| Effects of 5-azacytidine-2'-deoxycytidine on the proliferation, cell cycle, and apoptosis of fetal bovine fibroblast cells |
2. Key Laboratory of Agricultural Animal Genetic, Breeding, and Reproduction of Ministry of Education, College of Animal Science & Technology, Huazhong Agricultural University, Wuhan 430070, China
2. 华中农业大学动物科学技术学院,农业动物遗传育种与繁殖教育部重点实 验室,武汉 430070
Terminally differentiated somatic cell nuclei can be reprogrammed to totipotency when transferred into enucleated mature oocytes[1-2]. However, the success rate of somatic cell nuclear transfer (SCNT) remains quite low, frequently due to abnormal genomic imprinting. In some mammals, genomic imprinting is an important epigenetic mechanism that ensures the expression of parental origin-specific monoallelic genes. Imprinted genes play essential roles in diverse biological phenomena, notably in the regulation of placental and postnatal growth and/or adult behaviors[3].
Methylation imprints are established during germ cell development and are protected from genomewide demethylation and re-methylation during early embryonic development. DNA methylation plays an important role in gene expression, which influences early embryonic development and subsequent growth[4]. In natural reproduction, DNA methylation levels in gametes are relatively low, and further demethylation occurs during early development. However, terminally differentiated somatic cells exhibit higher levels of DNA methylation. In SCNT, these highly methylated somatic cells are used as donor cells, which will in turn result in abnormal hypermethylation in cloned embryos[5]. To improve the efficacy of SCNT, several epigenetic remodeling drugs such as the DNA methylation inhibitor 5-azacytidine-2'-deoxycytidines (5-AzaCdR) and histone deacetylase inhibitors as Trichostatin A have been used to enhance the early developmental potential of cloned embryos[6-7]. Although epigenetic modification agents such as 5-Aza-CdR can improve the cloning efficacy, the mechanisms underlying the developmental improvement in cloned embryos still require further investigation. In this study, we explored the effects of different concentrations of 5-Aza-CdR on common SCNT donor cells (fetal bovine fibroblast cells, FBFCs).
1 Materials and methods 1.1 ReagentsUnless otherwise stated, all chemicals and media were purchased from Sigma-Aldrich (St Louis, MO, USA).
1.2 Cell cultureThe isolation of FBFCs was performed as previously described[8]. Briefly, FBFCs were obtained from a bovine fetus of a slaughterhouse. The FBFCs were isolated from a skin biopsy and cultured in DMEM (Gibco, Life Technology, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, Life Technology) in a 37.5 ℃ incubator with the humidified atmosphere of 5% CO2 and 95% air.
1.3 Experimental designFBFCs at passage 6 were used. At 24 h after cell seeding, the FBFCs were treated with different concentrations (0, 0.01, 0.1, 0.5 and 1.0 μmol/L) of the DNA methyltransferase (DNMT1) inhibitor 5- AzaCdR. The cells at different time points were used for the analysis of proliferation, cell cycle, apoptosis, and mRNA expression of DNMT1.
1.4 Cell proliferation assayThe proliferation of FBFCs treated with 5-AzaCdR was detected[9]. Briefly, harvested FBFCs were seeded onto 96-well culture plates at a density of 5 000 cells/well and cultured for 24, 48 and 72 h. The cells treated with different concentrations of 5-AzaCdR were then incubated with 10 μL of Cell Counting Kit (CCK)-8 (Dojindo, Kumamoto, Japan) solution for 4 h at 37 ℃. The proliferation of FBFCs was measured using the CCK- 8 according to the manufacturer's protocol. Absorbance was measured at a wavelength of 450 nm using a spectrophotometer.
1.5 Cell viability assayThe viability of FBFCs treated with different concentrations of 5-Aza-CdR was measured[10]. Briefly, the FBFCs were seeded onto 96-well plates and treated with 0, 0.01, 0.1, 0.5 or 1.0 μmol/L of 5-Aza-CdR for 24, 48, 72 or 96 h. Then, 10 μL of CCK-8 solution was added, and the cells were incubated for 4 h. Cell viability was analyzed using the CCK-8. The optical density (OD) values were measured at 450 nm using a microplate reader (Model 680, Bio-Rad, Hercules, CA, USA).
1.6 Cell cycle assayThe cell cycle was analyzed as reported elsewhere[11] with some modifications. Briefly, the FBFCs were seeded onto six- well microplates at a final density of 1×106 cells/well. After 24 h incubation, the cells were treated with different concentrations of the DNMT1 inhibitor 5-Aza-CdR. At 24, 48 and 72 h after 5-Aza-CdR treatment, the cells were trypsinized, washed, and fixed in 70% ethanol at -20 ℃ for 24 h. For flow cytometric analysis, the cells were incubated in RNase at 37 ℃ for 30 min, treated with propidium iodide (PI) and suspended in 300 µL of Dulbecco's phosphate buffer solution (DPBS). The cell cycle distribution was examined by measuring the DNA content on a FACSCalibur (BD, Immunocytometry Systems, San José, CA, USA).
1.7 Detection of apoptosisThe procedure used to detect apoptotic cells was conducted as described[12] with some modifications. Briefly, the FBFCs were seeded onto sixwell microplates at a final density of 1 × 106 cells/ well in 100 μL of complete medium. After 24 h, the cells were treated with different concentrations of the DNMT1 inhibitor 5-Aza-CdR. After 24, 48 or 72 h, apoptotic FBFCs in the early and late stages were determined via annexin V- FITC and PI staining, respectively. Terminal deoxynucleotidyl transferase biotin- dUTP nick end labeling (TUNEL) was performed to analyze the apoptotic changes in the FBFCs. The rate of early apoptosis was defined as the sum of the apoptotic cells (Annexin V-FITCpositive/PI- negative)/total cells in the early stages. The rate of late apoptosis was defined as the sum of the apoptotic cells (Annexin V-FITC-positive/ PI-positive)/total cells in the late stages. The rate of total apoptosis was the sum of the rates of early apoptosis to late apoptosis.
1.8 Real-time polymerase chain reaction (PCR)Total RNA was isolated from fetal bovine fibroblasts at passage 6 using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Total RNA was measured using a NanoDrop-2000 spectrophotometer at wavelengths of 260 and 280 nm (Thermo Fisher Scientific Inc., Waltham, USA). Reverse transcription was performed with M-MuLV reverse transcriptase (Promega, WI, USA). The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. Amplification was performed using SYBR green master mix (Life Technologies, USA). The amplification conditions were as follows: 40 cycles of denaturation at 94 ℃ for 20 s, annealing at 60 ℃ for 20 s, and primer extension at 72 ℃ for 20 s. The primer sequences were as follows: 5′-GTAGTG GACGACAAGAAGTTTG-3′ (forward), 5′-ATCTC AGGGAGGTCAGACAT-3′ (reverse), 101 bp for DNMT1, and 5′- CTGCCCGTTCGACAGATAG- 3′ (forward), 5′-CTCCGACCTTCACCATCTTG-3′ (reverse), 76 bp for GAPDH.
1.9 Statistical analysisValues are shown as the mean±standard error of the mean (SEM). The statistical analysis was performed using Student 's t-test for two groups and one-way analysis of variance for multiple comparisons. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when P < 0.05.
2 Results 2.1 Effects of 5-Aza-CdR on the proliferation of FBFCsFBFCs were widely used in exploring the mechanisms of cellular reprogramming. Therefore, FBFCs were used as materials to explore the effects of 5-Aza-CdR on cellular physical events (Fig. 1A). the FBFCs at passage 6 were used for further experiments (Fig. 1B).
 |
| Fig. 1 Primary fetal bovine fibroblast cells (A) and fetal bovine fibroblast cells at passage 6 (B) |
It is possible that demethylation treatment in FBFCs may change cell proliferation. Therefore, the OD values of FBFCs treated with different concentrations of 5-Aza-CdR were assessed using the CCK-8 method. As shown in Fig. 2, 5-Aza-CdR produced inhibitory effects on FBFCs in a dosedependent manner. At 24 and 48 h after 5-Aza-CdR treatment, the OD value in the 1.0 μmol/L group was significantly lower than that in the control group (P < 0.05). At 72 h after 5- Aza- CdR treatment, the OD value in the 1.0 μmol/L group was significantly lower than that of the 0.5 μmol/L group. Similarly, at 96 h, the OD value of the 1.0 μmol/L treatment group was significantly lower than those of the control and the 0.5 μmol/L groups (P < 0.01). Meanwhile, the OD value of the 0.5 μmol/L treatment group was significantly lower than that of the control group. However, no differences were observed at different time within the same treatment group. These results suggest that the FBFCs with treatment of 5-AzaCdR produce a harmful effect on the proliferation of FBFCs in a dose- and time-dependent manner.
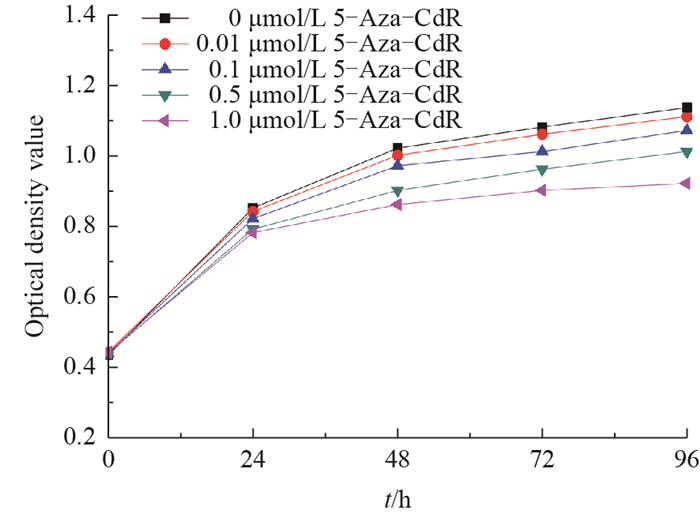 |
| Fig. 2 Effects of 5-Aza-CdR on the proliferation of FBFCs |
Demethylation drug treatment may exert harmful effects of cell characteristics, such as cell viability. Therefore, the effect of 5-Aza-CdR on the viability of FBFCs was detected using the CCK- 8 method at 450 nm. As shown in Fig. 3, 5- Aza- CdR inhibited the growth of FBFCs in a time- and dose- dependent manner. At 24 and 48 h after 5-Aza-CdR treatment, the viability of FBFCs in the 1.0 μmol/L group was significantly lower than that of the control group (P < 0.05). At 72 and 96 h after treatment, the cell viability in the 1.0 μmol/L group was significantly lower than those of the control and 0.01 μmol/L groups (P < 0.05). Similarly, the cell viability in the 0.5 μmol/L group was significantly lower than that of the control group (P < 0.05). Moreover, no significant differences were found among other groups and among the same treatment group at different time points.
 |
| Different lowercase letters above the bars indicate statistically significant differences among the groups at the 0.05 probability level. Fig. 3 Effects of 5-Aza-CdR on the viability of FBFCs |
The efficacy of reprogramming in the somatic cells vary with dependence on the different stages of cell cycles. Therefore, the cell cycle distribution of FBFCs treated by 5-Aza-CdR was measured by using flow cytometry. As indicated in Fig. 4, the rate of FBFCs arrested at the G0/G1 stage was significantly higher in the 1.0 μmol/L group than in the control and 0.01 μmol/L groups at 24, 48 and 72 h (P < 0.05 and 0.01). Meanwhile, the rate in the 0.5 μmol/L group was significantly higher than that in the control group (24 and 48 h, P < 0.05; 72 h, P < 0.01) and in the 0.01 μmol/ L group (24 and 48 h, P < 0.05; 72 h, P < 0.01). The rates of the FBFCs in the S stage in the 1.0 μmol/L and 0.5 μmol/L groups were significantly lower than that of the control group (P < 0.05) at 24 h. Similarly, at 48 h, the rates in the 1.0 and 0.5 μmol/L groups were significantly lower than that of the control group (P < 0.05), while the rate in the 1.0 μmol/L group was also significantly lower than that of the 0.01 μmol/L group (P < 0.05). These data indicate that 5-Aza-CdR treat-ment arrested the cells at the G0/G1 stage in a dose- and time-dependent manner.
 |
| Different lowercase letters above the bars indicate statistically significant differences among the groups at the 0.05 probability level; different uppercase letters above the bars indicate statistically highly significant differences among the groups at the 0.01 probability level. Fig. 4 Effects of 5-Aza-CdR on the cell cycle distribution of FBFCs |
Drug treatment in somatic cells may result in an increase of apoptosis. Therefore, the effect of 5-Aza CdR on the apoptosis of FBFCs was analyzed using TUNEL. As indicated in Fig. 5A, the effects of 5- Aza- CdR on the rate of early apoptosis in FBFCs were as follows. The rates in the 1.0 μmol/L group were significantly higher than those of the other four groups at 24, 48 and 72 h (P < 0.01). Meanwhile, the early apoptotic rate in the 0.5 μmol/L group was significantly higher than those in the control (48 and 72 h, P < 0.01) and 0.01 μmol/L (48 and 72 h, P < 0.01) groups. Similarly, the early apoptotic rates in the 0.1 and 0.01 μmol/L groups were significantly higher than those of the control group at 48 and 72 h (P < 0.05). The effects of 5-Aza-CdR on the rate of late apoptosis were as follows (Fig. 5B). The rate in the 1.0 μmol/L group was significantly higher than those in the control and 0.01 μmol/L groups at 24, 48 and 72 h, respectively (P < 0.05 and 0.01). The rate of late apoptosis in the 0.5 μmol/L group was also significantly higher than that in the control group (48 h, P < 0.05; 72 h, P < 0.01) and the 0.01 μmol/L group, respectively (48 h, P < 0.05). No significant differences were found among the other groups. The rate of total apoptosis showed no significant difference between the 0.01 μmol/L and the control group (24 h, P > 0.05), while the differences among the other groups were all significant (P < 0.01) (Fig. 5C). These results demonstrated that 5-Aza-CdR induced apoptosis of FBFCs in a dose- and time dependent manner.
 |
| Different lowercase letters above the bars indicate statistically significant differences among the groups at the 0.05 probability level; different uppercase letters above the bars indicate statistically highly significant differences among the groups at the 0.01 probability level. Fig. 5 Effects of 5-Aza-CdR on the apoptosis of FBFCs |
The effects of 5-Aza-CdR on the expression of DNMT1 mRNA were measured by using qPCR. As shown in Fig. 6, the mRNA expression levels of DNMT1 in the 0.01 and 0.1 μmol/L groups were not significantly different from the control group. In contrast, the DNMT1 mRNA levels in the 0.5 and 1.0 μmol/L groups were significantly decreased as comparison with that of the control group (decreasing by 14.0% and 24.0%, respectively).
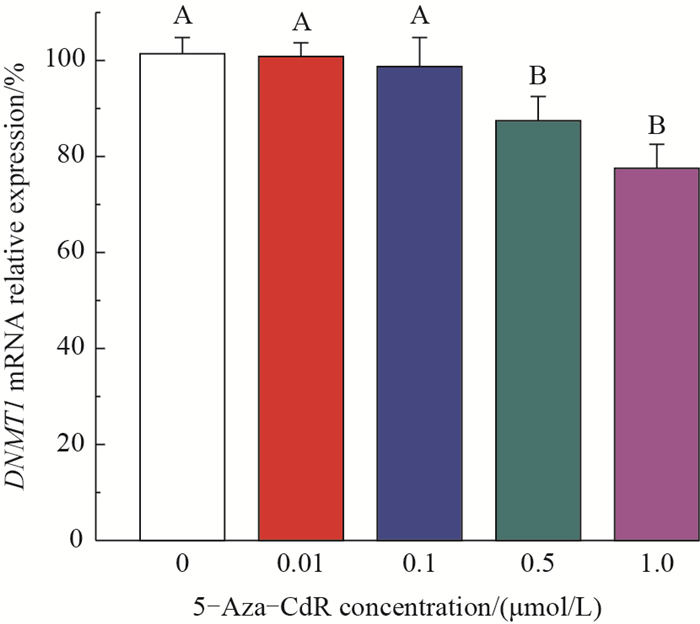 |
| Different uppercase letters above the bars indicate statistically highly significant differences among the groups at the 0.01 probability level. Fig. 6 Effects of 5-Aza-CdR on the mRNA expression of DNMT1 |
In the present study, we aimed to explore a method for treating donor cells for SCNTs. The results demonstrated that the FBFCs with the treatment of 5- Aza-CdR produce dose- and time-dependent effects.A concentration of 5-Aza-CdR of less than 0.1 μmol/L will not affect the cell cycle distribution, apoptosis, and DNMT1 mRNA expression level in the FBFCs. The optimal treatment time of FBFCs with 5- AzaCdR is 24 h.
The DNA demethylating agent 5-Aza-CdR has been used to activate the expression of silent imprinted genes[13]. Due to this characteristic, it has also been used to enhance genomic methylation reprogramming and regulate the transcription of early embryo developmentassociated genes in embryos undergoing SCNT, thereby resulting in the improvement of the efficacy of SCNT[14-15]. However, it is also a teratogenic agent and can result in defects in the developing embryos after implantation. Therefore, the effects of 5-Aza-CdR on SCNT donor cells were need to be explored. We first investigated the effects of different concentrations of 5- Aza-CdR on the cell viability of FBFCs. The OD values indicated that 5-Aza-CdR reduced the viability of FBFCs in a dose- and time-dependent manner, suggesting that 5-Aza-CdR exerted potential harmful effects on somatic cells. This result may explain to some extent the inhibitory effect of 5-Aza-CdR against the developmental potential of preimplantation[16] or implanted embryos[17]. Meanwhile, different concen trations of 5-Aza-CdR induced dose-dependent apop tosis in the FBFCs, the levels of which were significantly higher than those of the control group, further suggesting that 5-Aza-CdR at relatively high concentrations exerted harmful effects on the treated cells. These inhibitory effects induced by 5-Aza-CdR may be due to DNA damage and/or gene mutations that activated the expression of apoptosis-associated genes and inhibited the viability and proliferation of the treated cells.
Somatic cells at the G0/G1 stage were preferentially used as donor cells to enhance the efficacy of SCNT. Serum starvation was used to synchronize the cell cycle of donor cells. However, this method resulted in DNA damage and apoptosis. In the present study, the demethylating reagent 5-Aza-CdR also affected the cell cycle distribution in a dose-dependent manner. These results were consistent with a previous study that 5-Aza CdR at the concentration of less than 0.05 μmol/L had no deleterious effect on the chromosomes[18]. However, a previous study suggested that the concentration of 5- Aza-CdR for treating bovine donor cells for SCNT was less than 0.01 μmol/L[19]. The difference in the concentrations of 5-Aza-CdR may be due to the differences in the cell types, cell passages or cell culture conditions. The influence mechanism of 5-Aza-CdR on the cell cycle distribution may be due to the increased expression of p21, which inhibits the activity of cyclin dependent kinases and cell proliferation[20].
Apoptosis plays an important role during development, homeostasis, and many diseases. In the present study, 5-Aza-CdR treatment resulted in apoptosis of FBFCs in a dose-dependent manner. These results are consistent with a previous study, which showed that 5-Aza-CdR treatment led to in creased sensitivity to apoptotic signals in melanoma[21]. Similarly, in cancer cells, apoptosis induced by 5- Aza-CdR may be due to the activation of cancer inhibitory genes[22]. 5-Aza-CdR treatment will induce apoptosis in cells and is cumulative over time[23]. This apoptosis may be due to DNA damage or the interaction with apoptosis-inducing cytokines, which in turn activate pro-apoptosis cytokines. These data suggest that 5-Aza-CdR is best used at a relatively low concentration and over a short time period.
As a demethylating reagent, 5-Aza-CdR can sig nificantly reduce the methylation level of the genome via interactions with the key enzymes associated with genomic methylation. A previous study demonstrated that 5-Aza-CdR could covalently bind with DNMT1. This binding subsequently induced the degradation of DNMT1[24]. The down- regulation of DNMT1 induced by 5-Aza-CdR was also confirmed by the present study. However, 5-Aza-CdR at a concentration of 0.01 μmol/L or lower failed to down-regulate the ex pression of DNMT1 mRNA in the FBFCs. 5- Aza CdR was used to treat donor cells/embryos before SCNT to improve the cloning efficacy by reducing the methylation level of the genome. However, high-dose of 5-Aza-CdR inhibited the developmental potential and implantation of embryos[25]. Therefore, the concen tration of 5-Aza-CdR used to improve SCNT efficacy should be optimized according to the experimental conditions, including the donor cell type, species, and enucleation methods, among others.
4 ConclusionsThe DNA demethylating reagent 5- Aza- CdR can reduce cell viability, inhibit cell proliferation, increase apoptosis, and affect the cell cycle distribution. 5-Aza-CdR treatment (> 0.5 μmol/L) can significantly down-regulate the expression of DNMT1 (P < 0.01), which is beneficial for improving SCNT efficacy. Due to the cumulative effect of 5- Aza-CdR, its appropriate concentration and time for the treatment of FBFCs are less than 0.1 μmol/L for 24 h. Further investigations are needed to improve the efficacy of SCNT.
| [1] |
WILMUT I, SCHNIEKE A E, MCWHIR J, et al. Viable offspring derived from fetal and adult mammalian cells.
Nature, 1997,385 (6619):810–813. DOI: 10.1038/385810a0. |
| [2] |
LORTHONGPANICH C, SOLTER D, LIM C Y. Nuclear reprogramming in zygotes.
International Journal of Developmental Biology, 2011,54 (11/12):1631–1640. |
| [3] |
HIRASAWA R, CHIBA H, KANEDA M, et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development.
Genes & Development, 2008,22 (12):1607–1616. |
| [4] |
HIENDLEDER S, MUND C, REICHENBACH H D, et al. Tissue-specific elevated genomic cytosine methylation levels are associated with an overgrowth phenotype of bovine fetuses derived by in vitro techniques.
Biology of Reproduction, 2004,71 (1):217–223. DOI: 10.1095/biolreprod.103.026062. |
| [5] |
SELOKAR N L, SAINI M, AGRAWAL H, et al. Downregulation of DNA methyltransferase 1 in zona-free cloned buffalo (Bubalus bubalis) embryos by small interfering RNA improves in vitro development but does not alter DNA methylation level.
Cellular Reprogramming, 2015,17 (2):89–94. DOI: 10.1089/cell.2014.0056. |
| [6] |
DIAO Y F, NARUSE K J, HAN R X, et al. Treatment of fetal fibroblasts with DNA methylation inhibitors and/or histone deacetylase inhibitors improves the development of porcine nuclear transfer-derived embryos.
Animal Repro duction Science, 2013,141 (3/4):164–171. |
| [7] |
SELOKAR N L, SAINI M, AGRAWAL H, et al. Buffalo (Bubalus bubalis) SCNT embryos produced from somatic cells isolated from frozen-thawed semen:Effect of trichostatin A on the in vitro and in vivo developmental potential, quality and epigenetic status.
Zygote, 2016,24 (4):549–553. DOI: 10.1017/S0967199415000520. |
| [8] |
YU D W, ZHANG S F, DU W H, et al. Expression of intracellular interferon-alpha confers antiviral properties in transfected bovine fetal fibroblasts and does not affect the full development of SCNT embryos.
PLoS One, 2014,9 (7):e94444. DOI: 10.1371/journal.pone.0094444. |
| [9] |
CUI Y, SU W Y, XING J, et al. MiR-29a inhibits cell proliferation and induces cell cycle arrest through the downregulation of p42.3 in human gastric cancer.
PLoS One, 2011,6 (10):e25872. DOI: 10.1371/journal.pone.0025872. |
| [10] |
LIU Y, DU F Y, CHEN W, et al. Knockdown of dual specificity phosphatase 4 enhances the chemosensitivity of MCF-7 and MCF-7/ADR breast cancer cells to doxorubicin.
Experimental Cell Research, 2013,319 (20):3140–3149. DOI: 10.1016/j.yexcr.2013.08.023. |
| [11] |
KAFI M A, KIM T H, AN J H, et al. Fabrication of cell chip for detection of cell cycle progression based on electrochemical method.
Analytical Chemistry, 2011,83 (6):2104–2111. DOI: 10.1021/ac102895b. |
| [12] |
SOURANI Z M, POURGHEYSARI B, BESHKAR P M, et al. Gallic acid inhibits proliferation and induces apoptosis in lymphoblastic leukemia cell line (C121).
Iranian Journal of Medical Sciences, 2016,41 (6):525–530. |
| [13] |
EL K A, PIRAS G, STEWART C L. DNA demethylation reactivates a subset of imprinted genes in uniparental mouse embryonic fibroblasts.
Journal of Biological Chemistry, 2001,276 (12):8674–8680. DOI: 10.1074/jbc.M009392200. |
| [14] |
DING X S, WANG Y, ZHANG D, et al. Increased pre implantation development of cloned bovine embryos treated with 5-aza-2'-deoxycytidine and trichostatin A.
Therio genology, 2008,70 (4):622–630. DOI: 10.1016/j.theriogenology.2008.04.042. |
| [15] |
ENRIGHT B P, KUBOTA C, YANG X, et al. Epigenetic characteristics and development of embryos cloned from donor cells treated by trichostatin A or 5-aza-2'-deoxycytidine.
Biology of Reproduction, 2003,69 (3):896–901. DOI: 10.1095/biolreprod.103.017954. |
| [16] |
YU J N, XUE C Y, WANG X G, et al. 5-AZA-2'- deoxycytidine (5-AZA-CdR) leads to down-regulation of Dnmt1o and gene expression in preimplantation mouse embryos.
Zygote, 2009,17 (2):137–145. DOI: 10.1017/S0967199408005169. |
| [17] |
BRANCH S, FRANCIS B M, BROWNIE C F, et al. Teratogenic effects of the demethylating agent 5-aza-2'- deoxycytidine in the Swiss Webster mouse.
Toxicology, 1996,112 (1):37–43. DOI: 10.1016/0300-483X(96)88183-2. |
| [18] |
李凤珍.
5-氮-2'-脱氧胞苷对牛成纤维细胞周期、染色体和凋亡的影响. 武汉: 华中农业大学 , 2009 : 33 -34.
LI F Z. Effects of 5-aza-2'-deoxycytidine on cell cycle distribution, chromosome and apoptosis in bovine fibroblasts. Wuhan: Huazhong Agricultural University , 2009 : 33 -34. (in Chinese with English abstract) |
| [19] |
ENRIGHT B P, SUNG L Y, CHANG C C, et al. Methylation and acetylation characteristics of cloned bovine embryos from donor cells treated with 5-aza-2'-deoxycytidine.
Biology of Reproduction, 2005,72 (4):944–948. DOI: 10.1095/biolreprod.104.033225. |
| [20] |
WANG H Y, ZHAO Y, LI L, et al. An ATM-and Rad3- related (ATR) signaling pathway and a phosphorylation acetylation cascade are involved in activation of p53/ p21Waf1/Cip1 in response to 5-aza-2'-deoxycytidine treatment.
Journal of Biological Chemistry, 2008,283 (5):2564–2574. DOI: 10.1074/jbc.M702454200. |
| [21] |
SOENGAS M S, CAPODIECI P, POLSKY D, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma.
Nature, 2001,409 (6817):207–211. DOI: 10.1038/35051606. |
| [22] |
CHRISTMAN J K. 5-Azacytidine and 5-aza-2'- deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy.
Oncogene, 2002,21 (35):5483–5495. DOI: 10.1038/sj.onc.1205699. |
| [23] |
LIU R, ZHANG X H, ZHANG K, et al. 5-Aza-2'- deoxycytidine inhibits retinoblastoma cell by reactivating epigenetically silenced RASSF1A gene.
International Journal of Ophthalmology (English Edition), 2014,7 (1):51–56. |
| [24] |
PATEL K, DICKSON J, DIN S, et al. Targeting of 5-aza-2'- deoxycytidine residues by chromatin-associated DNMT1 induces proteasomal degradation of the free enzyme.
Nucleic Acids Research, 2010,38 (13):4313–4324. DOI: 10.1093/nar/gkq187. |
| [25] |
DING Y B, LONG C L, LIU X Q, et al. 5-aza-2'- deoxycytidine leads to reduced embryo implantation and reduced expression of DNA methyltransferases and essential endometrial genes.
PLoS One, 2012,7 (9):e45364. DOI: 10.1371/journal.pone.0045364. |
 2018, Vol. 44
2018, Vol. 44


