2. 复旦大学附属华山医院感染病科, 上海 200040
2. Department of Infectious Disease, Huashan Hospital, Fudan University, Shanghai 200040, China
共收集2009年至2012年上海市公共卫生临床中心艾滋病患者来自于痰液、支气管肺泡灌洗液、脑脊液、淋巴结、胸腔积液、腹腔积液、组织活检及血液标本培养得到的分枝杆菌菌株140株。菌株保存于-70 ℃,后接种于罗氏培养基37 ℃培养28 d。挑取生长菌株置于400 mL 1 mmol/L Tris-HCL和1 mmmol/L EDTA (酸碱度值8.0)的混合溶液中煮沸10 min后,11 750×g离心10 min,取上清液保存于-20 ℃待用[20]。
1.2 主要仪器及试剂S1000 PCR仪购自美国BIO-RAD公司,DY-A电泳仪购自上海西巴斯生物技术开发公司;DNA提取试剂盒购自德国Qiagen公司,引物由上海俊晟生物科技有限公司合成,MPB64抗原胶体金检测试剂盒购自杭州创新生物检控技术有限公司。
1.316 SrDNA基因扩增和测序检测菌株类型采用DNA提取试剂盒提取分枝杆菌培养阳性菌株DNA,合成16SrDNA通用引物。8FPL:5’-AGTTTGATCCTGGCTCAG-3’;1492:5’-GGTTACCTTACGACTT-3’。将抽提的DNA进行PCR扩增。50 μL PCR反应体系:37.5 μL双蒸水,1 μL模板DNA,5 μL 10×PCR含镁离子缓冲液,4 μL dNTPs(2.5 mmol/L),20 μmol/L引物8FPL和1492各1 μL,2.5 U rTaq DNA聚合酶。PCR反应条件:94 ℃5 min;依次95 ℃40 s、55 ℃40 s、72 ℃90 s,共35循环;72 ℃10 min延伸后置于4 ℃保存。凝胶电泳检测后送上海生工生物工程股份有限公司测序,序列比对后确定相应菌种。
1.4 MPB64抗原胶体金检测菌株类型MPB64抗原采用MPB64抗原胶体金检测试剂盒进行检测,操作流程按照试剂盒要求。采集1 μL菌体悬浊于0.2 mL生理盐水中并充分混匀,取100 μL加入到试剂检测孔中,15 min后观察,1 h内判定结果。检测结果判定:检测结果区和阳性对照区均出现条带则结果为阳性;阳性对照区出现条带,检测结果区无条带则结果为阴性。如检测结果为阳性,表明菌株中存在MTB;结果为阴性则表明菌株中不存在MTB,一般判定为NTM。
2 结果 2.116 SrDNA测序鉴定分型结果共有114份标本(114/140,81.43%)获得16SrDNA测序结果,26份标本扩增及测序结果为阴性(26/140,18.57%)。在获得鉴定结果的114份标本中,51份(43.86%)为MTB,63份(55.26%)为NTM,1份为奴卡菌。63份NTM菌株标本的亚型鉴定结果见图 1。其中戈登分枝杆菌、鸟分枝杆菌和堪萨斯分枝杆菌系前三位的NTM菌种。
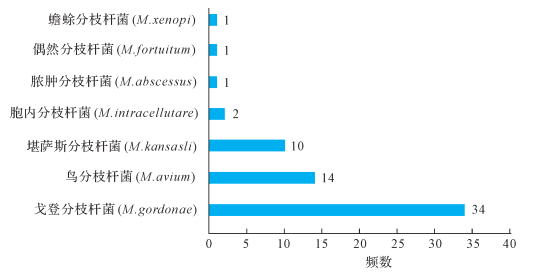
|
| 图 1 NTM测序鉴定分型结果 Fig. 1 The results of NTM species by gene sequencing |
在通过16SrDNA测序鉴定的113份分枝杆菌菌株中有50份经MPB64胶体金法检测结果为阳性(50/113,44.25%)。将16SrDNA测序结果作为金标准判定MBP64抗原胶体金法检测的敏感性和特异性分别为96.00%和96.84%,见表 1。
| [n(%)] | ||
| 16SrDNA测序结果 | MTB(n=50) | NTM(n=63) |
| MTB | 48(96.00) | 3(3.17) |
| NTM | 2(4.00) | 61(96.84) |
本研究分析了140株来自艾滋病患者的分枝杆菌培养阳性菌株,结果显示,艾滋病合并分枝杆菌感染中NTM所占的比例相当高。在一项来自中国广西壮族自治区的研究表明,艾滋病合并分枝杆菌感染患者43%为NTM感染[21]。在另一项印度的研究中,经过鉴定MTB和NTM的比例分别为42.8%和57.2%[22],与本研究结果相似。这表明对艾滋病患者的分枝杆菌培养阳性的标本进行MTB和NTM鉴别十分重要。
在中国的一些不发达地区,由于仪器设备的落后,分枝杆菌感染的诊断目前还仅仅依赖于临床症状和抗酸染色阳性结果。虽然痰涂片染色的方法简单而且经济,但阳性结果无法判别是何种分枝杆菌。NTM[23]和其他一些细菌如努卡菌等在自然界中广泛存在,所导致的肺部感染在影像学上极为相似,且抗酸染色均为阳性[24]。因此,需要同时进行培养以明确病原体种类并提供药物敏感性结果。目前分枝杆菌的液体培养已经在艾滋病合并分枝杆菌感染的检测中广泛应用,该方法可以提高菌株的检出率而且所需时间较短。在发达国家,快速培养及鉴定MTB与NTM对临床诊疗十分重要。MTB复合群在液体培养基中生长时的主要分泌蛋白是MPB64抗原,且后者不存在NTM培养滤液中[25]。日本研发了Capillia TB免疫层析技术,通过检测MPB64抗原可进行MTB复合群和NTM初步鉴定。该检测方法的特点为快速、经济[26-28],对MTB复合群的敏感度为98.4%,特异度为97.6%,阳性预测值和阴性预测值分别为97.7%和98.4%,Kappa值为0.96,但该技术仅限于对培养物的检测[29]。本研究结果显示,国产的MPB64抗原胶体金检测试剂盒对临床分枝杆菌培养菌株的敏感性和特异性分别为96.00%和96.84%,提示该试剂盒可以作为MTB与NTM初步鉴定的一种有效的方法;而且该试剂盒价格实惠,操作简便,特别适用于经济不发达地区。
本研究结果还显示,艾滋病合并结核患者中最常见的NTM菌种为戈登分枝杆菌、鸟分枝杆菌和堪萨斯分枝杆菌。1982年,在免疫缺陷患者中首次报道了播散性鸟分枝杆菌病[30-31]。这些患者后来被证实为HIV感染者。CD4+T细胞计数低于100/μL的严重艾滋病患者容易感染NTM,临床可在患者的血液、组织、痰液及粪便标本中分离出NTM[32-33]。自1985年以来,已鉴定出超过30种的分枝杆菌菌种[34],其中最常见的为鸟分枝杆菌。因此大部分鉴定为NTM的艾滋病患者都接受了抗鸟分枝杆菌的经验性治疗。首选的药物为克拉霉素或阿奇霉素加上乙胺丁醇。虽然鸟分枝杆菌感染占大多数,但临床发现越来越多的其他NTM导致的感染。本研究中,最常见的NTM类型为戈登分枝杆菌,第二位才是鸟分枝杆菌。体外研究结果提示,戈登分枝杆菌药物治疗方案包括阿米卡星、乙胺丁醇、利福平、复方磺胺甲噁唑以及利奈唑胺[35]。两种NTM的治疗药物存在差异,因此对所有鉴定为NTM的艾滋病合并分枝杆菌感染的患者进行经验性抗鸟分枝杆菌治疗可能导致临床治疗失败或疗效不佳。
在本研究中较为遗憾的是,虽然对140份标本进行了16SrDNA测序鉴定,但最终只有114份标本获得了测序结果。分析原因为:在提取阳性菌株的DNA及PCR扩增的过程中,由于标本抽提失败或DNA过少或其他操作原因可能导致了26份标本扩增及测序结果为阴性。这导致了最终样本量的减少。
总之,MPB64抗原胶体金法快速检测具有较好的特异性,且快速、经济、简便,适合用于临床对分枝杆菌的鉴别。艾滋病合并分枝杆菌感染的患者中NTM的比例较高,需进行菌种鉴定以指导临床治疗。
| [1] | World Health Organization. WHO global tuberculosis report 2015[M]. Geneva: World Health Organization, 2015 : 13 . |
| [2] | World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response[M]. Geneva: World Health Organization, 2012 : 17 -19. |
| [3] | JOHNSON J L, ELLNER J J. Tropical infectious diseases: principles, pathogens and practice[M]. Philadelphia: Churchill Livingstone, 2011 : 228 -247. |
| [4] | WOLINSKY E. Mycobacterial diseases other than tuberculosis[J]. Clin Infect Dis, 1992, 15 (1) :1–10. doi:10.1093/clinids/15.1.1 |
| [5] | HERZMANN C, LANGE C. Infections with Non-tuberculous mycobacteria and HIV[J]. Dtsch Med Wochenschr, 2010, 135 (23) :1192–1197. doi:10.1055/s-0030-1255130 |
| [6] | KAWANA M, STARR R S, TASHIMA K T, et al. Spontaneous perforation of the terminal ileum in an AIDS patient on highly active antiretroviral therapy with disseminated non-tuberculous mycobacterial infection[J]. Int J Infect Dis, 2008, 12 (6) :603–606. doi:10.1016/j.ijid.2007.12.013 |
| [7] | MIGUEZ-BURBANO M J, FLORES M, ASHKIN D, et al. Non-tuberculous mycobacteria disease as a cause of hospitalization in HIV-infected subjects[J]. Int J Infect Dis, 2006, 10 (1) :47–55. doi:10.1016/j.ijid.2004.11.005 |
| [8] | MIGUEZ-BURBANO M J, SHOR-POSNER G, HADRIGAN S. Non-tuberculous mycobacteria in HIV-infected patients: geographic, behavioural, and immunological factors[J]. Lancet Infect Dis, 2005, 5 (7) :394–395. doi:10.1016/S1473-3099(05)70146-8 |
| [9] | EL-SOLH A A, NOPPER J, ABDUL-KHOUDOUD M R, et al. Clinical and radiographic manifestations of uncommon pulmonary nontuberculous mycobacterial disease in AIDS patients[J]. Chest, 1998, 114 (1) :138–145. doi:10.1378/chest.114.1.138 |
| [10] | POKAM B T, ASUQUO A E. (2012) Acid-fast bacilli other than Mycobacteria in tuberculosis patients receiving directly observed therapy short course in cross river state, Nigeria[J]. Tuberc Res Treat, 2012, 2012 :301056. |
| [11] | AL JARAD N, DEMERTZIS P, JONES D J, et al. Comparison of characteristics of patients and treatment outcome for pulmonary non-tuberculous mycobacterial infection and pulmonary tuberculosis[J]. Thorax, 1996, 51 (2) :137–139. doi:10.1136/thx.51.2.137 |
| [12] | AFGHANI B, LIEBERMAN J M, DUKE M B, et al. Comparison of quantitative polymerase chain reaction, acid fast bacilli smear, and culture results in patients receiving therapy for pulmonary tuberculosis[J]. Diagn Microbiol Infect Dis, 1997, 29 (2) :73–79. doi:10.1016/S0732-8893(97)00114-4 |
| [13] | CRUCIANI M, SCARPARO C, MALENA M, et al. Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of Mycobacteria[J]. J Clin Microbiol, 2004, 42 (5) :2321–2325. doi:10.1128/JCM.42.5.2321-2325.2004 |
| [14] | KATILA M L, KATILA P, ERKINJUNTTI-PEKKANEN R. Accelerated detection and identification of Mycobacteria with MGIT 960 and COBAS AMPLICOR systems[J]. J Clin Microbiol, 2000, 38 (3) :960–964. |
| [15] | CLOUD J L, NEAL H, ROSENBERRY R, et al. Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries[J]. J Clin Microbiol, 2002, 40 (2) :400–406. doi:10.1128/JCM.40.2.400-406.2002 |
| [16] | REDDINGTON K, O'GRADY J, DORAI-RAJ S, et al. A novel multiplex real-time PCR for the identification of Mycobacteria associated with zoonotic tuberculosis[J/OL]. PLoS One, 2011, 6(8):e23481. https://www.researchgate.net/publication/51586948_A_Novel_Multiplex_Real-Time_PCR_for_the_Identification_of_Mycobacteria_Associated_with_Zoonotic_Tuberculosis |
| [17] | MOURE R, MARTIN R, ALCAIDE F. Effectiveness of an integrated real-time PCR method for detection of the Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples in an area of low tuberculosis prevalence[J]. J Clin Microbiol, 2012, 50 (2) :513–515. doi:10.1128/JCM.06467-11 |
| [18] | LING D I, FLORES L L, RILEY L W, et al. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression[J/OL]. PLoS One, 2008, 3(2): e1536. |
| [19] | WANG L, CHENG S, XU M, et al. Model collaboration between hospitals and public health system to improve tuberculosis control in China[J]. Int J Tuberc Lung Dis, 2009, 13 (12) :1486–1492. |
| [20] | VAN SOOLINGEN D, HWEMANS P W, DE HAAS P E, et al. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis[J]. J Clin Microbiol, 1991, 29 (11) :2578–2586. |
| [21] | LAN R, YANG C, LAN L, et al. Mycobacterium tuberculosis and Non-tuberculous mycobacteria isolates from HIV-infected patients in Guangxi, China[J]. Int J Tuberc Lung Dis, 2011, 15 (12) :1669–1675. doi:10.5588/ijtld.11.0036 |
| [22] | KHATTER S, SINGH U B, ARORA J, et al. Mycobacterial infections in human immuno-deficiency virus seropositive patients: role of Non-tuberculous mycobacteria[J]. Indian J Tuberc, 2008, 55 (1) :28–33. |
| [23] | BUIJTELS P C, VAN-DER-SANDE M A, DE-GRAAFF C S, et al. Non-tuberculous mycobacteria, Zambia[J]. Emerg Infect Dis, 2009, 15 (2) :242–249. doi:10.3201/eid1502.080006 |
| [24] | OLSON E S, SIMPSON A J, NORTON A J, et al. Not everything acid fast is Mycobacterium tuberculosis: a case report[J]. J Clin Pathol, 1998, 51 (7) :535–536. doi:10.1136/jcp.51.7.535 |
| [25] | NAGAI S, WIKER H G, HARBOE M, et al. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis[J]. Infect Immun, 1991, 59 (1) :372–382. |
| [26] | ABE C, HIRANO K, TOMIYAMA T. Simple and rapid identification of Mycobacterium tuberculosis complex by immunochromatographic assay using anti-MPB64 monoclonal antibodies[J]. J Clin Microbiol, 1999, 37 (11) :3693–3697. |
| [27] | HILLEMANN D, RVSCH-GERDES S, RICHTER E. Application of the Capilia TB assay for culture confirmation of Mycobacterium tuberculosis complex isolates[J]. Int J Tuberc Lung Dis, 2005, 9 (12) :1409–1411. |
| [28] | MUYOYETA M, DE HAAS P E, MUELLER D H, et al. Evaluation of the Capilia TB assay for culture confirmation of Mycobacterium tuberculosis infections in Zambia and South Africa[J]. J Clin Microbiol, 2010, 48 (10) :3773–3775. doi:10.1128/JCM.01688-09 |
| [29] | YAMAGUCHI R, MATSUO K, YAMAZAKI A, et al. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG[J]. Infect Immun, 1989, 57 (1) :283–288. |
| [30] | TAMADA Y, KANDA S, YOSHIDOME A, et al. Diagnosis of active tuberculosis using MPB64, a specific antigen of Mycobacterium bovis[J]. Microbiol Immunol, 2012, 56 (11) :740–747. doi:10.1111/mim.2012.56.issue-11 |
| [31] | ZAKOWSKI P, FLIGIEL S, BERLIN G W, et al. Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency[J]. JAMA, 1982, 248 (22) :2980–2982. doi:10.1001/jama.1982.03330220024029 |
| [32] | HOSBURGH C R, HAVLIK J A, ELLIS D A, et al. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy[J]. Am Rev Respir Dis, 1991, 144 (3 Pt 1) :557–559. |
| [33] | NIGHTINGALE S D, BYRD L T, SOUTHERN P M, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients[J]. J Infect Dis, 1992, 165 (6) :1082–1085. doi:10.1093/infdis/165.6.1082 |
| [34] | TORTOLI E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s[J]. Clin Microbiol Rev, 2003, 16 (2) :319–354. doi:10.1128/CMR.16.2.319-354.2003 |
| [35] | BROWN-ELLIOTT B A, CRIST C J, MANN L B, et al. In vitro activity of linezolid against slowly growing Non-tuberculous mycobacteria[J]. Antimicrob Agents Chemother, 2003, 47 (5) :1736–1738. doi:10.1128/AAC.47.5.1736-1738.2003 |




