文章信息
- 奥氮平治疗阿片类止痛药所致恶心呕吐的临床观察
- Clinical Observation of Olanzapine in Treatment of Nausea and Vomiting Caused by Opioid Analgesics
- 肿瘤防治研究, 2018, 45(5): 326-328
- Cancer Research on Prevention and Treatment, 2018, 45(5): 326-328
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.17.1275
- 收稿日期: 2017-10-11
- 修回日期: 2017-12-11
2. 443003 宜昌,宜昌市中心人民医院肿瘤科;
3. 443003 宜昌,三峡大学肿瘤研究所
2. Department of Oncology, Yichang Central People's Hospital, Yichang 443003, China;
3. China Three Gorges University Oncology Institute, Yichang 443003, China
癌痛是恶性肿瘤患者常见的、亦是患者最畏惧的症状,止痛治疗是姑息治疗的主要内容之一。合理应用阿片类止痛药能有效缓解患者的痛苦。阿片类镇痛药的不良反应主要发生于用药初期及过量用药时,少数患者的不良反应如呕吐、嗜睡、头晕、便秘等甚至发生于用药剂量尚未达到镇痛作用剂量水平时。其中,阿片类药物所致恶心呕吐高达31.4%~38.7%,严重影响患者生活质量,引起患者恐惧、排斥的心理,降低医从性,甚至放弃阿片类药物治疗致使不能有效止痛,既往使用甲氧氯普胺等治疗并不理想,奥氮平作为一种抗精神病药物,可抑制多种呕吐相关性神经递质与受体结合,为其用于阿片类药物所致恶心呕吐提供了依据。2013—2017年我们将85例服用阿片类药物所致恶心呕吐的患者,随机分为两组,治疗组予奥氮平,对照组使用甲氧氯普胺。现将研究结果报道如下。
1 资料与方法 1.1 病例选择85例均为2013年1月—2017年7月在宜昌市中心人民医院应用阿片类镇痛药所致恶心呕吐的恶性肿瘤患者,其中男47例、女38例,年龄30~75岁;肺癌37例、胃癌12例、大肠癌12例、食管癌10例、鼻咽癌9例、淋巴瘤5例。将全部病例随机分为两组,奥氮平组予奥氮平片,对照组予甲氧氯普胺注射液。两组之间年龄、性别、所患恶性肿瘤比率差异无统计学意义。
止痛方案:依据三阶梯止痛原则,对于中、重度疼痛患者使用羟考酮缓释片,起始剂量20~30 mg,每12 h一次,使用吗啡即释片处理爆发痛,依据滴定原则调整羟考酮缓释片剂量。
1.2 止吐药的给药方法奥氮平组:奥氮平片5 mg呕吐发生后给一次,以后每晚一次,连用5天;对照组:甲氧氯普胺20 mg肌注,呕吐发生后每日2次,连用3~5天。
1.3 疗效评价标准按WHO评价标准进行,止吐作用分级:0级:完全有效,无呕吐;1级:显著有效,呕吐次数每天1~2次;2级:轻度有效,呕吐次数每天3~5次;3级:无效,呕吐次数每天 > 5次。抑制恶心作用分级:0级:完全有效,无恶心;1级:显著有效,轻度恶心不影响进食;2级:轻度有效,中度恶心影响进食;3级:无效,频频严重恶心。使用3天后,以控制呕吐和恶心完全有效及显著有效病例数、每组的全部病例数计算有效率,评价疗效。
1.4 统计学方法应用SPSS17.0软件包,用χ2检验对资料进行统计分析。P < 0.05为差异有统计学意义。
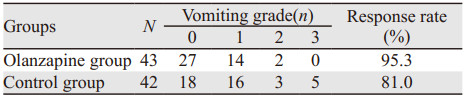
2 结果 2.1 临床疗效 2.1.1 两组对阿片类药物引起呕吐的疗效比较奥氮平组的有效率高于对照组,分别为95.3%、81.0%,差异有统计学意义(P=0.039),见表 1。
|
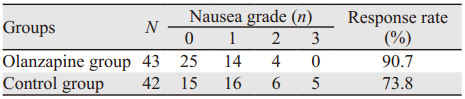
奥氮平组的有效率高于对照组,分别为90.7%、73.8%,差异有统计学意义(P=0.041),见表 2。
|
两组患者因恶心、呕吐反应而导致的阿片类药物中断率差异有统计学意义(P=0.020),见表 3。

|
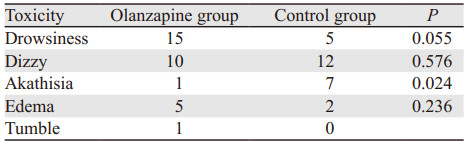
两组治疗方案引起的嗜睡、头晕、水肿等不良反应发生率相仿,差异无统计学意(P > 0.05),但对照组锥体外系反应(静坐不能)明显高于奥氮平组(P=0.024),奥氮平组发生1例跌倒,见表 4。
阿片类药物可直接兴奋位于延髓的呕吐化学感受器而引起恶心和呕吐,这种作用可因前庭的兴奋而增强,而阿片类药物可以提高前庭的敏感度,所以临床常用的μ受体激动剂都会引起一定程度的恶心和呕吐[1],其发生率高达31.4%~38.7%[2],少数转化为顽固性恶心、呕吐,是早期阿片类药物最常见的不良反应。甲氧氯普胺片、昂丹司琼片等药物被推荐用于阿片类药物相关恶心呕吐的处理,肠梗阻时则推荐奥氮平片(尤其在锥体外系反应方面明显优于氟哌啶醇)[3]。甲氧氯普胺是多巴胺受体和5-HT3受体拮抗剂,是临床使用广泛的止吐药物。奥氮平是精神科常用药物,多个研究显示,奥氮平与多巴胺D1-4、5-HT2a、5-HT2c、5-HT3、5-HT6、毒蕈碱M1-5、肾上腺素α1和组胺H1受体有一定结合力[4],尤其是D2、5-HT2c及5-HT3受体,与恶心、呕吐发生机制重叠[5],从而发挥拮抗作用,成为一种新型的多途径止吐药物,多个临床研究显示,奥氮平可明显降低化疗所致恶心、呕吐,特别是延迟性、突破性呕吐疗效显著[6-9],其不良反应主要包括神经系统症状(如恶性综合征、锥体外系反应、意识障碍、迟发型运动障碍)、下肢水肿、血糖升高、低血钾、转氨酶升高、中性粒细胞减少、鼻出血等,停药后对症处理多可缓解或消失;以迟发性为主(仅有6%发生于1天之内,75%发生于1周以后)[10]。本试验予奥氮平片5 mg,连用5天,明显提高了阿片类药物所致恶心、呕吐的控制率,减少了患者因不能耐受而导致的阿片类药物中断率,提高了患者的生活质量及医从性,与对照组相比,未增加嗜睡、头晕、水肿等不良反应发生率,锥体外系反应较甲氧氯普胺组明显降低,与文献报道的不良反应相仿[8-9],关于奥氮平使用的剂量,虽然在预防化疗所致呕吐的Ⅰ期临床研究中以10 mg组为佳[7],本组患者在治疗阿片类所致恶心、呕吐时予5 mg,每日一次,取得了较好疗效,值得进一步探讨与临床推广。
| [1] | Owusu Obeng A, Hamadeh I, Smith M. Review of Opioid Pharmacogenetics and Considerations for Pain Management[J]. Pharmacotherapy, 2017, 37(9): 1105–21. DOI:10.1002/phar.2017.37.issue-9 |
| [2] | Fearon JA, Dimas V, Ditthakasem K, et al. A Randomized Controlled Trial of Oral Versus Intravenous Administration of a Nonnarcotic Analgesia Protocol Following Pediatric Craniosynostosis Corrections on Nausea and Vomiting Rates[J]. J Craniofac Surg, 2015, 26(6): 1951–3. DOI:10.1097/SCS.0000000000002009 |
| [3] | Swarm RA, Abernethy AP, Anghelescu DL, et al. Adult cancer pain[J]. J Natl Compr Canc Netw, 2013, 11(8): 992–1022. DOI:10.6004/jnccn.2013.0119 |
| [4] | Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer[J]. J Pain Symptom Manage, 2003, 25(6): 578–82. DOI:10.1016/S0885-3924(03)00143-X |
| [5] | Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting[J]. Eur J Pharmacol, 2014, 722: 180–6. DOI:10.1016/j.ejphar.2013.08.048 |
| [6] | Mukhopadhyay S, Kwatra G, Alice KP, et al. Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients:a randomized controlled study[J]. Support Care Cancer, 2017, 25(1): 145–54. DOI:10.1007/s00520-016-3386-9 |
| [7] | Passik SD, Navari RM, Jung SH, et al. A Phase Ⅰ trail of olanzapine (zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study[J]. Cancer Invest, 2004, 22(3): 383–8. DOI:10.1081/CNV-200029066 |
| [8] | Navari RM, Nagy CK, Gray SE. The use of Olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy[J]. Support Care Cancer, 2013, 21(6): 1655–63. DOI:10.1007/s00520-012-1710-6 |
| [9] | Mizukami N, Yamauchi M, Koike K, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study[J]. J Pain Symptom Manage, 2014, 47(3): 542–50. DOI:10.1016/j.jpainsymman.2013.05.003 |
| [10] | Flank J, Schechter T, Gibson P, et al. Olanzapine for prevention of chemotherapy-induced nausea and vomiting in children and adolescents: a multi-center, feasibility study[J]. Support Care Cancer, 2018, 26(2): 549–55. DOI:10.1007/s00520-017-3864-8 |
 2018, Vol. 45
2018, Vol. 45