文章信息
- 松油烯-4-醇对人多发性骨髓瘤U266细胞增殖及凋亡的影响
- Effect of Terpinen-4-ol on Proliferation and Apoptosis of Human Multiple Myeloma Cells U266
- 肿瘤防治研究, 2016, 43(5): 355-359
- Cancer Research on Prevention and Treatment, 2016, 43(5): 355-359
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2016.05.008
- 收稿日期: 2015-07-18
- 修回日期: 2015-12-11
2. 550004 贵阳,贵州医科大学微生物学教研室;
3. 550025 贵阳,贵州大学生命科学学院贵州省中药(民族药)创制工程中心
2. Department of Microbiology, Guiyang Medical University, Guiyang 550004, China;
3. Guizhou Province Chinese Medicine and Ethnic Medicine Creation Engineering Center, College of Life Sciences, Guizhou University, Guiyang 550025, China
多发性骨髓瘤(multiple myeloma,MM)为发生于B淋巴细胞的恶性浆细胞病。好发于中老年,且近年有发病率增高及发病年龄提前的趋势[1, 2]。尽管造血干细胞移植、联合化疗及新的靶向药物治疗取得了较好效果,但该病目前仍无法治愈[3, 4],因此,研发新型治疗药物变得尤为重要。珊瑚姜为苗医常用药材,具有抗细菌、抗真菌及抗肿瘤等活性[5, 6],松油烯-4-醇(Terpinen-4-ol,T4O)是其主要成分之一[7, 8],它具有抗菌、抗肿瘤及杀虫等活性[9]。近年来,T4O在抗肿瘤方面的作用已被广泛研究[10-12],但对人多发性骨髓瘤U266细胞的影响尚未见报道,本研究利用T4O作用于U266细胞,观察其对U266细胞增殖及凋亡的影响,并探讨其作用机制。
1 材料与方法 1.1 材料与试剂T4O(上海古朵生物科技有限公司,完全培养液:T4O:DMSO体积以1000:10:0.1的比例混合,用培养液稀释成10 mmol/ml,过滤除菌,4℃冰箱保存备用),U266细胞(贵阳医学院血液科惠赠),四季青胎牛血清(浙江天杭生物科技股份有限公司),RPMI1640及青链霉素双抗(上海生工生物工程股份有限公司),CCK-8(日本同仁公司),线粒体膜电位JC-1检测试剂盒、AO/EB染色试剂盒、Annexin ⅤFITC细胞凋亡检测试剂盒(上海贝博生物公司),ELX800酶联免疫检测仪(美国宝特公司),Caspase-3(南京建成生物工程研究所),奥林巴斯倒置荧光显微镜(日本OLYMPUS IX51),BD FACS Verse 流式细胞仪(美国BD Biosciences)。
1.2 方法 1.2.1 细胞培养U266悬浮细胞用完全培养液(RPMI 1640、10%胎牛血清及1%双抗),置37℃、5%CO2培养箱中培养,每2~3天传代1次,取对数生长期细胞进行实验。
1.2.2 抑制率测定CCK-8法测抑制率,取对数生长期细胞,调整密度为每毫升1.5×105个细胞接种于96孔板,培养12 h,实验组加入T4O,使其终浓度分别为5、10、20、40、80 μmol/L;每孔总体积为100 μl,设细胞对照组及空白对照组,每一浓度复6孔,继续培养24、48及72 h,培养结束1.5 h前,加入每孔10 μl CCK-8溶液孵育,酶标仪测波长450 nm吸光度(A)值,并计算细胞增殖抑制率=(细胞对照组A450-实验组A450 /细胞对照组A450-空白对照组A450)×100%。
1.2.3 凋亡形态观察调整细胞密度为1.0×104/ml,接种到6孔板,培养12 h,实验组加入T4O,使终浓度为5、10、20、40、80 μmol/L,设细胞对照组,培养48 h,经AO/EB染色,倒置显微镜观察细胞形态。
1.2.4 Caspase-3酶活性检测实验组加入T4O,使其终浓度为5、10、20、40、80 μmol/L,设细胞对照组,每孔总体积为2 ml,继续培养48 h,离心收集细胞。按照Caspase-3酶活性检测试剂盒步骤操作,酶标仪检测波长为450 nm时的吸光度(A)值。Caspase-3酶活化程度=实验组A450/对照组A450。
1.2.5 凋亡及周期检测细胞培养方法同1.2.4,离心收集细胞,参考涂云华等[13]实验方法,行细胞固定,按AnnexinⅤFITC细胞凋亡检测试剂盒和细胞周期检测试剂盒染色,然后上机检测。
1.2.6 线粒体膜电位检测细胞培养方法同1.2.4,调整细胞密度为1.3×105/ml,按JC-1线粒体膜电位检测试剂盒染色,流式细胞仪检测。
1.3 统计学方法采用SPSS13.0,结果以(x ±s)表示,同一时间段不同浓度组间采用单因素方差分析(One-Way ANOVA),不同时间段不同浓度组间采用重复测量方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 T4O对U266细胞增殖的影响T4O对U266细胞增殖有抑制作用,呈时间-剂量依赖性。其增殖抑制作用与对照组相比差异有统计学意义(P < 0.05),见表 1。
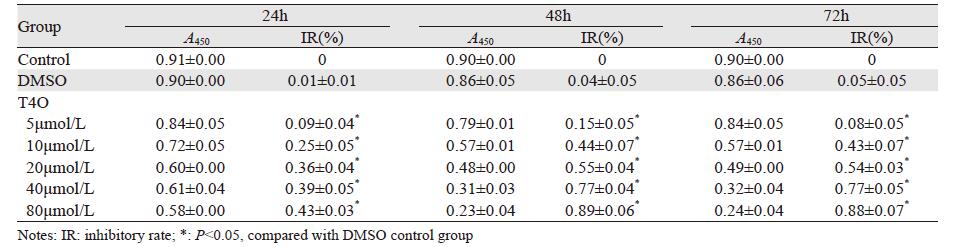 |
T4O对U266细胞形态的影响
对照组细胞呈圆形、椭圆形悬浮生长,生长活跃,荧光呈现致密绿色荧光,见图 1A;T4O不同浓度组间,可见数量不等黄绿色荧光,由低浓度至高浓度,红色荧光逐渐增多,细胞逐渐变得大小不均,胞间隙增大,并有大量坏死细胞及细胞碎片,见图 1B~F。

|
| A: Control group, well-distributed green fluorescence; B-F: 5, 10, 20, 40 and 80μmol/L of T4O respectively(×200), with the increasing of drug concentration, red fluorescence were gradually increased and the number of cells was gradually decreased as well as its size. Meanwhile, a large number of necrotic cells and cell debris were presented 图 1 T4O处理48h后对U266细胞形态变化影响 (×200) Figure 1 Morphology changes of U266 cells after treated with T4O for 48h (×200) |
2.3 T4O对U266细胞Caspase-3酶活性影响
低浓度至高浓度T4O作用U266细胞48 h后,可见Caspase-3酶活性随药物浓度增加而增强,见表 2。
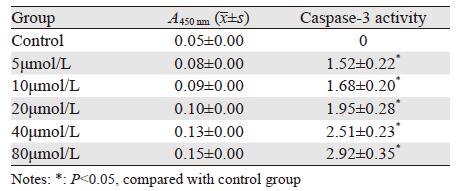 |
2.4 T4O对U266细胞凋亡影响
低浓度至高浓度T4O作用U266细胞48 h后,细胞出现不同程度凋亡,晚期凋亡逐渐增多(P < 0.05),早期凋亡及坏死细胞亦增多,见图 2、表 3。
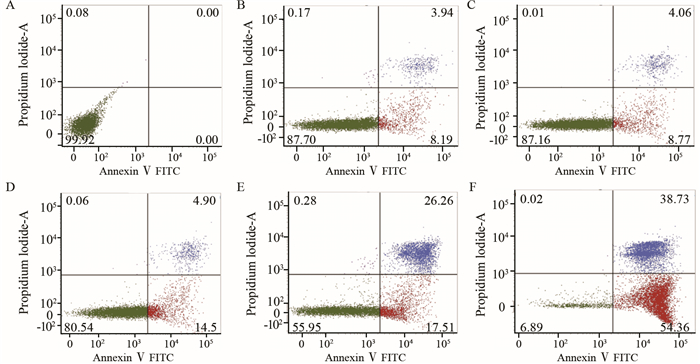
|
| A: Control group; B-F: 5, 10, 20, 40, 80μmol/L of T4O respectively. The apoptosis rate was increased in a dose-dependent manner 图 2 作用48 h时T4O对U266细胞凋亡的诱导作用 Figure 2 Apoptosis of U266 cells after treated with T4O for 48h |
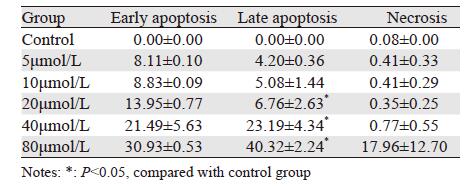 |
2.5 T4O对U266细胞周期的影响
随药物浓度增加,G1期细胞数减少,G2期细胞数明显减少,S期细胞数增加,细胞周期被阻滞在G2期,见图 3。
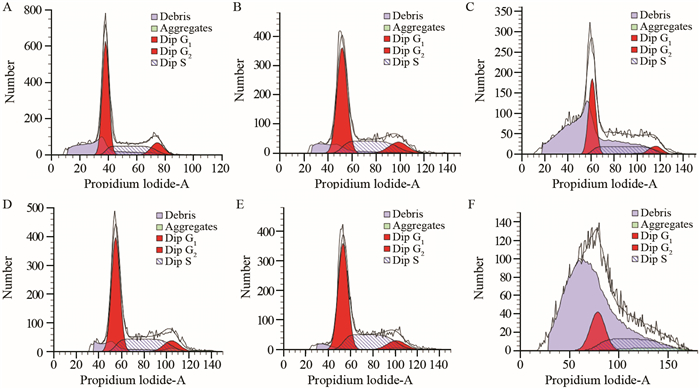
|
| A: Control group; B-F:5, 10, 20, 40, 80μmol/L of T4O respectively. The U266 cells were decreased in G1 and G2 phases while increased in S phase 图 3 T4O对U266细胞周期的影响 Figure 3 Changes of U266 cell cycles after treated with T4O |
2.6 JC-1检测T4O对U266线粒体膜电位的影响
T4O作用U266细胞48 h后,膜电位由低浓度至高浓度逐渐降低,见图 4。

|
| A: Control group; B-F:5, 10, 20, 40, 80μmol/L of T4O respectively; with the increasing drug concentration, mitochondrial membrane potential were (42.35±1.56), (39.94±0.58)*, (37.66±1.24)*, (34.33±0.48)*, (31.46±2.09)*, respectively, *: P<0.05, compared with control group (46.30±2.92) 图 4 T4O对细胞线粒体膜电位的 影响 Figure 4 Changes of mitochondrial membrane potential after treated with T4O |
3 讨论
T4O通过不同作用方式发挥抗癌活性[14-16],本实验数据表明,T4O通过线粒体途径及影响凋亡相关分子而对U266细胞有抑制增殖及促凋亡作用。
本研究中,CCK-8检测结果显示T4O能抑制U266细胞增殖,其作用方式呈时间-剂量依赖性,尤其作用48 h时,随药物浓度增加,T4O通过诱导U266细胞凋亡,增殖抑制作用变得越加明显。凋亡是由特定基因调控的细胞自杀过程,以细胞DNA发生特异性降解、形态表现为核固缩、核碎裂、核溶解及凋亡小体形成等为特征。诸多生理及病理过程均有凋亡参与[17]。倒置相差显微镜观察时,吖啶橙可透过正常细胞膜,使细胞核呈绿色或黄绿色均匀荧光;而在凋亡细胞中,因染色质固缩或断裂为大小不等的片段,形成凋亡小体。吖啶橙使其染上致密浓染的黄绿色荧光,或黄绿色碎片颗粒;而坏死细胞黄色荧光减弱甚至消失。吖啶橙AO常与溴化乙锭EB合用双染,因EB只染色死细胞使之产生桔黄色荧光,由此可以区分出正常细胞、凋亡细胞及坏死细胞[18]。本实验中,由低浓度至高浓度,黄绿色荧光逐渐减少,而红色荧光逐渐增多,并见数量不等的漂浮细胞及细胞碎片,说明T4O具有诱导U266细胞凋亡的作用。PARP(Poly ADP-Ribose Polymerase)是Caspase-3酶的主要底物之一,参与DNA修复及维持基因完整性[19]。凋亡启动时,PARP被Caspase-3酶剪切成两个片段,使受PARP负调控的核酸内切酶活性增高,损害核小体DNA,引起细胞凋亡[20]。本研究结果显示:Caspase-3酶活性增高呈剂量依赖性,证实了T4O激活凋亡途径关键酶Caspase-3的活性,进而诱导细胞凋亡。
细胞周期中,G1、G2期分别为S期和M期做准备,当细胞周期发生紊乱时,其可使细胞出现恶性增殖,导致肿瘤的发生[21]。本实验中,随药物浓度增加,G1期细胞数减少、G2期细胞数明显减少、S期细胞数增加,细胞周期被阻滞在G2期。
JC-1为线粒体膜电位检测的理想荧光探针。线粒体膜电位较高时,JC-1在线粒体基质中形成聚合物,其产生红色荧光;而线粒体膜电位较低时,JC-1不能聚集在线粒体的基质中,单体JC-1可以产生绿色荧光。线粒体膜电位的下降是细胞凋亡的一个标志性事件[22]。本研究中,JC-1检测结果随药物浓度增加,线粒体膜电位逐渐降低,提示T4O通过线粒体途径诱导细胞凋亡。
综上所述,珊瑚姜主要成分T4O通过使细胞线粒体膜电位降低及激活了凋亡途径关键酶Caspase-3诱导细胞凋亡,同时使细胞周期阻滞在G2期,从而抑制肿瘤细胞的分化与增殖。T4O显示良好的抗肿瘤活性,有希望成为临床用药之一,本研究为下一步临床研究提供了理论基础。
| [1] | Guidetti A, Prada CP, Laubach JP, et al. Pomalidomide for the treatment of relapsed and refractory multiple myeloma[J]. Expert Opin Orphan Drugs, 2014, 2 (10) : 1089–108. |
| [2] | Petrucci MT, Giraldo P, Corradini P, et al. A prospective, international phase 2 study of bortezomib retreatment in patients with relapsed multiple myeloma[J]. Br J Haematol, 2013, 160 (5) : 649–59. |
| [3] | Popat R, Khan I, Dickson J, et al. An alternative dosing strategy of lenalidomide for patients with relapsed multiple myeloma[J]. Br J Haematol, 2015, 168 (1) : 148–51. |
| [4] | Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib[J]. Blood, 2013, 121 (11) : 1961–7. |
| [5] | Zhou LL, Yang C, Wang HY, et al. Anti-infective effect of Zingiber corallinum Hance oil on drug-resistant bacteria and its acute toxicity[J]. Di San Jun Yi Da Xue Xue Bao, 2010, 32 (2) : 111–4. [周琳琳, 杨策, 王海燕, 等. 珊瑚姜油对常见耐药菌的抑菌作用及急性毒性实验[J]. 第三军医大学学报,2010, 32 (2) : 111–4. ] |
| [6] | Cao Y. Observation of clinical effect and antifungal activities on wild Zingiber corallinum[J]. Zhonghua Pi Fu Ke Za Zhi, 1989, 22 (2) : 103–5. [曹煜. 野生植物珊瑚姜抗真菌研究及临床疗效观察[J]. 中华皮肤科杂志,1989, 22 (2) : 103–5. ] |
| [7] | Luo SQ, Peng QC, Yang XO, et al. Volatiles and inhibitory phytopathogens fungi activities of essential oil extracted from Zingiber corallinum Hance[J]. Guangdong Nong Ye Ke Xue, 2013, 40 (17) : 84–6. [罗世琼, 彭全材, 杨雪鸥, 等. 珊瑚姜精油化学成分及其对4种植物病原真菌的抑制活性[J]. 广东农业科学,2013, 40 (17) : 84–6. ] |
| [8] | Chen WH, Cao Y, Yang ZN, et al. Analysis of chemical constituents in volatile oils from Zingiber coraflinum Hance[J]. Huaxi Yao Xue Za Zhi, 2008, 23 (6) : 718–9. [陈文慧, 曹煜, 杨占南. 珊瑚姜中挥发油的化学成分分析[J]. 华西药学杂志,2008, 23 (6) : 718–9. ] |
| [9] | Pan Q, Shi Y, Ye XF, et al. Effect of Terpinen-4-ol on the proliferation of lung adenocarcinoma A-549 cells in vitro and in vivo[J]. Ju Jie Shou Shu Xue Za Zhi, 2014, 23 (5) : 481–5. [潘乾, 石瑶, 叶秀峰, 等. 松油烯-4-醇体内外对人肺癌细胞系A-549增殖抑制作用[J]. 局解手术学杂志,2014, 23 (5) : 481–5. ] |
| [10] | Wu CS, Chen YJ, Chen JJ, et al. Terpinen-4-ol induces apoptosis in human nonsmall cell lung cancer in vitro and in vivo[J]. Evid Based Complement Alternat Med, 2012, 2012 : 818261. |
| [11] | Banjerdpongchai R, Khaw-On P. Terpinen-4-ol induces autophagic and apoptotic cell death in human leukemic HL-60 cells[J]. Asian Pac J Cancer Prev, 2013, 14 (12) : 7537–42. |
| [12] | Calcabrini A, Stringaro A, Toccacieli L, et al. Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells[J]. J Investi Dermatol, 2004, 122 (2) : 349–60. |
| [13] | Tu YH, Kang YQ, Zhou Y, et al. Effect of turmeric volatile oil on the proliferation and apoptosis of human leukemia cell line THP-1[J]. Shandong Da Xue Xue Bao(Yi Xue Ban), 2015, 53 (5) : 46–51. [涂云华, 康颖倩, 周英, 等. 姜黄挥发油对THP-1细胞增殖及凋亡的影响[J]. 山东大学学报(医学版),2015, 53 (5) : 46–51. ] |
| [14] | Pereira TS, de Sant'Anna JR, Silva EL, et al. In vitro genotoxicity of Melaleuca alternifolia essential oil in human lymphocytes[J]. J Ethnopharmacol, 2014, 151 (2) : 852–7. |
| [15] | Chinembiri TN, du Plessis LH, Gerber M, et al. Review of natural compounds for potential skin cancer treatment[J]. Molecules, 2014, 19 (8) : 11679–721. |
| [16] | Ji YB, Yu L. N-butanol extract of Capparis spinosa L. induces apoptosis primarily through a mitochondrial pathway involving mPTP open, cytochrome C release and caspase activation[J]. Asian Pac J Cancer Prev, 2014, 15 (21) : 9153–7. |
| [17] | H?cker G. The morphology of apoptosis[J]. Cell Tissue Res, 2000, 301 (1) : 5–17. |
| [18] | Atale N, Gupta S, Yadav UC, et al. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques[J]. J Microsc, 2014, 255 (1) : 7–19. |
| [19] | Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death[J]. Mutat Res, 2001, 477 (1-2) : 97–110. |
| [20] | Ferrucci A, Leboffe L, Agamennone M, et al. Ac-tLeu-Asp-H is the minimal and highly effective human caspase-3 inhibitor: biological and in silico studies[J]. Amino Acids, 2015, 47 (1) : 153–62. |
| [21] | Ezoe S, Matsumura I, Satoh Y, et al. Cell cycle regulation in hematopoietic stem/progenitor cells[J]. Cell Cycle, 2004, 3 (3) : 312–8. |
| [22] | Yokosuka T, Goto H, Fujji H, et al. Flow cytometric chemosensitivity assay using JC-1, a sensor of mitochondrial transmembrane potential, in acute leukemia[J]. Cancer Chemother Pharmacol, 2013, 72 (6) : 1335–42. |
 2016, Vol. 43
2016, Vol. 43


