文章信息
- miR-205对胃癌细胞侵袭迁移的调控作用及其潜在机制
- Regulatory Effects of miR-205 on Invasion and Migration of Gastric Cancer Cells and Potential Mechanism
- 肿瘤防治研究, 2018, 45(5): 280-284
- Cancer Research on Prevention and Treatment, 2018, 45(5): 280-284
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2018.17.1217
- 收稿日期: 2017-09-25
- 修回日期: 2017-12-28
2. 471000 洛阳,洛阳职业技术学院护理学院;
3. 471000 洛阳,96531部队医院内科;
4. 471000 洛阳,洛阳职业技术学院药学与检验系;
5. 810000 西宁,青海卫生职业技术学院人事处
2. School of Nursing, Luoyang Vocational & Technical College, Luoyang 471000, China;
3. Department of Medicine, No.96531 Army Hospital, Luoyang 471000, China;
4. School of Pharmaceutical Inspection, Luoyang Vocational & Technical College, Luoyang 471000, China;
5. Department of Personnel, Qinghai Institute of Health Sciences, Xining 810000, China
胃癌作为消化系统的常见恶性肿瘤之一,发病率及死亡率一直居高不下,胃癌的侵袭和迁移是患者术后复发及导致患者死亡的主要原因[1],故揭示胃癌侵袭和迁移发生的相关分子机制并积极寻找有效的干预靶点对于提高患者的生存率具有十分重要的意义。miR-205定位于1q32.2,在肿瘤组织中存在差异性表达,从而发挥抑癌或促癌的作用[2];据报道miR-205在乳腺癌和卵巢癌中高表达,而在食管癌和胃癌中低表达[2-5]。且miR-205可靶向VEGFA及FGF2调控乳腺癌化疗耐药[6];在卵巢癌细胞中,miR-205则靶向TCF21促进细胞侵袭迁移[7];宫颈癌中,miR-205通过调控IGF-1R影响细胞的增殖及迁移[8]。由此可见miR-205可通过不同的信号通路及靶基因调控肿瘤细胞的增殖、凋亡、侵袭及迁移等生物学特性,且Xu等[9]研究显示,在胃癌NCI-H87细胞中,miR-205可能通过靶向ZEB1基因抑制细胞的迁移及EMT进程。考虑到基因调控的复杂性,miR-205对胃癌细胞侵袭迁移的具体作用及分子机制,还需要大量的实验进行验证。本研究拟通过实时荧光定量PCR,初步检测miR-205在几种胃癌细胞中的表达,并通过体外干预miR-205的表达进一步观察其对胃癌细胞HGC27侵袭、迁移能力的影响并探讨其分子机制。
1 材料与方法 1.1 材料与试剂人胃癌细胞系AGS、MKN74、HGC27、SGC7901及永生化胃黏膜上皮细胞株GES-1均由本实验室自行冻存;胎牛血清(fetal bovine serum, FBS)、RPMI 1640培养液及Opti-MEM培养液购自美国Gibco公司;Transwell小室及Matrigel胶购自美国Corning公司;Lipofectamine2000及相关转染试剂购自美国Invitrogen公司;E-cadherin、β-catenin、N-cadherin、vimentin、Notch、snail及GAPDH抗体购自美国Abcam公司;miR-205 mimic、negative control miR及SYBR Green Ⅰ real-time PCR kit由上海吉玛制药技术有限公司提供;其余试剂均为国产分析纯。
1.2 实验方法 1.2.1 细胞培养、转染和分组所有实验用细胞株均常规培养于含10%胎牛血清的RPMI 1640培养液(含100 u/ml青霉素、100 μg/ml链霉素)中,培养箱条件为37℃、5%CO2。正常状态下,细胞呈单层贴壁生长,每2天换液一次。待3~5天细胞生长至90%融合后可用胰酶常规消化进行传代。转染:对数生长期的细胞消化后接种于6孔板中,待细胞生长至60%~80%融合,更换为不含血清的培养液,同步化12 h,随后进行转染。实验设置为空白对照组(control)、阴性对照组(negative control miR,miR-NC)、转染组(miRNA-205 mimic,miR-205)。转染方法按说明书进行:将miR-205或者miR-NC溶解于Opti-MEM培养液中室温孵育5 min,同时另取Lipofectamine2000加Opti-MEM培养液室温孵育5 min,然后将两者轻柔混合室温反应20 min。最后将混合物加入到相应组别的细胞中,置于培养箱中孵育6 h后更换为完全培养液继续培养48 h。收集细胞蛋白测定转染效率并进行后续实验分析。
1.2.2 细胞划痕实验取对数生长期的细胞消化后接种至6孔板中,待细胞长至80%~90%融合时,用10 μl枪头垂直方向划痕,PBS溶液冲洗细胞两遍后加入培养基,于倒置相差显微镜下观察24 h,每隔10 min拍照记录,观察细胞划痕的修复情况。每组设三个复孔取均值。
1.2.3 Transwell细胞侵袭实验将各组细胞消化后加不含血清的培养基重悬制成单细胞悬液(密度2×103个每毫升)。接种细胞于Transwell小室(预先铺好Matrigel胶)上室中,并在下室内加入完全培养液,置于37℃培养箱中培养24 h。取出小室后用PBS冲洗并用棉签轻轻擦除上室内细胞,然后将小室置于90%酒精中固定30 min,加0.1%结晶紫染色10 min,PBS漂洗10 min×3次。倒置显微镜下拍照观察并计算染色细胞的个数,每组细胞计数5个视野取均值。
1.2.4 Western blot测定蛋白表达水平加含有蛋白酶抑制剂的RIPA裂解液提取各组细胞蛋白,并用BCA蛋白定量试剂盒测定蛋白浓度。每组取60 μg蛋白样品,加4倍体积的上样缓冲液后,95℃加热变性5 min,进行SDS-PAGE凝胶电泳,然后200 mA恒流电将蛋白转移至PVDF膜上。室温下,加5%脱脂奶粉封闭1 h,依据说明书要求加入相应比例的一抗,4℃孵育过夜,复温后TBST洗涤5 min×3次。室温下分别加入二抗,继续孵育2 h,TBST洗涤10 min×3次。然后于暗房中将ECL发光试剂均匀撒在PVDF膜上激发荧光,压X线片后显影、定影。结果使用ImageJ进行蛋白半定量灰度分析。
1.2.5 实时荧光定量PCR按照TRIzol说明书要求裂解细胞提取总RNA,测定所提RNA样品的纯度并定量。依据mRNA反转录试剂盒说明书,取2 μg总RNA合成cDNA,采用SYBR Green Ⅰreal-time PCR的方法检测mRNA的相对表达量,U6作为内参。引物设计如下:miR-205-F: 5’-GCAATCCTTACTTCCACCG-3’,miR-205 -R: 5’-GTGCGTGTCGTGGAGTCG-3’;U6-F: 5’-CTCGCTTCGGCAGCACA-3’,U6-R: 5’-AACGCTTCACGAATTTGCGT-3’。PCR循环条件为95℃ 30 s;95℃ 5 s,60℃ 30 s,40个循环。记录CT值并采用2-ΔΔCT法分析mRNA的相对表达量。
1.3 统计学方法所得数据均采用SPSS13.0统计软件进行分析。实验结果计量资料用均数±标准差(x±s)表示。两组计量资料的组间差异采用t检验,多组计量资料行单因素方差分析,多组计量资料的两两比较采用Bonferroni校正的t检验,计数资料的比较采用χ2检验,P < 0.05为差异有统计学意义。
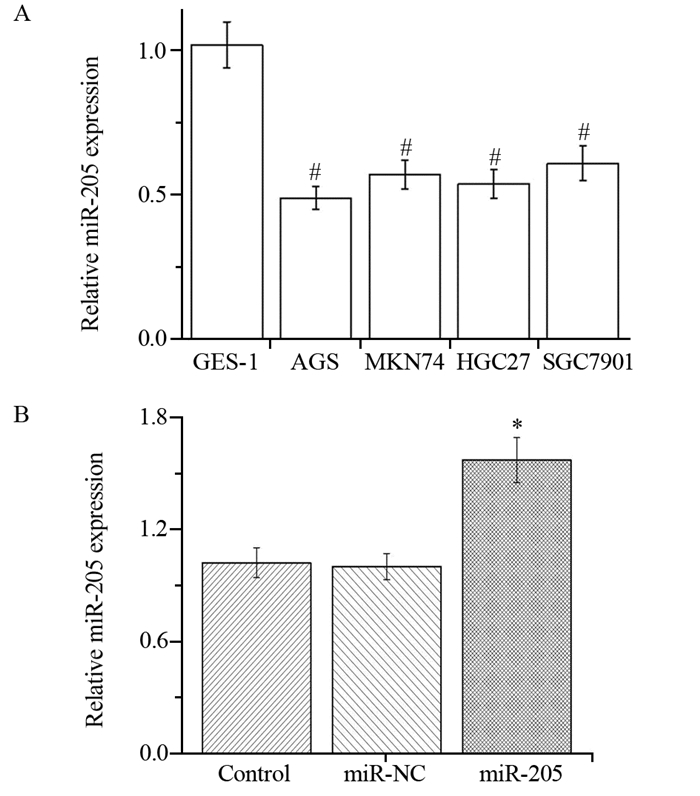
2 结果 2.1 miR-205在胃癌细胞中低表达相较于人永生化胃黏膜上皮细胞GES-1中miR-205表达(1.02±0.14),胃癌细胞系AGS(0.49±0.05)、MKN74(0.64±0.07)、HGC27(0.55±0.06)及SGC7901(0.68±0.08)中miR-205的表达水平均明显降低(PAGS=0.001, PMKN74=0.002, PHGC27=0.002, PSGC7901=0.004),见图 1A。
|
| A: the expression of miR-205 in different gastric cancer cells; B: the expression of miR-205 in HGC27 cells after miR-205 mimic transfection; #: P < 0.05, compared with GES-1 group; *: P < 0.05, compared with Control group 图 1 qRT-PCR检测胃癌细胞中miR-205的表达水平以及miR-205 mimic的转染效率 Figure 1 miR-205 expression in different gastric cancer cells and transfection efficacy of miR-205 mimic detected by qRT-PCR assay |
通过脂质体将negative control miR、miR-205 mimic分别转染至HGC27细胞中,采用qRT-PCR检测miR-205表达水平的变化。结果显示,转染miR-205 mimic后,miR-205组HGC27细胞中的miR-205表达水平显著升高(P=0.018),见图 1B。
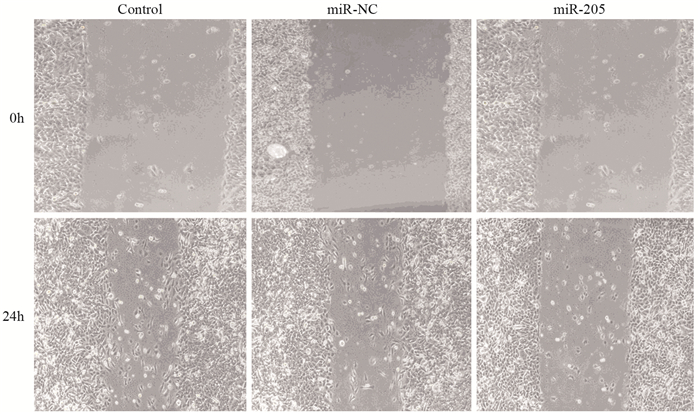
采用划痕实验测定转染miR-205 mimic后细胞迁移率的变化。结果显示,划痕后24 h,转染组细胞迁移率由对照组的(54.7±4.1)%降低至(34.1±4.5)%(P=0.005),见图 2。
|
| 图 2 划痕实验检测过表达miR-205对HGC27细胞迁移的影响 Figure 2 Effect of miR-205 overexpression on HGC27 cells migration detected by scratch test |
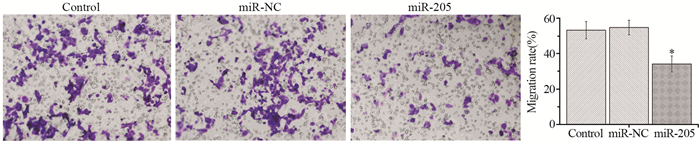
采用Transwell法检测细胞侵袭能力,发现过表达miR-205后,转染组细胞侵袭率由对照组的(52.8±6.3)%降低至(32.2±4.9)%(P=0.001),见图 3。
|
| *: P < 0.05, compared with Control group 图 3 Transwell实验检测过表达miR-205对HGC27细胞侵袭的影响 Figure 3 Effect of miR-205 overexpression on HGC27 cells invasion detected by Transwell assay |
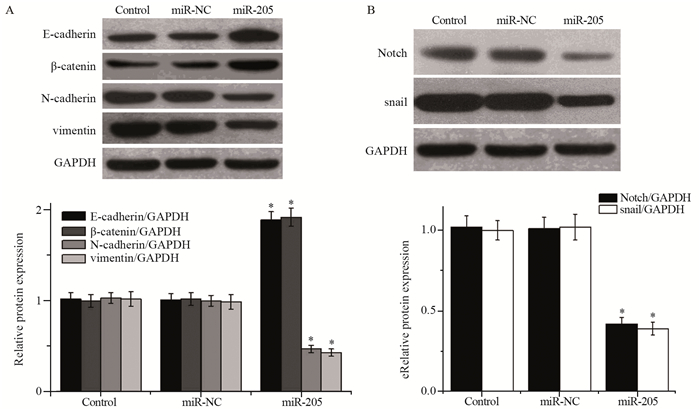
为探讨miR-205影响细胞侵袭迁移的可能作用机制,我们通过Western blot检测过表达miR-205对HGC27细胞EMT表达的影响。结果显示,与对照组(E-cadherin(1.01±0.08)、β-catenin(0.97±0.11)、N-cadherin(1.03±0.09)、vimentin(1.02±0.13))比较,过表达miR-205后上皮细胞标志物E-cadherin(1.82±0.12)及β-catenin的表达(1.93±0.21)显著升高(P=0.002, P=0.003),而间质细胞标志物N-cadherin(0.54±0.05)及vimentin的表达(0.46±0.06)明显降低(P=0.005, P=0.004),见图 4A。
|
| *: P < 0.05, compared with Control group 图 4 Western blot检测过表达miR-205对HGC27细胞EMT进程及Notch信号通路的影响 Figure 4 Effect of miR-205 overexpression on EMT progress and activation of Notch signal pathway in HGC27 cells detected by Western blot |
与对照组(Notch(1.03±0.11)、snail(0.98±0.13))比较,转染miR-205 mimic后Notch(0.42±0.05)和snail的表达(0.37±0.06)明显降低(P=0.002, P=0.003),见图 4B。
3 讨论已有研究证明miR-205在胃癌中发挥抑癌因子样作用,下调miR-205可靶向YY1基因促进胃癌细胞AGS及NCI-N87的增殖[5];而本实验中实时荧光定量PCR的结果发现,miR-205在不同类型的胃癌细胞中均呈现一定程度的低表达,这与以前的研究结果一致。随后,本实验通过体外细胞实验进一步探讨了miR-205对胃癌细胞HGC27侵袭迁移能力的影响。结果显示,转染miR-205 mimic之后,HGC27细胞的侵袭及迁移能力明显受到抑制。
miRNA的调控作用多是通过相应的靶基因影响相关信号通路,形成复杂的调控网络来实现的。在肿瘤细胞侵袭迁移的过程中,上皮间充质转化是关键的环节,即在侵袭的过程中癌细胞由上皮细胞形态转化为间质细胞形态,细胞间的极性丢失且黏附性降低,同时由于间质细胞结构疏散,运动能力强,故而容易迁移[10-12]。已有大量研究证实miR-205可影响肿瘤细胞的EMT表达[13-15]。Wang等[13]在喉鳞状细胞癌SNU899细胞中的研究表明,miR-205可通过影响AKT介导的EMT进程调控细胞的侵袭迁移。曹罗元等[14]研究显示,miR-205可通过下调ZEB1和ZEB2的表达抑制肾小管HK-2细胞的EMT进程。本研究通过Western blot检测了过表达miR-205对胃癌HGC27细胞中EMT的影响,结果发现转染miR-205 mimic之后,上皮细胞标志物E-cadherin及β-catenin的蛋白表达显著升高而间质细胞标志物N-cadherin及vimentin的蛋白表达明显降低,说明过表达miR-205后HGC27细胞的EMT被抑制。上皮间充质转化的过程中也涉及到多条信号通路的交互作用,Notch信号通路就是其中一条[16]。Notch信号通路是一条多调控的复杂通路,研究显示,Notch信号可通过上调snail、twist、slug、ZEB1及ZEB2等转录因子的表达,抑制E-cadherin和E-box的结合,进而上调波形蛋白的表达,促进细胞EMT的发生[16-18]。本研究的检测结果发现,过表达miR-205可抑制Notch/snail信号通路的活性。
综上所述,miR-205在本研究各型胃癌细胞中呈现低表达,且在胃癌细胞HGC27中上调miR-205可抑制细胞的侵袭迁移;其分子机制可能与抑制EMT及下调Notch/snail信号通路的活性有关。Notch信号通路及肿瘤细胞EMT均涉及众多复杂的调控机制,miR-205在其中对各种转录因子的具体调控方式还有待进一步深入的研究。
| [1] | Yan H, Liu J, Ming X, et al. Metastatic gastric carcinoma to the breast: A case report and review of the Chinese literature[J]. Mol Clin Oncol, 2017, 7(2): 221–4. |
| [2] | Vosgha H, Salajegheh A, Smith RA, et al. The important roles of miR-205 in normal physiology, cancers and as a potential therapeutic target[J]. Curr Cancer Drug Targets, 2014, 14(7): 621–37. DOI:10.2174/156800961407140926105634 |
| [3] | Orang AV, Safaralizadeh R, Hosseinpour Feizi MA. Insights into the diverse roles of miR-205 in human cancers[J]. Asian Pac J Cancer Prev, 2014, 15(2): 577–83. DOI:10.7314/APJCP.2014.15.2.577 |
| [4] | Teoh SL, Das S. The Role of MicroRNAs in Diagnosis, Prognosis, Metastasis and Resistant Cases in Breast Cancer[J]. Curr Pharm Des, 2017, 23(12): 1845–59. DOI:10.2174/1381612822666161027120043 |
| [5] | Yin WZ, Li F, Zhang L, et al. Down-regulation of microRNA-205 promotes gastric cancer cell proliferation[J]. Eur Rev Med Pharmacol Sci, 2014, 18(7): 1027–32. |
| [6] | Hu Y, Qiu Y, Yagüe E, et al. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer[J]. Cell Death Dis, 2016, 7(6): e2291. DOI:10.1038/cddis.2016.194 |
| [7] | Wei J, Zhang L, Li J, et al. MicroRNA-205 promotes cell invasion by repressing TCF21 in human ovarian cancer[J]. J Ovarian Res, 2017, 10(1): 33. DOI:10.1186/s13048-017-0328-1 |
| [8] | Pang H, Yue X. MiR-205 serves as a prognostic factor and suppresses proliferation and invasion by targeting insulin-like growth factor receptor 1 in human cervical cancer[J]. Tumour Biol, 2017, 39(6): 1010428317701308. |
| [9] | Xu C, Li M, Zhang L, et al. MicroRNA-205 suppresses the invasion and epithelial-mesenchymal transition of human gastric cancer cells[J]. Mol Med Rep, 2016, 13(6): 4767–73. DOI:10.3892/mmr.2016.5118 |
| [10] | He SJ, Xiang CQ, Zhang Y, et al. Recent progress on the effects of microRNAs and natural products on tumor epithelial-mesenchymal transition[J]. Onco Targets Ther, 2017, 10: 3435–51. DOI:10.2147/OTT |
| [11] | Sun C, Tao Y, Gao Y, et al. F-box protein 11 promotes the growth and metastasis of gastric cancer via PI3K/AKT pathway-mediated EMT[J]. Biomed Pharmacother, 2017, 98: 416–23. |
| [12] | 胡文兵, 王钢胜, 陈曦, 等. siRNA干扰KLF8表达对鼻咽癌细胞上皮间质转化的作用[J]. 肿瘤防治研究, 2016, 43(12): 1055–8. [ Hu WB, Wang GS, Chen X, et al. Effect of down-regulating KLF8 expression by siRNA on EMT in nasopharyngeal carcinoma cells[J]. Zhong Liu Fang Zhi Yan Jiu, 2016, 43(12): 1055–8. DOI:10.3971/j.issn.1000-8578.2016.12.009 ] |
| [13] | Wang B, Lv K, Chen W, et al. miR-375 and miR-205 Regulate the Invasion and Migration of Laryngeal Squamous Cell Carcinoma Synergistically via AKT-Mediated EMT[J]. Biomed Res Int, 2016, 2016: 9652789. |
| [14] | 曹罗元, 杨菁, 富显果, 等. miR-205通过下调ZEB1和ZEB2表达抑制肾小管上皮细胞转分化[J]. 南方医科大学学报, 2016, 36(12): 1700–5, 11. [ Cao LY, Yang J, Fu XG, et al. The microRNA miR-205 inhibits epithelial-messenchymal transition in HK-2 cells by downregulating ZEB1 and ZEB2 expressions[J]. Nanfang Yi Ke Da Xue Xue Bao, 2016, 36(12): 1700–5, 11. DOI:10.3969/j.issn.1673-4254.2016.12.19 ] |
| [15] | Wang X, Yu M, Zhao K, et al. Upregulation of MiR-205 under hypoxia promotes epithelial-mesenchymal transition by targeting ASPP2[J]. Cell Death Dis, 2016, 7(12): e2517. DOI:10.1038/cddis.2016.412 |
| [16] | Ito T, Kudoh S, Ichimura T, et al. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1[J]. Hum Cell, 2017, 30(1): 1–10. DOI:10.1007/s13577-016-0149-3 |
| [17] | Yang G, Zhao Z, Zhang X, et al. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice[J]. Drug Des Devel Ther, 2017, 11: 1065–79. DOI:10.2147/DDDT |
| [18] | Rodriguez-Vita J, Tetzlaff F, Fischer A. Notch controls endothelial cells[J]. Oncoscience, 2017, 4(5-6): 45–6. |
 2018, Vol. 45
2018, Vol. 45
