文章信息
- 中低位直肠癌腹腔镜与开放全系膜切除合并侧方淋巴结清扫围手术期临床分析
- Perioperative Outcomes Between Laparoscopic and Conventional Open Lateral Pelvic Lymph Node Dissection following Total Mesorectal Excision for Mid-low Rectal Cancer
- 肿瘤防治研究, 2017, 44(6): 418-422
- Cancer Research on Prevention and Treatment, 2017, 44(6): 418-422
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2017.17.0256
- 收稿日期: 2017-03-10
- 修回日期: 2017-05-11
直肠癌的发病率及死亡率在国内仍高居不下,且近年有明显上升趋势[1]。目前全直肠系膜切除术(total mesorectal excision, TME)是直肠癌手术治疗的金标准术式。TME基础上的盆腔侧方淋巴结清扫用于治疗腹膜返折以下进展期直肠癌,目前在国内外仍有争论。近年国内多个学者报道了直肠癌患者在TME基础上行侧方淋巴结清扫,对这些报道的Meta分析结果显示开放手术下侧方淋巴结清扫可以降低局部复发率,提高患者的5年生存率,改善患者预后,但会增加手术时间及术中出血量[2]。腹腔镜手术由于有术中出血量少、创伤小、术后恢复快等优点,已被广泛用于直肠癌患者的治疗[3-4]。国内关于腹腔镜侧方淋巴结清扫的报道非常有限[5-6]。本研究回顾性分析了浙江省肿瘤医院腹腔镜与开放手术下围手术期结果,探讨中低位直肠癌患者在行腹腔镜TME基础上的侧方淋巴结清扫手术的安全性及可行性,并分析与侧方淋巴结转移有关的临床病理指标。
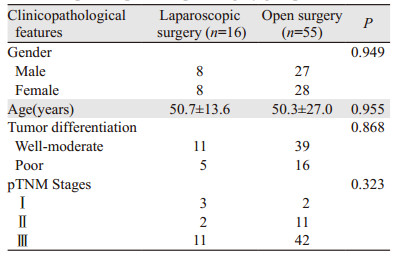
1 资料与方法 1.1 一般资料收集2005年12月至2014年12月间浙江省肿瘤医院结直肠外科71例中低位直肠癌住院患者,因术前CT及MR等影像学检查或术中探查发现盆腔侧方淋巴结肿大,临床诊断淋巴结可疑转移而行侧方淋巴结清扫术。两组患者临床病理特征上差异无统计学意义,见表 1。
 |
两组患者均在行全直肠系膜切除术(TME)基础上做单侧或双侧盆腔侧方淋巴结清扫术。腹腔镜组有12例行单侧清扫,4例行双侧清扫。开放手术组有34例行单侧清扫,21例行双侧清扫。两组单双侧清扫比例相似(P=0.354)。清扫的淋巴结主要包括肠系膜下动脉根部、髂总血管、髂外血管、腹股沟深、髂内血管、闭孔及部分患者的骶前淋巴结。腹腔镜组有5例、开放组有10例患者接受术前新辅助放化疗,两组接受新辅助治疗比例相似(P=0.260)。盆腔放疗方案为DT(45~50.4)Gy/((25~28)F.(5~5.5)w)-1,放疗期间同步口服卡培他滨片(1 650 mg/(m2.d))-1。
1.3 围手术期指标比较腹腔镜与开放手术两组患者的总手术时间、术中出血量、术后并发症、术后住院时间、肿瘤大小、分化程度、所获淋巴结的数目及阳性淋巴结转移数,并分析术前放化疗、肿瘤大小、分化程度、脉管瘤栓等临床病理因素与侧方淋巴结转移的相关性。
1.4 统计学方法采用SAS9.3软件分析。计量数据采用t检验,计数指标采用卡方检验。临床病理因素与侧方淋巴结转移的相关性采用逻辑回归分析。双边P < 0.05为差异有统计学意义。
2 结果 2.1 两组手术方式比较腹腔镜组16例患者中,行低位前切除术5例、腹会阴联合切除术9例、Hartmann手术2例。开放组55例患者中,行低位前切除术20例、腹会阴联合切除术32例、Hartmann手术3例。两组手术方式上的差异无统计学意义(P=0.173)。
2.2 从TME开始至侧方清扫结束手术全程平均时间、术中出血量及术后住院时间比较腹腔镜组患者平均手术时间(218.6±71.6)min,显著长于开放组的(181.3±57.9)min(P=0.035)。腹腔镜组和开放组患者术中出血量分别为(190.6±86.1)和(344.9±295.2)ml(P=0.044)。腹腔镜组患者术后住院时间(10.9±3.5天)与开放组(13.8±7.1天)比较,差异无统计学意义(P=0.125)。腹腔镜组患者无中转开腹。所有患者无围手术期死亡。
2.3 术后并发症比较腹腔镜组有4例患者出现术后并发症,包括尿路感染1例、会阴部感染2例及小肠不全性梗阻1例。开放手术组有11例患者出现术后并发症,包括子宫直肠旁积液2例、淋巴囊肿2例、盆腔感染2例、小肠不全性梗阻3例及短暂性尿潴留2例。分别予以抗炎、囊肿穿刺、留置小肠减压管及留置导尿管等保守处理后康复。两组并发症阳性率差异无统计学意义(25% vs. 20%, P=0.666)。
2.4 侧方淋巴结情况腹腔镜组患者切除侧方淋巴结数目平均为9.8±6.1枚,开放组患者为11.0±9.7枚,两组淋巴结切除数目差异无统计学意义(P=0.642)。腹腔镜组患者有25.0%(4/16)的侧方淋巴结转移,而开放组患者有34.5%(19/55)侧方淋巴结转移, 两组侧方淋巴结转移阳性率差异无统计学意义(P=0.473)。
在行双侧淋巴结清扫的25例患者中,3例(12.0%)有双侧淋巴结转移,6例(24.0%)有单侧淋巴结转移,其他16例(64.0%)双侧淋巴结均无转移。
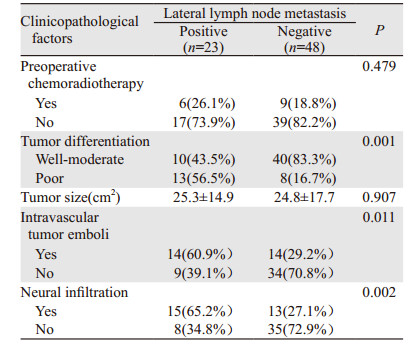
2.5 临床病理因素与侧方淋巴结转移的关系术前放化疗未能显著降低侧方淋巴结转移阳性率(P=0.479)。相比高中分化肿瘤,低分化癌可能更容易发生侧方淋巴结转移(P=0.001)。肿瘤的大小与是否发生侧方淋巴结转移无相关性(P=0.907)。有脉管瘤栓(P=0.011)和神经侵犯(P=0.002)的患者侧方淋巴结转移风险显著提高,见表 2。
 |
直肠癌全系膜切除术最早于1982年由英国的Heald提出,强调完整切除盆腔筋膜脏层包绕的直肠及其周围淋巴、脂肪和血管组织,同时切除的远端直肠系膜达提肛肌水平或超过肿瘤下缘5 cm。因TME手术能较彻底地清除沿上方引流途径转移的淋巴结,目前已经成为进展期直肠癌的“金标准”术式。除上方途径外,Gerota最早提出,直肠横襞(相当于腹膜返折处)以下的直肠还存在侧方淋巴结引流途径,因此TME不能取代侧方淋巴结清扫。
目前对中低位直肠癌患者TME基础上的侧方淋巴结清扫治疗仍有争议。研究表明侧方淋巴结有无转移与患者的总生存率、无病生存期、局部或远处转移无关[7]。相应的侧方淋巴结清扫手术不能降低局部复发率及延长患者5年生存率。且该手术延长了手术时间、增加术中出血,并带来一系列术后并发症,如尿滞留、性功能障碍等[7-9]。也有报道TME加术前放化疗在局部进展期直肠癌患者中能达到侧方淋巴结清扫相似的临床效果[10-11]。而在日本,侧方淋巴结转移则被认为是局限性疾病。2010年日本结直肠癌协会结直肠癌治疗指南建议对腹膜返折以下的进展期直肠癌作侧方淋巴结清扫[12]。Sugihara等报道TME加侧方淋巴结清扫使局部复发率降低了50%并且使5年生存率提高了8%[13]。国内的研究结果表明,开放TME加侧方淋巴结清扫有助于降低局部复发,提高患者的生存率[2]。保留盆腔内脏神经(splanchnic nerves)术式的应用,也减少严重并发症的发生[9-14]。本组资料显示,腹腔镜和开放TME加侧方淋巴结清扫的所有患者均未发生严重并发症及手术死亡。且侧方淋巴结清扫有助于提高低位直肠癌淋巴结转移的诊断准确性,更好指导术后治疗和评估预后。
腹腔镜已逐渐在直肠癌手术中得到广泛应用。凭借高清晰放大的图像以及照明系统,腹腔镜在深而狭窄的盆腔内对直肠癌患者行侧方淋巴结清扫具有一定的优势。Akiyoshi[15]对关于腹腔镜TME基础上侧方淋巴结清扫的多个报道进行了总结。这些报道中含12~68例手术患者,平均(或中位)的手术时间是52~678 min,术中出血量25~380 ml,切除侧方淋巴结数目为6~23枚。没有患者中转开放手术。术后并发症的发生率是7%~36%。本研究包括16例患者行腔镜侧方淋巴结清扫,5/16(31.3%)患者接受了术前放化疗,平均手术时间为218.6 min,术中平均出血量为190.6 ml,切除淋巴结的平均数目为9.8枚,有25.0%患者出现常见轻度的术后并发症,如创口感染和小肠梗阻等,与报道[15]的数据相似。
本研究进一步比较了腹腔镜与开放TME基础上行侧方淋巴结清扫后的围手术期结果。结果显示腹腔镜组比开放组需要更长的手术时间,但术中出血量少。腹腔镜及开放组有相似比例的单侧或双侧清扫。两组切除的侧方淋巴结数目、侧方淋巴结转移阳性的比例、并发症发生率及严重程度、术后住院时间均无显著差别。围手术期无患者死亡。Nagayoshi等[16]报道腹腔镜组患者切除的侧方淋巴结数目更多,术后住院时间缩短,其他围手术期结果与本文报道相似。他们也发现腹腔镜组与开放手术患者有相似的肿瘤复发率、3年总生存及无病生存率。这些结果说明腹腔镜下的侧方淋巴结清扫与开放手术有可比的安全性及可行性。
由于选择淋巴结清扫患者的标准不同,报道的侧方淋巴结转移率大不一致。有些文献报道超过50%的侧方淋巴结转移[17-19],更多的文献报道侧方淋巴结转移率为0~28%[5, 16, 20-22]。本研究的71例低位直肠癌患者中23例患者(32.4%)存在侧方淋巴结转移。但是,双侧侧方淋巴结清扫的患者中,仅12%有两侧侧方淋巴结转移,24%患者有单侧转移。我们样本数不足以分析哪些临床病理因素决定患者出现两侧侧方淋巴结转移,或单侧转移发生于哪一侧。TME基础上的直肠癌根治术不能清除转移的侧方淋巴结。这些侧方淋巴结转移是引起局部复发的重要原因。已有报道侧方淋巴结转移患者术后局部复发率显著高于无转移患者,并与生存期相关[2, 16, 23]。另一方面,这些结果提示有相当比例的直肠癌患者行侧方淋巴结清扫术后并未发现淋巴结转移,术前的CT或MRI影像甚至PET-CT诊断侧方淋巴结转移是低位直肠癌患者选择清扫的主要指征。这些结果提示需要进一步提高术前诊断准确率,减少不必要的侧方淋巴结清扫手术。
本研究结果显示侧方淋巴结转移与肿瘤的分化程度、有无脉管瘤栓及淋巴结神经侵犯相关,但与肿瘤的大小无关。其他文献也报道了上述或其他与侧方淋巴结转移相关的临床病理指标[13, 16, 23-24],有性别、肿瘤部位、肿瘤浸润深度[13]、直肠系膜内淋巴结转移[16]、侧方淋巴结大小[23]等。这些临床病理相关指标结合术前的影像学检查可能会有助于指导患者的进一步治疗。与Akiyoshi等[25]的报道一致,本研究也发现术前放化疗并没有降低所清扫的侧方淋巴结数目及侧方淋巴结转移的阳性率,提示单纯盆腔放化疗并不能彻底消除转移淋巴结中的肿瘤组织,仍有行盆腔侧方淋巴结清扫的必要性。
总之,腹腔镜TME加侧方淋巴结清扫与开放手术一样是安全可行的,能达到和开放手术一样的临床效果。
| [1] | Liu S, Zheng R, Zhang M, et al. Incidence and mortality of colorectal cancer in China, 2011[J].Chin J Cancer Res, 2015, 27(1): 22–8. |
| [2] | 朱明达, 殷红专, 苏琪. 侧方淋巴结清扫在国内低位直肠癌治疗中应用的Meta分析[J].世界华人消化杂志, 2016, 24(21): 3270–80. [Zhu MD, Yin HZ, Su Q. Meta-analysis of application of lateral lymph node dissection for low rectal cancer in China[J].Shi Jie Hua Ren Xiao Hua Za Zhi, 2016, 24(21): 3270–80. ] |
| [3] | Jin K, Wang J, Lan H, et al. Laparoscopic surgery for colorectal cancer in China: an overview[J].Int J Clin Exp Med, 2014, 7(12): 4635–45. |
| [4] | Chen W, Li Q, Fan Y, et al. Factors Predicting Difficulty of Laparoscopic Low Anterior Resection for Rectal Cancer with Total Mesorectal Excision and Double Stapling Technique[J].PLoS One, 2016, 11(3): e0151773. DOI:10.1371/journal.pone.0151773 |
| [5] | Liu T, Zhang C, Yu P, et al. Laparoscopic radical correction combined with extensive lymphadenectomy and pelvic autonomic nerve preservation for mid-to-low rectal cancer[J].Clin Colorectal Cancer, 2011, 10(3): 183–7. DOI:10.1016/j.clcc.2011.03.025 |
| [6] | 邓建中, 彭翔, 余思, 等. 保留自主神经的腹腔镜侧方淋巴结清扫在中低位直肠癌的应用[J].中华胃肠外科杂志, 2011, 14(8): 640–1. [Deng JZ, Peng X, Yu S, et al. Laparoscopic lateral lymph node dissection with autonomic nerve preservation in mid-low rectal cancer[J].Zhonghua Wei Chang Wai Ke Za Zhi, 2011, 14(8): 640–1. ] |
| [7] | Georgiou P, Tan E, Gouvas N, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis[J].Lancet Oncol, 2009, 10(11): 1053–62. DOI:10.1016/S1470-2045(09)70224-4 |
| [8] | 吴小剑, 黄美近, 何晓生, 等. 直肠癌侧方淋巴结清扫术安全性与有效性的系统评价[J].中华胃肠外科杂志, 2009, 12(3): 229–35. [Wu XJ, Huang MJ, He XS, et al. Systematic review on safety and efficacy of lateral node dissection in rectal cancer[J].Zhonghua Wei Chang Wai Ke Za Zhi, 2009, 12(3): 229–35. ] |
| [9] | Fujita S, Akasu T, Mizusawa J, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stageⅡ or stage Ⅲ lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial[J].Lancet Oncol, 2012, 13(6): 616–21. DOI:10.1016/S1470-2045(12)70158-4 |
| [10] | Kim JC, Takahashi K, Yu CS, et al. Comparative outcome between chemoradiotherapy and lateral pelvic lymph node dissection following total mesorectal excision in rectal cancer[J].Ann Surg, 2007, 246(5): 754–62. DOI:10.1097/SLA.0b013e318070d587 |
| [11] | Kusters M, Beets GL, van de Velde CJ, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence[J].Ann Surg, 2009, 249(2): 229–35. DOI:10.1097/SLA.0b013e318190a664 |
| [12] | Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer[J].Int J Clin Oncol, 2015, 20(2): 207–39. DOI:10.1007/s10147-015-0801-z |
| [13] | Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer[J].Dis Colon Rectum, 2006, 49(11): 1663–72. DOI:10.1007/s10350-006-0714-z |
| [14] | Moriya Y, Hojo K, Sawada T, et al. Significance of lateral node dissection for advanced rectal carcinoma at or below the peritoneal reflection[J].Dis Colon Rectum, 1989, 32(4): 307–15. DOI:10.1007/BF02553486 |
| [15] | Akiyoshi T. Technical feasibility of laparoscopic extended surgery beyond total mesorectal excision for primary or recurrent rectal cancer[J].World J Gastroenterol, 2016, 22(2): 718–26. DOI:10.3748/wjg.v22.i2.718 |
| [16] | Nagayoshi K, Ueki T, Manabe T, et al. Laparoscopic lateral pelvic lymph node dissection is achievable and offers advantages as a minimally invasive surgery over the open approach[J].Surg Endosc, 2016, 30(5): 1938–47. DOI:10.1007/s00464-015-4418-0 |
| [17] | Liang JT. Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy[J].Ann Surg Oncol, 2011, 18(1): 153–9. DOI:10.1245/s10434-010-1238-2 |
| [18] | Konishi T, Kuroyanagi H, Oya M, et al. Multimedia article. Lateral lymph node dissection with preoperative chemoradiation for locally advanced lower rectal cancer through a laparoscopic approach[J].Surg Endosc, 2011, 25(7): 2358–9. DOI:10.1007/s00464-010-1531-y |
| [19] | Park JS, Choi GS, Lim KH, et al. Laparoscopic extended lateral pelvic node dissection following total mesorectal excision for advanced rectal cancer: initial clinical experience[J].Surg Endosc, 2011, 25(10): 3322–9. DOI:10.1007/s00464-011-1719-9 |
| [20] | Obara S, Koyama F, Nakagawa T, et al. Laparoscopic lateral pelvic lymph node dissection for lower rectal cancer: initial clinical experiences with prophylactic dissection[J].Gan To Kagaku Ryoho, 2012, 39(12): 2173–5. |
| [21] | Bae SU, Saklani AP, Hur H, et al. Robotic and laparoscopic pelvic lymph node dissection for rectal cancer: short-term outcomes of 21 consecutive series[J].Ann Surg Treat Res, 2014, 86(2): 76–82. DOI:10.4174/astr.2014.86.2.76 |
| [22] | Furuhata T, Okita K, Nishidate T, et al. Clinical feasibility of laparoscopic lateral pelvic lymph node dissection following total mesorectal excision for advanced rectal cancer[J].Surg Today, 2015, 45(3): 310–4. DOI:10.1007/s00595-014-0906-4 |
| [23] | Kim TH, Jeong SY, Choi DH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection[J].Ann Surg Oncol, 2008, 15(3): 729–37. DOI:10.1245/s10434-007-9696-x |
| [24] | Wang Z, Loh KY, Tan KY, et al. The role of lateral lymph node dissection in the management of lower rectal cancer[J].Langenbecks Arch Surg, 2012, 397(3): 353–61. DOI:10.1007/s00423-011-0864-x |
| [25] | Akiyoshi T, Ueno M, Matsueda K, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging[J].Ann Surg Oncol, 2014, 21(1): 189–96. DOI:10.1245/s10434-013-3216-y |
 2017, Vol. 44
2017, Vol. 44
