文章信息
- SNAI2基因对低转移潜能乳腺癌细胞恶性特征的影响及机制
- Effects and Regulatory Mechanisms of SNAI2 on Malignant Hallmarks of Non-Invasive Breast Tumor Cells
- 肿瘤防治研究, 2018, 45(8): 528-532
- Cancer Research on Prevention and Treatment, 2018, 45(8): 528-532
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.17.1408
- 收稿日期: 2017-11-07
- 修回日期: 2018-01-02
2. 443000 宜昌,湖北省宜昌市三峡大学第一临床医学院感染性疾病科;
3. 443000 宜昌,湖北省宜昌市三峡大学第一临床医学院中心实验室
2. Department of Infectious Disease, The First College of Clinical Medical Science, China Three Gorges University, Yichang 443000, China;
3. Center Lab, The First College of Clinical Medical Science, China Three Gorges University, Yichang 443000, China
乳腺癌是女性最常见的恶性肿瘤。全世界每年约有120万妇女罹患乳腺癌,并还在以每年2%的速度递增[1]。我国乳腺癌的总体发病率在过去30年中上升了近96%,严重威胁女性健康[1]。近年来随着肿瘤临床理论及相关诊疗技术的快速发展,乳腺癌患者的无病生存率得到了明显提高,但仍面临较高的肿瘤复发和远处转移风险,这也是导致患者死亡的主要原因[2]。
SNAI2是锌指转录因子Snail家族成员之一,主要参与肿瘤细胞侵袭迁移等恶性特征的调控[3-5]。SNAI2在多种类型的人类肿瘤组织中均有表达,并且表达量与肿瘤低分化、肿瘤的复发转移及不良预后密切相关[6]。相关临床危险因素相关性分析表明,SNAI2表达增高是多种肿瘤转移后复发的主要危险因素[7-8],这些研究均聚焦在具有较高转移潜能的肿瘤细胞中,而SNAI2对低转移潜能肿瘤细胞的影响目前尚不清楚。低转移潜能肿瘤细胞的转移潜能虽然极弱,但研究表明绝大部分该类型的肿瘤患者最终都由于转移而导致死亡。因此研究极低转移潜能肿瘤细胞如何获得转移潜能并完成转移对肿瘤的防治具有重要的意义。
本研究采用慢病毒载体构建GV367-SNAI2用于转染低转移潜能的乳腺癌MCF-7和T-47D细胞,并进一步探索SNAI2基因对低转移潜能的肿瘤细胞恶性特征的影响,为将SNAI2作为乳腺癌的基因靶向治疗提供新的方法和理论支撑。
1 材料与方法 1.1 细胞培养人MCF7和T-47D细胞均为低转移潜能的乳腺癌细胞[9],购于中国科学院上海细胞库。Lipofectamine2000购自美国Thermo公司,GV367、GV367-SNAI2、pHelper 1.0和pHelper 2.0载体质粒购自上海吉凯基因生物有限公司。细胞采用含10%胎牛血清(Gibco公司, 南美)的DMEM培养液于37℃、5%CO2培养箱中培养。
1.2 稳定表达SNAI2基因的乳癌细胞系的建立(1)转染前2 h将乳癌细胞培养液更换为无血清培养液;(2)将DNA溶液(GV367载体质粒20 μg、pHelper 1.0载体质粒15 μg、pHelper 2.0载体质粒10 μg),与相应体积的Lipofectamine2000混匀,调整总体积为1 ml,室温下温育15 min;(3)将以上混合液缓慢滴加至已预处理的乳癌细胞培养液中混匀,于37℃、5%CO2细胞培养箱中培养;(4)培养6 h用无菌PBS洗三次,常规培养液继续培养48 h后于倒置显微镜下观察。转染后的肿瘤细胞经过嘌呤霉素筛选成功后再进行下一步的实验,具体操作流程参考产品说明书。
1.3 MTT法检测细胞增殖能力将处于对数生长期的细胞接种于96孔板中,每孔细胞数量约(1.5~2.0)×103个,经处理后采用Cell Titer 96® AQueous One Solution细胞增殖检测试剂盒Promega检测细胞增殖水平,具体操作方式详见说明书。实验分组为:(1)对照组(Control):MCF-7或T-47D;(2)空白载体对照组(Control-vector):转染GV367空载体后的MCF-7或T-47D细胞;(3)高表达SNAI2基因(SNAI2)组:转染GV367-SNAI2后的MCF-7或T-47D细胞。
1.4 SNAI2 shRNA慢病毒转染肿瘤细胞根据产品说明书,采用SNAI2 shRNA慢病毒载体、空白对照shRNA颗粒(Santa Cruz Biotech, Inc.)沉默肿瘤细胞MCF-7和T-47D内SNAI2的表达。转染后的肿瘤细胞经过嘌呤霉素筛选成功后再进行下一步的实验。
1.5 体外细胞迁移实验将处于对数生长期的肿瘤细胞接种于12孔板中,每孔细胞数量为(4~5)×105个,DMEM(含10%胎牛血清)培养过夜,待细胞充分贴壁后,用无菌牙签在贴壁细胞上划“一”字划痕,PBS清洗3次后,用无血清DMEM培养24 h,吸去培养液,PBS清洗3次后,倒置荧光显微镜下观察并测量细胞迁移距离,拍照分析。不同组细胞的迁移能力以迁移指数来比较,迁移指数=实验组的迁移距离/对照组的迁移距离[10]。
1.6 细胞凋亡能力检测将细胞接种于24孔板中培养,按1:100稀释比例滴加适量的Hoechst 33342活细胞染色液(×100),轻柔混匀后,置于培养箱内孵育10 min,弃含染料的培养液,PBS洗涤2~3次后荧光显微镜下观察并计数阳性染色细胞数。
1.7 Real-time PCR检查基因表达水平首先用TRIzol(Invitrogen, Carlsbad, CA)从细胞中提取总RNA,然后采用real-time RT-PCR技术检测细胞内mRNA的相对表达水平[9],GAPDH为内参。引物序列如下所示:GAPDH, 正义:5'-TCATTGACCTCAACTA CATGGTTT-3', 反义:5'-GAAGATGGTGATGGGA TTTC-3'; Cyclin D1, 正义:5’-CTACACCGACAACTCCATC-3', 反义:5’-TCCAGCAGGGCTTCG ATC-3’; MMP9, 正义:5′-CAGTCCACCCTTGTGCTCTTCC-3′, 反义:5′-CTGCCAC CCGAGTGTAACCAT-3′; VIM, 正义:5'-CCAGGCAAAGCAGGAGTC-3', 反义:5'-GGGTATCAACCAGAGGGAGT-3'; E-cadherin, 正义:5'-ACGCATTGCCACATACACTC-3', 反义:5'- GTAGGGAAACTCT CTCGGTC-3'。
1.8 统计学方法实验数据结果的获得至少通过3次独立的重复实验,应用SPSS17.0软件,采用配对t检验的方式对数据进行分析,数据经处理后以(x±s)表示,P < 0.05为差异有统计学意义。
2 结果 2.1 稳定表达SNAI2基因的细胞株鉴定根据产品说明书,将GV367-SNAI2转染MCF-7和T-47D细胞,观察病毒转染48 h后的细胞,荧光显微镜观察可见大量绿色荧光,提示转染成功,结果见图 1。
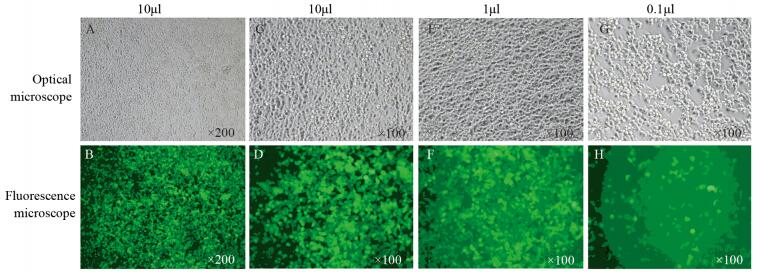
|
| A, B, C, D: breast cancer cells transfected with 10μl GV367-SNAI2; E, F: breast cancer cells transfected with 1μl GV367-SNAI2; G, H: breast cancer cells transfected with 0.1μl GV367-SNAI2 图 1 荧光显微镜观察转染GV367-SNAI2的乳腺癌细胞 Figure 1 Breast cancer cells transfected with GV367-SNAI2 observed by fluorescence microscope |
MTT法检测结果显示:相对于Control和Control-vector组,SNAI2组细胞的增殖能力明显增强,且差异有统计学意义(均P < 0.05),见图 2A。反之,MCF-7细胞经SNAI2-shRNA沉默SNAI2后,细胞增殖能力较对照组稍有降低,但差异无统计学意义(均P > 0.05),见图 2B。
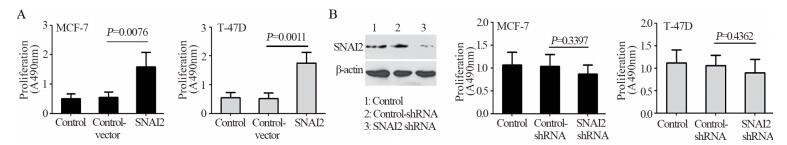
|
| A: tumor cells were transfected with or without control-vector or GV367-SNAI2. The proliferation abilities of MCF-7 and T-47D cells were tested by MTT assay; B: SNAI2 expression in control, control shRNA and SNAI2 shRNA groups of MCF-7 cells were detected by Western blot. The proliferative potential of MCF-7 and T-47D cells in control, control shRNA and SNAI2 shRNA groups were tested by MTT assay 图 2 MTT法检测SNAI2对低转移潜能乳腺癌细胞增殖能力的影响 Figure 2 Effect of SNAI2 on proliferation ability of non-invasive breast tumor cells detected by MTT assay |
划痕实验结果显示:相对于Control和Control-vector组,SNAI2组细胞的迁移能力明显增强,且差异有统计学意义(均P < 0.001),见图 3。
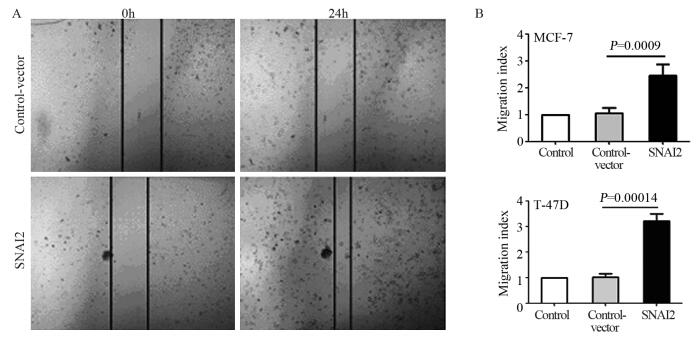
|
| 图 3 划痕实验检测SNAI对低转移潜能乳腺癌细胞迁移能力的影响 Figure 3 Effect of SNAI2 on migration ability of non-invasive breast tumor cells detected by wound healing assay |
细胞核染料Hoechst 33342检查结果显示:相对于Control和Control-vector组,SNAI2组细胞的抗凋亡能力明显增强,且差异有统计学意义(均P < 0.05),见图 4。
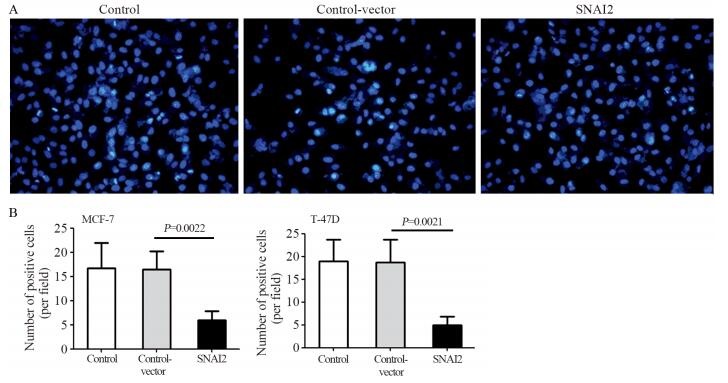
|
| 图 4 Hoechst 33342检查SNAI2对低转移潜能乳腺癌细胞抗凋亡能力的影响 Figure 4 Effect of SNAI2 on anti-apoptosis ability of non-invasive breast tumor cells detected by Hoechst 33342 assay |
Real-time PCR法检测结果显示:相对于Control和Control-vector组,SNAI2组细胞内的Cyclin D1、MMP9和VIM基因表达水平明显提高,而E-CDH基因的表达水平降低,且差异有统计学意义(均P < 0.05),见图 5。
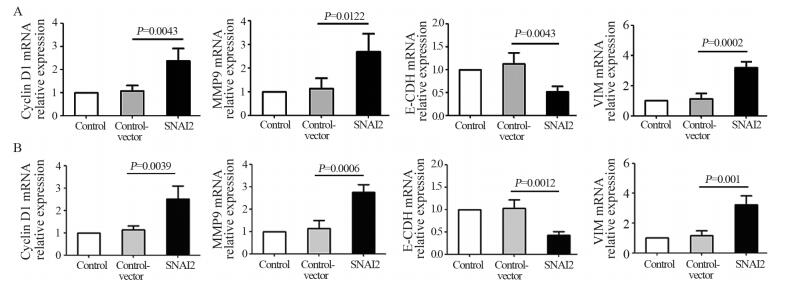
|
| A: MCF-7; B: T-47D 图 5 Real-time PCR法检测SNAI2基因对低转移潜能肿瘤细胞恶性特征相关基因表达的影响 Figure 5 Effect of SNAI2 on malignant hallmarks-related gene expression in non-invasive breast tumor cells detected by real-time PCR |
SNAI2是具有锌指结构EMT关键转录因子之一,在肿瘤EMT进程和肿瘤细胞的侵袭、迁移和转移中起到重要作用。然而,SNAI2是否在低转移潜能的乳腺癌细胞获得较高转移潜能中发挥重要作用目前尚不清楚。
本研究通过构建的慢病毒载体GV367-SNAI2,使低转移潜能的乳腺癌MCF-7细胞的SNAI2基因稳定高表达,证实了SNAI2能有效增强低转移潜能的乳腺癌MCF-7细胞的恶性特征,包括细胞的增殖能力、迁移能力和抗凋亡能力。有研究表明下调SNAI2表达能抑制直肠癌、前列腺癌、食管癌细胞的增殖能力[6, 11],而本研究结果发现下调SNAI2表达并不能有效降低MCF-7细胞的增殖能力(P > 0.05),这可能与我们采用的为低转移潜能的肿瘤细胞有关,一方面该细胞自身转移潜能极弱,另一方面该细胞SNAI2基因本底的表达极低[9]。
既然SNAI2对低转移潜能的乳腺癌细胞的增殖和迁移能力均有促进作用,那么其潜在机制可能是什么呢?有研究表明SNAI2可上调原发肿瘤细胞的细胞周期蛋白Cyclin D1,促进细胞周期G1/S转换,进而启动肿瘤细胞增殖[12],同样,本研究结果也证实了低转移潜能的MCF-7和T-47D细胞高表达SNAI2基因后,不仅其增殖潜能和迁移能力显著增强,同时增殖相关基因CyclinD1表达上调,迁移相关基因中E-CDH表达下调,MMP9和VIM均表达明显上调,进一步从基因水平证实了SNAI2能增强低转移潜能的乳腺癌细胞恶性特征。细胞黏附分子(如E-CDH)表达减少,间质细胞“特征”分子(VIM)表达上调是肿瘤细胞发生上皮间质转化(epithelial-mesenchymal transition, EMT)的关键所在[13-14],而目前研究已证实EMT在肿瘤细胞的转移过程中扮演了重要的角色,SNAI2是肿瘤细胞发生EMT最关键转录因子之一[13-14]。因此,我们推测SNAI2基因的稳定表达可能是通过促进低转移潜能乳腺癌MCF-7细胞发生EMT而获得更强的增殖和迁移能力。
总之,本研究通过慢病毒载体GV367-SNAI2使低转移潜能的乳腺癌细胞中SNAI2基因持续表达增强,进一步证实了SNAI2对低转移潜能的肿瘤细胞的增殖、迁移和抗凋亡能力有明显促进作用,通过进一步基因转移相关基因检测推测:SNAI2可能是通过促进低转移潜能乳腺癌细胞发生EMT而获得更强的增殖和迁移能力,为将SNAI2作为乳腺癌的基因靶点提供新的方法和理论支撑。
| [1] | 卫生部新闻办公室. 第三次全国死因调查的主要情况[J]. 中国肿瘤, 2008, 17(5): 344–5. [ Information Office of National Health and Family Planning Commission of the People's Republic of China. The third cause survey of national death[J]. Zhongguo Zhong Liu, 2008, 17(5): 344–5. DOI:10.3969/j.issn.1004-0242.2008.05.001 ] |
| [2] | Friedenreich CM, Shaw E, Neilson HK, et al. Epidemiology and biology of physical activity and cancer recurrence[J]. J Mol Med (Berl), 2017, 95(10): 1029–41. DOI:10.1007/s00109-017-1558-9 |
| [3] | Esposito S, Russo MV, Airoldi I, et al. SNAI2/Slug gene is silenced in prostate cancer and regulates neuroendocrine differentiation, metastasis-suppressor and pluripotency gene expression[J]. Oncotarget, 2015, 6(19): 17121–34. |
| [4] | Liao H, Bai Y, Qiu S, et al. MiR-203 downregulation is responsible for chemoresistance in human glioblastoma by promoting epithelial-mesenchymal transition via SNAI2[J]. Oncotarget, 2015, 6(11): 8914–28. |
| [5] | Atmaca A, Wirtz RW, Werner D, et al. SNAI2/SLUG and estrogen receptor mRNA expression are inversely correlated and prognostic of patient outcome in metastatic non-small cell lung cancer[J]. BMC Cancer, 2015, 15: 300. DOI:10.1186/s12885-015-1310-1 |
| [6] | Pulkka OP1, Nilsson B2, Sarlomo-Rikala M, et al. SLUG transcription factor: a pro-survival and prognostic factor in gastrointestinal stromal tumour[J]. Br J Cancer, 2017, 116(9): 1195–202. DOI:10.1038/bjc.2017.82 |
| [7] | Lee HH, Lee SH, Song KY, et al. Evaluation of Slug expression is useful for predicting lymph node metastasis and survival in patients with gastric cancer[J]. BMC Cancer, 2017, 17(1): 670. DOI:10.1186/s12885-017-3668-8 |
| [8] | Hasan MR, Sharma R, Saraya A, et al. Slug is a predictor of poor prognosis in esophageal squamous cell carcinoma patients[J]. PloS One, 2013, 8(12): e82846. DOI:10.1371/journal.pone.0082846 |
| [9] | Zhou YH, Liao SJ, Li D, et al. TLR4 ligand/H2O2 enhances TGF-β1 signaling to induce metastatic potential of non-invasive breast cancer cells by activating non-Smad pathways[J]. PLos One, 2013, 8(5): e65906. DOI:10.1371/journal.pone.0065906 |
| [10] | Thompson CC1, Ashcroft FJ, Patel S, et al. Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility[J]. Gut, 2007, 56(1): 95–106. DOI:10.1136/gut.2005.083691 |
| [11] | Zhou W, Lv R, Qi W, et al. Snail contributes to the maintenance of stem cell-like phenotype cells in human pancreatic cancer[J]. PLoS One, 2014, 9(1): e87409. DOI:10.1371/journal.pone.0087409 |
| [12] | Casimiro MC, Velasco-Velázquez M, Aguirre-Alvarado C, et al. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present[J]. Expert Opin Inv Drug, 2014, 23(3): 295–304. DOI:10.1517/13543784.2014.867017 |
| [13] | Jolly MK, Ware KE, Gilja S, et al. EMT and MET: necessary or permissive for metastasis[J]. Mol Oncol, 2017, 11(7): 755–69. DOI:10.1002/mol2.2017.11.issue-7 |
| [14] | Santamaria PG, Moreno-Bueno G, Portillo F, et al. EMT: Present and future in clinical oncology[J]. Mol Oncol, 2017, 11(7): 718–38. DOI:10.1002/mol2.2017.11.issue-7 |
 2018, Vol. 45
2018, Vol. 45


