml的Dox处理(以加入1 μl/ml蒸馏水处理的细胞作为对照组);继续培养48 h后采用蛋白质印迹法检测Foxo3a及LSD1蛋白的表达水平。用预冷的PBS洗涤细胞2次,加入100 μl含蛋白酶抑制剂和磷酸酶抑制剂的裂解液,置于冰上5~10 min,将裂解的细胞液转移至EP管中,每隔5 min涡旋15 s,共3次,离心机上12 000×g,4℃离心15 min,收集上清液(总蛋白)。蛋白浓度采用BCA(上海康成生物工程有限公司产品)法检测。各取50 ng蛋白,在浓度为10%的SDS-PAGE分离胶分离蛋白,然后将分离后的蛋白在300 mA恒流的条件下(1~2 h)转移至PVDF膜(美国Bio-Rad公司产品)上。PVDF膜在5%脱脂奶粉中封闭1 h,分别加入一抗为兔抗人LSD1多克隆抗体(体积稀释比为1:1 000)、兔抗人α-tubulin单克隆抗体(内参照)(体积稀释比为1:4 000)、兔抗人Foxo3a多克隆抗体(体积稀释比为1:2 000),4℃反应过夜。第二天用TBST洗膜3次,接着加入二抗[辣根过氧化物酶标记的羊抗兔IgG(体积稀释比均为1:1 000)],室温条件下反应1 h,TBST洗膜3次,ECL显影。蛋白质印迹法检测EMT相关蛋白的表达水平,实验方法同上。一抗分别加入兔抗人LSD1多克隆抗体(体积稀释比为1:1 000)、兔抗人α-tubulin单克隆抗体(内参照)(体积稀释比为1:4 000)、兔抗人Foxo3a多克隆抗体(体积稀释比为1:2 000)、兔抗人E-cadherin抗体(体积稀释比为1:1 000)、兔抗人N-cadherin抗体(体积稀释比为1:1 000)、兔抗人Snail抗体(体积稀释比为1:1 000)。
1.4 CCK-8方法检测细胞增殖能力取对数生长期HO8910-LSD1-shRNA细胞,PBS缓冲液洗2遍,胰酶消化3~4 min,加入含10%胎牛血清的DMEM培养液3~5 ml吹打,调整细胞密度为8×105个/毫升后将细胞接种至96孔板中,每孔种植约8 000个细胞,在37℃、5%CO2条件下培养24 h。待细胞贴壁后分别加入蒸馏水(1 μl/ml)、Dox(100 ng/ml)、A6730(20 µmol/L)、Dox(100 ng/ml)+A6730(20 µmol/L),以此分为4组,每组3个复孔。孵育(0、24、48和72 h),A6730(20 µmol/L)每天重复加一次,Dox(100 ng/ml)每两天加一次。检测前每孔加入10 µl CCK-8溶液,将培养板在37℃、5%CO2条件下孵育2 h,用酶标仪在450 nm波长处测定吸光度值,实验重复3次。
1.5 Transwell检测细胞迁移能力ECM胶提前一天放于4℃冰箱融化过夜。铺胶(冰上操作):枪头在使用前需先在冰上预冷半小时,每孔加入稀释好的ECM胶30 μl,37℃、5%CO2孵育5 h。水化基底膜:吸去小室内的ECM胶,每个小室加入50 μl无血清DMEM培养液,37℃、5%CO2孵育30 min后吸去培养液。制备细胞悬液:将HO8910-LSD1-shRNA细胞用胰酶消化3~4 min,吸去胰酶,无血清DMEM悬浮,细胞调至1×105个/毫升,每孔加入150 μl细胞悬液。下室加入600 µl含10%血清的DMEM,设置4组小室,每组重复3次。待细胞贴壁后分别在4组小室中加入Dox(100 ng/ml)+A6730(20 μmol/L)、Dox(100 ng/ml)、A6730(20 µmol/L)、蒸馏水(1 μl/ml)。继续培养24 h后吸弃小室中液体,取出小室,置于500 μl 4%多聚甲醛中室温固定30 min,吸弃小室内液体,置于500 μl 0.1%结晶紫液体中室温15 min,将小室于PBS中洗数次至透明,用棉签轻轻擦掉上层未迁移的细胞,注意不要擦去下层已经迁移的细胞,在显微镜下随机选取5个视野观察细胞,计数取平均值。
1.6 统计学方法应用SPSS13.0和GraphPad Prism 5统计学软件对所有的实验数据进行统计学分析和做图整理,所有实验均独立重复3次,数据以(x±s)表示。Student t检验用于2组数据间的比较,多组间均数比较采用单因素方差分析,组内两两比较采用LSD-t检验,P < 0.05为差异有统计学意义。
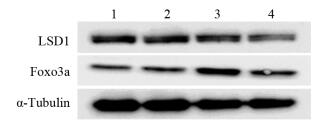
2 结果 2.1 干扰LSD1表达上调Foxo3a蛋白水平蛋白质印迹法结果显示,与对照组比较,观察组随Dox浓度增加LSD1蛋白水平逐渐下降,而Foxo3a蛋白表达水平逐渐增加。100 ng/ml Dox处理的细胞中LSD1表达水平下降及Foxo3a表达水平增加最显著。表明抑制LSD1表达可上调Foxo3a蛋白水平,见图 1。
|
| 1: control group; 2-4: 1, 10 and 100 ng/ml Dox groups 图 1 干扰LSD1表达后Foxo3a蛋白水平变化 Figure 1 Knockdown of LSD1 upregulated Foxo3a protein levels |
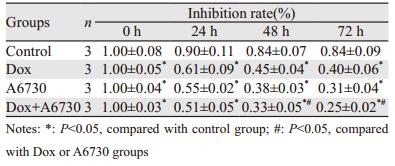
CCK-8结果表明,与对照组比较,联合组、Dox组、A6730组细胞增殖率均显著下降(抑制率分别为29.8%、47.6%、36.9%),差异有统计学意义(P=0.019、0.002、0.013)。
联合组与Dox组和A6730组比较,细胞增殖率均显著下降(抑制率分别为62.5%、80.6%),差异有统计学意义(P=0.003、0.016),见表 1。
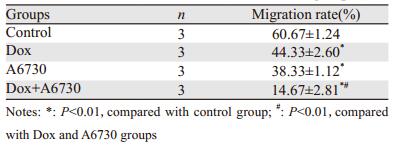
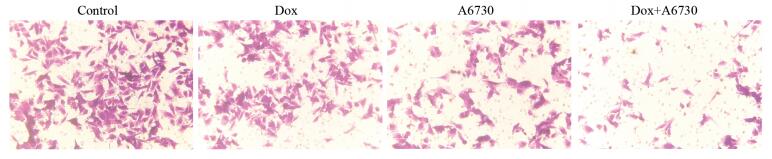
Transwell结果显示,与对照组比较,Dox组、A6730组、联合组细胞穿过小室基底膜数间差异有统计学意义(P=0.001、0.001、0.000)。此外,与Dox组和A6730组比较,联合组细胞迁移率显著降低(P=0.000、0.001)。提示LSD1通过抑制Foxo3a,增强了卵巢癌细胞的迁移能力,见表 2、图 2。
|
| 图 2 Transwell实验检测四组卵巢癌细胞的迁移情况(×200) Figure 2 Metastasis of ovarian cancer cells in four groups detected by Transwell assay (×200) |
蛋白质印迹法结果显示,Dox组和A6730组与对照组相比,E-cadherin表达量增加,而N-cadherin及Snail表达量下降。此外,与Dox组或A6730组比较,联合组Foxo3a和E-cadherin表达量明显增加,而N-cadherin和Snail表达量下降。表明Foxo3a介导了LSD1对EMT标志物表达具有调节作用,见图 3。
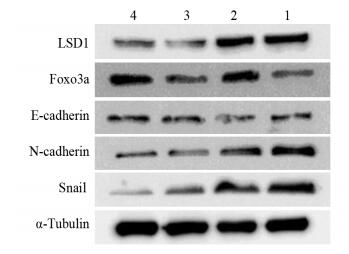
|
| 1: control group; 2: A6730 group; 3: Dox group; 4: Dox+A6730 group 图 3 Foxo3a介导LSD1对EMT相关蛋白表达的调控 Figure 3 Foxo3a mediated the regulation of LSD1 on EMT-related protein expression |
LSD1的调控涉及肿瘤的多个病理过程,在肿瘤的发生、增殖、转移和凋亡过程中都起着举足轻重的作用,并且对肿瘤细胞的维持必不可少。LSD1首先被证实与肿瘤的分化及生长有关,Schulte等[13]研究提示,LSD1与成神经细胞瘤分化密切相关,LSD1在低分化的成神经细胞瘤中高表达,实验采用siRNA干扰LSD1基因的表达后,肿瘤细胞的生长受到明显抑制;体内研究进一步证实,沉默LSD1基因的表达可以抑制成神经细胞瘤的生长。接着LSD1被证实与肿瘤的凋亡相关,Huang等[14]研究提示,LSD1的特异性抑制物能够诱导人结肠癌细胞的凋亡,并导致异常沉默的基因恢复表达。进一步LSD1被证实与肿瘤的侵袭与转移相关,Lv等[15]发现,LSD1可以促进肺癌细胞的增殖、侵袭和转移。
Fox转录因子家族的共同特征是拥有Fox(Forkhead Box)结构域,Fox是一个保守的DNA结合结构域,该结构域由3个α螺旋、3个β折叠以及2个翼状结构组成的翼螺旋结构域[16]。Fox家族成员众多,分为A-S共19个亚族,Foxo3a即属于FoxO家族。Foxo3a是研究最为完善的成员,现已发现,Foxo3a在几乎所有常见肿瘤中均表达,如肝癌[17]、肺癌[18]、胃癌[19]、前列腺癌[20]、乳腺癌[21]、淋巴瘤[22]、白血病等。如在乳腺癌细胞[23]中,Foxo3a能够抑制乳腺癌细胞上皮间质转化从而抑制细胞的侵袭与转移;同时在尿路上皮细胞肿瘤[24]中,Foxo3a下调促进TWIST1、YBX1表达增高,抑制了E-cadherin的表达从而抑制肿瘤细胞迁移。由此可见,Foxo3a调控肿瘤细胞的侵袭与转移能力在不同肿瘤中调控的结果有所不同。据此我们推测LSD1是否通过调控Foxo3a表达来调控卵巢癌细胞的侵袭与迁移。
本研究发现,抑制LSD1表达可以上调Foxo3a转录因子的表达,从而抑制卵巢癌HO8910细胞的迁移能力。有研究表明,LSD1能够通过Akt通路影响肿瘤细胞的侵袭与转移[25],同时Akt通路能够通过促进Foxo3a从细胞核内迁移至胞质内[26],从而抑制Foxo3a转录因子的作用。结合本研究的数据推测,LSD1可能通过Akt通路,抑制Foxo3a表达,从而促进了卵巢癌细胞的迁移。但LSD1是如何通过Akt通路调控Foxo3a的表达,以及LSD1-AKT-Foxo3a通路在卵巢癌细胞侵袭转移的作用还有待进一步研究。
综上所述,LSD1与Foxo3a对卵巢癌的发生与发展起着重要的作用,LSD1通过抑制Foxo3a表达促进卵巢癌HO8910细胞增殖与迁移,LSD1-Foxo3a通路有望为卵巢癌的治疗提供新依据。
| [1] | Maldonado L, Hoque MO. Epigenomics and ovarian carcinoma[J]. Biomark Med, 2010, 4(4): 543–70. DOI:10.2217/bmm.10.72 |
| [2] | Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010[J]. CA Cancer J Clin, 2010, 60(5): 277–300. DOI:10.3322/caac.20073 |
| [3] | Cao C, Vasilatos SN, Bhargava R, et al. Functional interaction of histone deacetylase 5 (hdac5) and lysine-specific demethylase 1 (lsd1) promotes breast cancer progression[J]. Oncogene, 2017, 36(1): 133–45. DOI:10.1038/onc.2016.186 |
| [4] | Amente S, Milazzo G, Sorrentino MC, et al. Lysine-specific demethylase (lsd1/kdm1a) and mycn cooperatively repress tumor suppressor genes in neuroblastoma J][J]. Oncotarget, 2015, 6(16): 14572–83. |
| [5] | Sakamoto A, Hino S, Nagaoka K, et al. Lysine demethylase lsd1 coordinates glycolytic and mitochondrial metabolism in hepatocellular carcinoma cells[J]. Cancer Res, 2015, 75(7): 1445–56. DOI:10.1158/0008-5472.CAN-14-1560 |
| [6] | Zhang X, Zhang X, Yu B, et al. Oncogene lsd1 is epigenetically suppressed by mir-137 overexpression in human non-small cell lung cancer[J]. Biochimie, 2017, 137: 12–9. DOI:10.1016/j.biochi.2017.02.010 |
| [7] | Kauffman EC, Robinson BD, Downes MJ, et al. Role of androgen receptor and associated lysine-demethylase coregulators, lsd1 and jmjd2a, in localized and advanced human bladder cancer[J]. Mol Carcinog, 2011, 50(12): 931–44. DOI:10.1002/mc.20758 |
| [8] | Mould DP, McGonagle AE, Wiseman DH, et al. Reversible inhibitors of lsd1 as therapeutic agents in acute myeloid leukemia: Clinical significance and progress to date[J]. Med Res Rev, 2015, 35(3): 586–618. DOI:10.1002/med.2015.35.issue-3 |
| [9] | Konovalov S, Garcia-bassets I. Analysis of the levels of lysine-specific demethylase 1 (lsd1) mrna in human ovarian tumors and the effects of chemical lsd1 inhibitors in ovarian cancer cell lines[J]. J Ovarian Res, 2013, 6(1): 75. DOI:10.1186/1757-2215-6-75 |
| [10] | 刘秀文, 魏野, 李袁霞, 等. 沉默LSD1基因的表达可抑制卵巢癌H0890细胞的增殖并诱导其凋亡[J]. 肿瘤, 2016, 36(5): 485–95. [ Liu XW, Wei Y, Li YX, et al. LSD1 gene-silencing inhibits proliferation and promotes apoptosis of ovarian cancer H08910 cells[J]. Zhong Liu, 2016, 36(5): 485–95. ] |
| [11] | Lin H, Dai T, Xiong H, et al. Unregulated mir-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor foxo3a[J]. PLoS One, 2010, 5(12): [1] Maldonado L, Hoque MO. Epigenomics and ovarian carcinoma[J]. Biomark Med, 2010, 4(4): 543-70.. |
| [12] | Mandinova A, Lefort K, Tommasi di Vignano A, et al. The FoxO3a gene is a key negative target of canonical notch signalling in the keratinocyte UVB response[J]. EMBO J, 2008, 27(8): 1243–54. DOI:10.1038/emboj.2008.45 |
| [13] | Schulte JH, Lim S, Schramm A, et al. Lysine-specific demethy lase1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy[J]. Cancer Res, 2009, 65(5): 2065–71. |
| [14] | Huang Y, Nayak S, Jankowitz R, et al. Epigenetics in breast cancer: What's new?[J]. Breast Cancer Res, 2011, 13(6): 225. DOI:10.1186/bcr2925 |
| [15] | Lv T, Yuan D, Miao X, et al. Over-expression of lsd1 promotes proliferation, migration and invasion in non-small cell lung cancer[J]. PLoS One, 2012, 7(4): e35065. DOI:10.1371/journal.pone.0035065 |
| [16] | Katoh M, Igarashi M, Fukuda H, et al. Cancer genetics and genomics of human fox family genes[J]. Cancer Lett, 2013, 328(2): 198–206. DOI:10.1016/j.canlet.2012.09.017 |
| [17] | Boix L, López-Oliva JM, Rhodes AC, et al. Restoring mir122 in human stem-like hepatocarcinoma cells, prompts tumor dormancy through smad-independent tgf-β pathway[J]. Oncotarget, 2016, 7(44): 71309–29. |
| [18] | Cheng CW, Chen PM, Hsien YH, et al. Foxo3a-mediated overexpression of microrna-622 suppresses tumor metastasis by repressing hypoxia-inducible factor-1α in erk-responsive lung cancer[J]. Oncotarget, 2015, 6(42): 44222–38. |
| [19] | Yang XB, Zhao JJ, Huang CY, et al. Decreased expression of the foxo3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients[J]. PLoS One, 2013, 8(10): e78158. DOI:10.1371/journal.pone.0078158 |
| [20] | Das TP, Suman S, Alatassi H, et al. Inhibition of akt promotes foxo3a-dependent apoptosis in prostate cancer[J]. Cell Death Dis, 2016, 7: e2111. DOI:10.1038/cddis.2015.403 |
| [21] | Kocak C, Kocak FE, Bayhan Z, et al. Value of immunohistochemical detection of foxo3a as a prognostic marker in human breast carcinoma[J]. Inter J Clin Expe Pathol, 2016, 9(10): 9938–50. |
| [22] | Obradorhevia A, Serra-sitjar M, Rodríguez J, et al. The tumour suppressor foxo3 is a key regulator of mantle cell lymphoma proliferation and survival[J]. Br J Haematol, 2012, 156(3): 334–45. DOI:10.1111/bjh.2012.156.issue-3 |
| [23] | Jiang Y, Zou L, Lu WQ, et al. Foxo3a expression is a prognostic marker in breast cancer[J]. PLoS One, 2013, 8(8): e70746. DOI:10.1371/journal.pone.0070746 |
| [24] | Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease[J]. Brain Res, 2010, 1338: 89–99. DOI:10.1016/j.brainres.2010.03.035 |
| [25] | Feng S, Jin Y, Cui M, et al. Lysine-specific demethylase 1 (lsd1) inhibitor s2101 induces autophagy via the akt/mtor pathway in skov3 ovarian cancer cells[J]. Med Sci Monit, 2016, 22: 4742–8. DOI:10.12659/MSM.898825 |
| [26] | Marlow LA, von Roemeling CA, Cooper SJ, et al. Foxo3a drives proliferation in anaplastic thyroid carcinoma through transcriptional regulation of cyclin a1: A paradigm shift that impacts current therapeutic strategies[J]. J Cell Sci, 2012, 125(18): 4253–63. DOI:10.1242/jcs.097428 |
 2018, Vol. 45
2018, Vol. 45