| 葡萄糖和茉莉酸甲酯对芥蓝芽菜芥子油苷积累及其抗氧化特性的影响 |
2. 四川农业大学园艺学院,成都 611130
2. College of Horticulture, Sichuan Agricultural University, Chengdu 611130, China
芥蓝(Brassica oleracea var. alboglabra Bailey)是原产于中国的十字花科芸薹属甘蓝类蔬菜,主要分布在中国南部。芥蓝通常以肥嫩的花薹及嫩叶作为食用部分。如今,其芽菜由于具有高营养价值且富含生物活性物质而日渐成为餐桌新宠。据报道,芥蓝芽菜中芥子油苷的含量至少10倍于花薹和莲座叶[1]。研究证明,经常摄入芥子油苷有助于维持人体健康,降低患癌症的风险[2-4]。因此,探究有效调控芥蓝芽菜中芥子油苷合成积累的化学手段,对其品质改良具有重大意义。
芥子油苷是一类主要存在于十字花科植物中的次生代谢物质,其含量除了受遗传因素的影响外,还受外源信号的调节,如糖信号[5-6]和植物激素[7-10]。葡萄糖不仅是植物碳和能量的来源,也是一种重要的信号分子,在植物整个生命进程中发挥着调控作用[11]。前期研究表明,葡萄糖可以显著地增加拟南芥和芸薹属蔬菜中芥子油苷的含量[5-6, 12]。植物激素对芥子油苷合成积累的调控作用也已有报道。其中,茉莉酸甲酯通过MYC-MYB模型调控拟南芥中芥子油苷合成的机制已被阐明[13-14]。此外,GUO等[10]的研究发现,葡萄糖与茉莉酸甲酯在拟南芥的芥子油苷合成过程中发挥协同调控作用。然而,葡萄糖和茉莉酸甲酯在作物中的互作研究还鲜有报道。基于此,本文分析了芥蓝芽菜中葡萄糖和茉莉酸甲酯在芥子油苷合成积累过程中的作用,并探究了二者共同处理对芥蓝芽菜中维生素C、花青素和多酚含量,以及总抗氧化活性的影响,以期为运用低毒高效的化学调控手段改善芸薹属蔬菜中芥子油苷的组分和含量、提高营养品质提供理论依据和技术支撑。
1 材料与方法 1.1 试验材料与培养方法试验材料:所用芥蓝种子为商业种“正源大笋迟花”。
培养方法:芥蓝种子经0.7%乙醇灭菌30 min后,用无菌蒸馏水冲洗4~5次,在25 ℃培养箱中催芽24 h后,播种在铺有3层湿润无菌滤纸的培养皿中,于23 ℃培养室中进行光照培养。培养室光周期为16 h光照/8 h黑暗。
1.2 处理方法培养4 d后,在处理组培养皿中加入5 mL 0.03 g/mL葡萄糖溶液,或者5 mL 5 μmol/L茉莉酸甲酯溶液,或者5 mL含有5 μmol/L茉莉酸甲酯的0.03 g/mL葡萄糖溶液;在对照组培养皿中加入5 mL蒸馏水。处理3 d后取样,去除根部。样品用液氮速冻后,将用于检测维生素C含量的材料保存于-80 ℃超低温冰箱中;其余材料经真空冷冻干燥机(Vir Tis公司,美国)冷冻干燥72 h,粉碎成粉后置于-20 ℃储存,用于检测芥子油苷、花青素、总多酚的含量和总抗氧化能力。
1.3 测定方法和数据分析 1.3.1 芥子油苷的提取纯化和分析参照SUN等[8]的方法并加以改进。将30 mg冷冻干燥的样品置于离心管中,加入2 mL沸腾的ddH2O,100 ℃水浴10 min后,7 000 g离心5 min转移上清液至新的离心管中。重复提取1次。合并提取液并进行纯化后,使用高效液相色谱仪(Shimadzu公司,日本)检测芥子油苷含量。结果表示为每毫克鲜样中含有的芥子油苷纳摩尔数,nmol/mg。
1.3.2 花青素含量的测定取0.2 g芥蓝冻干粉,加入2 mL含1% HCl的甲醇,冰浴研磨,其余参照TENG等[15]的方法进行测定。
1.3.3 总多酚含量的测定参照AINSWORTH等[16]的方法,略加改进。将0.2 g芥蓝冻干粉加入5 mL 30%乙醇中,37 ℃水浴1 h,然后室温下7 000 r/min离心10 min,吸取上清液作为多酚提取液。采用Folin-Ciocalteau溶剂法测定多酚化合物的含量。以没食子酸为标样,结果表示为每克干物质中没食子酸的含量。
1.3.4 维生素C含量的测定参照YUAN等[17]的方法,并加以改进。取0.2 g冷冻的样品于研钵中,加入2 mL 1%草酸溶液于冰上研磨,残渣用1 mL 1%草酸溶液洗涤2次,合并滤液,7 000 r/min离心10 min,取上清液,过滤后使用高效液相色谱法(high performance liquid chromatography, HPLC)进行维生素C含量分析。
HPLC条件:C18色谱柱(5 μm, 250 mm×4.6 mm),柱温30 ℃,流动相为0.1%的草酸溶液,流速为1.0 mL/min,检测波长为243 nm。将不同浓度的维生素C标样进行HPLC分析,依所得结果绘制标准曲线,以外标法计算样品中维生素C的含量。
1.3.5 抗氧化能力的测定参照BENZIE等[18]的铁离子还原/抗氧化能力(ferric reducing antioxidant potential assay, FRAP)法,并加以改进。取0.2 g芥蓝芽菜冻干粉于2 mL离心管中,加入1 mL 30%乙醇和2粒钢珠,使用研磨仪在60 Hz下振荡研磨60 s后转移到10 mL离心管中,用30%乙醇定容至6 mL,80 ℃水浴1 h,然后7 500 r/min离心10 min,吸取上清液作为提取液。取提取液0.3 mL,加入预热的2.7 mL FRAP工作液,混匀,37 ℃水浴10 min后,在593 nm波长下测定吸光度。以硫酸亚铁溶液作为标样,样品的抗氧化活性(FRAP值)以达到同样吸光度所需的硫酸亚铁的微摩尔数表示。
1.3.6 数据统计分析数据分析所用软件为SPSS 11.5,采用单因素方差分析(one-way analysis of variance, ANOVA)和独立样本t检验对数据进行显著性分析,显著性水平为P<0.05。
2 结果与分析 2.1 芥蓝芽菜中芥子油苷的种类和含量从图 1可以看出,在芥蓝芽菜中共检测到11种芥子油苷。其中:7种为脂肪类芥子油苷,分别为3-甲基硫氧丙基芥子油苷(glucoiberin, GIB)、2-羟基-3-丁烯基芥子油苷(progoitrin, PRO)、烯丙基芥子油苷(sinigrin, SIN)、4-甲基硫氧丁基芥子油苷(glucoraphanin, GRA)、3-丁烯基芥子油苷(gluconapin, GNA)、4-甲基硫丁基芥子油苷(glucoerucin, GER)和2-羟基-4-戊烯基芥子油苷(gluconapoleiferin, GNL),分别占总脂肪类芥子油苷的2.7%、24.4%、14.3%、8.8%、37.9%、11.4%和0.4%(图 1A);4种为吲哚类芥子油苷,分别为吲哚- 3-甲基芥子油苷(glucobrassicin, GBS)、4-甲氧基-吲哚-3-甲基芥子油苷(4-methoxy glucobrassicin, 4-OMGBS)、4-羟基-吲哚-3-甲基芥子油苷(4- hydroxy glucobrassicin, 4-OHGBS)和1-甲氧基-吲哚- 3-甲基芥子油苷(1- methoxy glucobrassicin, NGBS),分别占总吲哚类芥子油苷的23.1%、49.2%、21.4%和6.2%(图 1B)。由图 1C可知,芥蓝芽菜中芥子油苷以脂肪类芥子油苷为主,约占总芥子油苷含量的95.5%。
 |
| GIB:3-甲基硫氧丙基芥子油苷;PRO:2-羟基-3-丁烯基芥子油苷;SIN:烯丙基芥子油苷;GRA:4-甲基硫氧丁基芥子油苷;GNA:3-丁烯基芥子油苷;GER:4-甲基硫丁基芥子油苷;GNL:2-羟基-4-戊烯基芥子油苷;GBS:吲哚-3-甲基芥子油苷;4-OMGBS:4-甲氧基-吲哚-3-甲基芥子油苷;4-OHGBS:4-羟基-吲哚-3-甲基芥子油苷;NGBS:1-甲氧基-吲哚-3-甲基芥子油苷;TAGS:总脂肪类芥子油苷;TIGS:总吲哚类芥子油苷。 图1 芥蓝芽菜中各种芥子油苷在总脂肪类芥子油苷(A)、总吲哚类芥子油苷(B)中所占比重及总脂肪类和总吲哚类芥子油苷在总芥子油苷中所占比重(C) Fig. 1 Percentage of individual glucosinolate in total aliphatic glucosinolates (A) or total indolic glucosinolates (B), and proportion of total aliphatic glucosinolates and total indolic glucosinolates in total glucosinolates (C) |
如图 2所示,茉莉酸甲酯单独处理可以显著提高芥蓝芽菜中除GIB和GRA以外的所有种类脂肪类芥子油苷的含量,而0.03 g/mL葡萄糖单独处理对脂肪类芥子油苷的积累没有显著影响。然而,当共同施加葡萄糖和茉莉酸甲酯时,芥蓝芽菜中的GIB和GRA含量得到了显著的提升,与对照相比分别增加了63%和52%(图 2A、D)。此外,葡萄糖可以进一步促进茉莉酸甲酯诱导的GNA的积累(图 2E)。
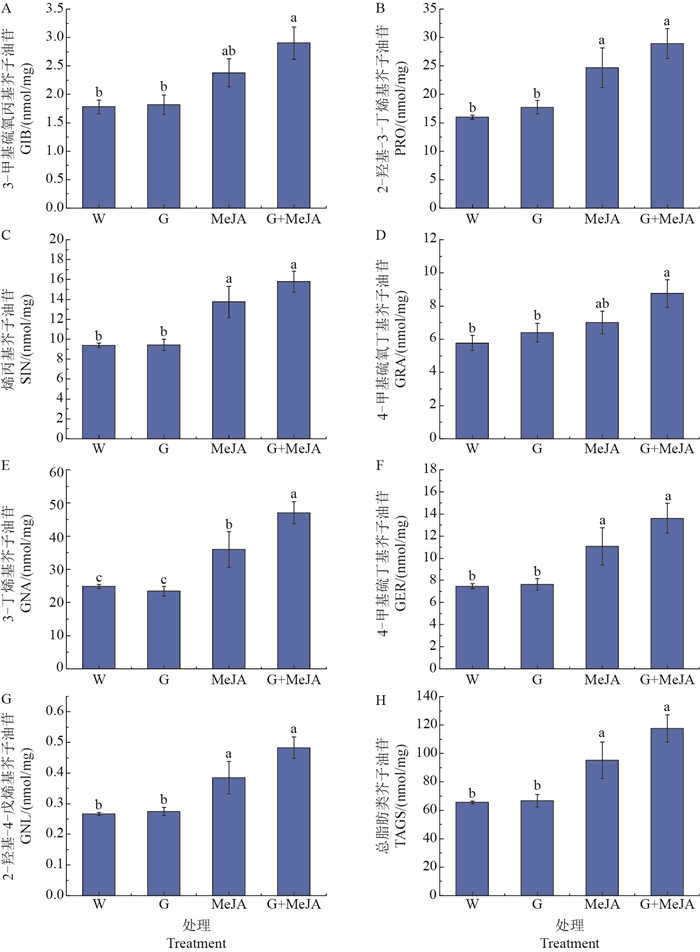 |
| W:蒸馏水;G:0.03 g/mL葡萄糖;MeJA:5 μmol/L茉莉酸甲酯。每个数据代表 3个独立生物学重复的平均值(平均值±标准误);短栅上的不同小写字母表示不同处理之间在P<0.05水平差异有统计学意义。 W: Distilled water; G: 0.03 g/mL glucose; MeJA: 5 μmol/L methyl jasmonate. Each data point represents the mean of three independent biological replicates per treatment (mean±standard error); different lowercase letters above bars indicate significant difference among different treatments at the 0.05 probability level. 图2 葡萄糖和茉莉酸甲酯(MeJA)单独或者共同处理对脂肪类芥子油苷积累的影响 Fig. 2 Effect of glucose or/and methyl jasmonate (MeJA) on accumulation of aliphatic glucosinolates |
葡萄糖单独处理可以显著促进芥蓝芽菜中GBS的合成(图 3A);茉莉酸甲酯单独处理可以显著增加所有吲哚类芥子油苷的积累(图 3A~D);葡萄糖的添加可增强茉莉酸甲酯对4-OMGBS合成的促进作用,与茉莉酸甲酯单独处理时4-OMGBS的积累量相比达到显著水平,比对照提高了50%(图 3B)。
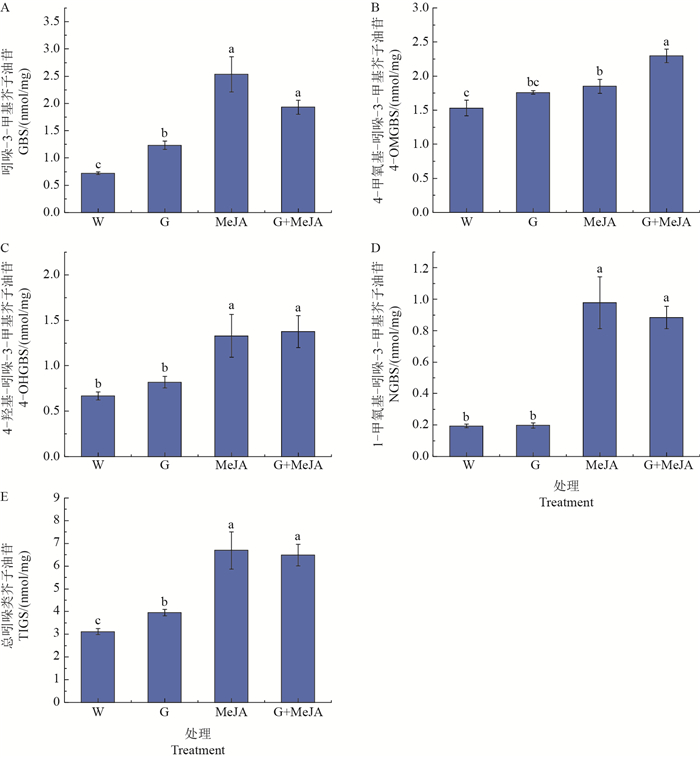 |
| W:蒸馏水;G:0.03 g/mL葡萄糖;MeJA:5 μmol/L茉莉酸甲酯。每个数据代表 3个独立生物学重复的平均值(平均值±标准误);短栅上的不同小写字母表示不同处理之间在P<0.05水平差异有统计学意义。 W: Distilled water; G: 0.03 g/mL glucose; MeJA: 5 μmol/L methyl jasmonate. Each data point represents the mean of three independent biological replicates per treatment (mean ± standard error); different lowercase letters above bars indicate significant difference among different treatments at the 0.05 probability level. 图3 葡萄糖和茉莉酸甲酯单独或者共同处理对吲哚类芥子油苷积累的影响 Fig. 3 Effect of glucose or/and MeJA on accumulation of indolic glucosinolates |
由图 4可知,与对照相比,茉莉酸甲酯单独处理可以显著促进芥蓝芽菜中总芥子油苷的积累,葡萄糖可以在一定程度上增强茉莉酸甲酯对总芥子油苷合成的促进作用。
 |
| W:蒸馏水;G:0.03 g/mL葡萄糖;MeJA:5 μmol/L茉莉酸甲酯。每个数据代表 3个独立生物学重复的平均值(平均值±标准误);短栅上的不同小写字母表示不同处理之间在P<0.05水平差异有统计学意义。 W: Distilled water; G: 0.03 g/mL glucose; MeJA: 5 μmol/L methyl jasmonate. Each data point represents the mean of three independent biological replicates per treatment (mean ± standard error); different lowercase letters above bars indicate significant difference among different treatments at the 0.05 probability level. 图4 葡萄糖和茉莉酸甲酯单独或者共同处理对总芥子油苷积累的影响 Fig. 4 Effect of glucose or/and MeJA on accumulation of total glucosinolates |
由表 1可知:葡萄糖和茉莉酸甲酯共同处理对芥蓝芽菜中花青素的积累和总抗氧化活性有一定的促进作用,其中对花青素含量的提升作用达到显著水平;但是,二者共同处理对维生素C和总多酚含量几乎没有影响。
| 表1 葡萄糖和茉莉酸甲酯共同处理对芥蓝芽菜中维生素C、花青素、总多酚含量和抗氧化活性的影响 Table 1 Effect of glucose and MeJA on contents of vitamin C, anthocyanins, total phenols, and antioxidant activity of Chinese kale sprouts |
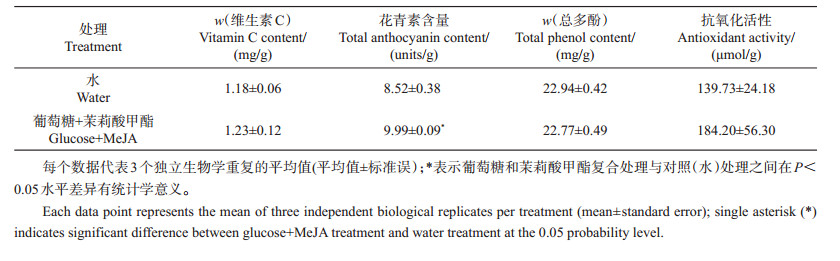 |
| 点击放大 |
本研究采用浓度较低的葡萄糖溶液(0.03 g/mL)和植物激素(5 μmol/L茉莉酸甲酯)进行外源复合处理,既经济又安全。作为一种营养价值很高的中国特产蔬菜,芥蓝具有很大的消费群体,其价格低廉的种子使得商业化生产芽菜成为可能。本文以芥蓝芽菜为材料,探究了有效改良芸薹属蔬菜中芥子油苷组分和含量、提高其抗氧化活性的化学调控手段。
前期研究表明,葡萄糖可作为信号分子有效促进芥子油苷的合成积累[5-6]。WEI等[12]的研究表明,5%葡萄糖可以显著提升芥蓝芽菜中脂肪类和吲哚类芥子油苷的积累。在本研究中,0.03 g/mL葡萄糖处理仅对吲哚类芥子油苷的合成有显著促进作用,这可能与使用的葡萄糖浓度不同有关,也说明芥蓝芽菜中吲哚类芥子油苷的合成对葡萄糖的响应更敏感。迄今为止,已知多种植物激素,如生长素、脱落酸、茉莉酸、油菜素内酯、水杨酸和乙烯等参与了芥子油苷的代谢调控[17, 19-20]。其中,SCHWEIZER等[13]已阐明了茉莉酸对拟南芥芥子油苷生物合成的调控机制。MOREIRA-RODRÍGUEZ等[21]的研究发现,茉莉酸可有效促进青花菜芽菜中吲哚类芥子油苷的积累。本研究表明,茉莉酸甲酯处理可以显著提高芥蓝芽菜中绝大多数种类的脂肪类和吲哚类芥子油苷的积累;当与葡萄糖共同处理时,茉莉酸甲酯对4-OMGBS的促进作用得到了进一步的增强,比对照增加了50%。据报道,在人体摄食过程中,4-OMGBS的降解产物——4-甲氧基吲哚-3-甲醇可抑制人结肠癌细胞的增殖,在十字花科蔬菜预防癌症功能中具有重要作用[22]。此外,本研究还发现,茉莉酸甲酯单独处理可以促进大多数脂肪类芥子油苷的合成,唯独不能提升GIB和GRA的积累,而这2种芥子油苷的降解产物——烷基异硫代氰酸盐具有较强的抗癌活性,可以抑制癌细胞的增殖和促进组氨酸乙酰化[23-24]。并且,GRA的降解产物萝卜硫素被认为是最具潜力的天然抗癌化合物[25-26],可以有效抑制致癌代谢中的阶段Ⅰ酶活性,并诱导具有解毒作用的阶段Ⅱ酶活性,从而阻遏癌症的发生[27-29],同时还能诱导癌症细胞的程序化死亡[30-33]。然而,茉莉酸甲酯与葡萄糖共同处理使这2种芥子油苷的含量得到了显著增高,与对照相比分别提升了63%和52%。
维生素C和多酚类化合物是十字花科植物中含量高,且具有抗氧化能力的重要生物活性物质。临床试验表明,食用含有此类物质的食物有助于延缓衰老,降低多种疾病发生的风险[34]。因此,本研究探究了葡萄糖和茉莉酸甲酯复合处理对于芥蓝芽菜中此类抗氧化物含量的影响。我们发现,每百克芥蓝芽菜中维生素C高达100 mg,再次证明芥蓝芽菜是抗氧化物质维生素C的重要来源[1, 12]。有研究表明,在拟南芥和烟草Bright Yellow-2(BY-2)悬浮细胞中,茉莉酸可以促进维生素C的从头合成[35]。但本研究表明,葡萄糖与茉莉酸甲酯复合处理没有显著促进芥蓝芽菜维生素C的积累,可能是由于葡萄糖对维生素C积累的抑制作用抵消了茉莉酸甲酯对它的促进效果[12]。花青素是一类重要的天然色素,属于多酚类化合物。前人的研究表明,糖类可以促进青花菜中花青素的合成[36]。FLORES等[37]的研究显示,茉莉酸可以促进黑加仑中花青素的积累。此外,LORETI等[38]阐明了蔗糖和茉莉酸对花青素合成的协同调控作用。本研究发现,葡萄糖和茉莉酸共同处理也同样可以显著提升芥蓝芽菜中花青素的含量,然而,二者复合处理对总多酚的含量没有显著影响。WEI等[12]的研究表明,葡萄糖可能通过调控多酚类化合物合成过程中的关键酶——苯丙氨酸解氨酶的活性来影响十字花科蔬菜中多酚物质的合成,但是关于茉莉酸对多酚化合物的调控作用则存在不同的见解。有报道指出,茉莉酸对青花菜芽菜中的多酚类化合物具有负向调控作用[21, 39];而KIM等[40]在萝卜芽菜中的研究则证明了茉莉酸可以促进多酚类化合物的合成。这其中的具体作用机制还有待阐明。此外,葡萄糖和茉莉酸复合处理对芥蓝芽菜的总抗氧化能力没有显著影响,再次印证了关于酚类化合物的含量和抗氧化活性之间具有高度相关性的报道[40]。
以上分析表明,0.03 g/mL葡萄糖与5 μmol/L茉莉酸甲酯共同处理可以增加芥蓝芽菜中芥子油苷的积累,并且可以显著提高花青素的含量,因此,这不失为一种有效改善芥蓝芽菜品质的化学调控手段。
| [1] |
SUN B, LIU N, ZHAO Y T, et al. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chemistry, 2011, 124(3): 941-947. DOI:10.1016/j.foodchem.2010.07.031 |
| [2] |
MAZUMDER A, DWIVEDI A, DU PLESSIS J. Sinigrin and its therapeutic benefits. Molecules, 2016, 21(4): 416. DOI:10.3390/molecules21040416 |
| [3] |
CARTEA M E, VELASCO P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochemistry Reviews, 2008, 7(2): 213-229. DOI:10.1007/s11101-007-9072-2 |
| [4] |
DINKOVA-KOSTOVA A T, KOSTOV R V. Glucosinolates and isothiocyanates in health and disease. Trends in Molecular Medicine, 2012, 18(6): 337-347. DOI:10.1016/j.molmed.2012.04.003 |
| [5] |
MIAO H Y, WEI J, ZHAO Y T, et al. Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. Journal of Experimental Botany, 2013, 64(4): 1097-1109. DOI:10.1093/jxb/ers399 |
| [6] |
MIAO H Y, CAI C X, WEI J, et al. Glucose enhances indolic glucosinolate biosynthesis without reducing primary sulfur assimilation. Scientific Reports, 2016, 6: 31854. DOI:10.1038/srep31854 |
| [7] |
ZANG Y X, GE J L, HUANG L H, et al. Leaf and root glucosinolate profiles of Chinese cabbage (Brassica rapa ssp. pekinensis) as a systemic response to methyl jasmonate and salicylic acid elicitation. Journal of Zhejiang University: SCIENCE B, 2015, 16(8): 696-708. DOI:10.1631/jzus.B1400370 |
| [8] |
SUN B, YAN H Z, ZHANG F, et al. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of Chinese kale. Food Research International, 2012, 48(2): 359-366. DOI:10.1016/j.foodres.2012.04.021 |
| [9] |
GUO R F, QIAN H M, SHEN W S, et al. BZR1 and BES1 participate in regulation of glucosinolate biosynthesis by brassinosteroids in Arabidopsis. Journal of Experimental Botany, 2013, 64(8): 2401-2412. DOI:10.1093/jxb/ert094 |
| [10] |
GUO R F, SHEN W S, QIAN H M, et al. Jasmonic acid and glucose synergistically modulate the accumulation of glucosinolates in Arabidopsis thaliana. Journal of Experimental Botany, 2013, 64(18): 5707-5719. DOI:10.1093/jxb/ert348 |
| [11] |
SHEEN J. Master regulators in plant glucose signaling networks. Journal of Plant Biology, 2014, 57(2): 67-79. DOI:10.1007/s12374-014-0902-7 |
| [12] |
WEI J, MIAO H Y, WANG Q M. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Scientia Horticulturae, 2011, 129(4): 535-540. DOI:10.1016/j.scienta.2011.04.026 |
| [13] |
SCHWEIZER F, FERNÁNDEZ-CALVO P, ZANDER M, et al. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. The Plant Cell, 2013, 25(8): 3117-3132. DOI:10.1105/tpc.113.115139 |
| [14] |
FRERIGMANN H, BERGER B, GIGOLASHVILI T. bHLH05 is an interaction partner of MYB51 and a novel regulator of glucosinolate biosynthesis in Arabidopsis. Plant Physiology, 2014, 166(1): 349-369. DOI:10.1104/pp.114.240887 |
| [15] |
TENG S, KEURENTJES J, BENTSINK L, et al. Sucrosespecific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiology, 2005, 139(4): 1840-1852. DOI:10.1104/pp.105.066688 |
| [16] |
AINSWORTH E A, GILLESPIE K M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols, 2007, 2(4): 875-877. DOI:10.1038/nprot.2007.102 |
| [17] |
YUAN G F, SUN B, YUAN J, et al. Effect of 1- methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chemistry, 2010, 118(3): 774-781. DOI:10.1016/j.foodchem.2009.05.062 |
| [18] |
BENZIE I F F, STRAIN J J. The ferric reducing ability of plasma (FRAP) as a measure of"antioxidant power": The FRAP assay. Analytical Biochemistry, 1996, 239(1): 70-76. DOI:10.1006/abio.1996.0292 |
| [19] |
KIM H H, BAE H, KIM S, et al. Influence of auxins on glucosinolate biosynthesis in hairy root cultures of broccoli (Brassica oleracea var. italica). Asian Journal of Chemistry, 2013, 25(11): 6099-6101. |
| [20] |
FRERIGMANN H, GIGOLASHVILI T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Molecular Plant, 2014, 7(5): 814-828. DOI:10.1093/mp/ssu004 |
| [21] |
MOREIRA-RODRÍGUEZ M, NAIR V, BENAVIDES J, et al. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. International Journal of Molecular Sciences, 2017, 18(11): 2330. DOI:10.3390/ijms18112330 |
| [22] |
KRONBAK R, DUUS F, VANG O. Effect of 4-methoxyindole- 3-carbinol on the proliferation of colon cancer cells in vitro, when treated alone or in combination with indole-3-carbinol. Journal of Agricultural and Food Chemistry, 2010, 58(14): 8453-8459. DOI:10.1021/jf101806t |
| [23] |
SMITH T K, LUND E K, PARKER M L, et al. Allylisothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis, 2004, 25(8): 1409-1415. DOI:10.1093/carcin/bgh149 |
| [24] |
LEA M A, RANDOLPH V M, LEE J E, et al. Induction of histone acetylation in mouse erythroleukemia cells by some organosulfur compounds including allyl isothiocyanate. International Journal of Cancer, 2001, 92(6): 784-789. DOI:10.1002/(ISSN)1097-0215 |
| [25] |
SHARMA R, SHARMA A, CHAUDHARY P, et al. Role of lipid peroxidation in cellular responses to D, L-sulforaphane, a promising cancer chemopreventive agent. Biochemistry, 2010, 49(14): 3191-3202. DOI:10.1021/bi100104e |
| [26] |
ZHANG Y, TALALAY P, CHO C G, et al. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proceedings of the National Academy of Sciences of the USA, 1992, 89(6): 2399-2403. DOI:10.1073/pnas.89.6.2399 |
| [27] |
CONAWAY C C, YANG Y M, CHUNG F L. Isothiocyanates as cancer chemopreventive agents: Their biological activities and metabolism in rodents and humans. Current Drug Metabolism, 2002, 3(3): 233-255. DOI:10.2174/1389200023337496 |
| [28] |
DINKOVA-KOSTOVA A T, FAHEY J W, WADE K L, et al. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiology, Biomarkers & Prevention, 2007, 16(4): 847-851. |
| [29] |
HECHT S S. Inhibition of carcinogenesis by isothiocyanates. Drug Metabolism Reviews, 2000, 32(3/4): 395-411. |
| [30] |
GAMET-PAYRASTRE L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Current Cancer Drug Targets, 2006, 6(2): 135-145. DOI:10.2174/156800906776056509 |
| [31] |
HERMAN-ANTOSIEWICZ A, XIAO H, LEW K L, et al. Induction of p21 protein protects against sulforaphaneinduced mitotic arrest in LNCaP human prostate cancer cell line. Molecular Cancer Therapeutics, 2007, 6(5): 1673-1681. DOI:10.1158/1535-7163.MCT-06-0807 |
| [32] |
PARNAUD G, LI P F, CASSAR G, et al. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutrition and Cancer, 2004, 48(2): 198-206. DOI:10.1207/s15327914nc4802_10 |
| [33] |
SINGH S V, HERMAN-ANTOSIEWICZ A, SINGH A V, et al. Sulforaphane-induced G(2)/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. Journal of Biological Chemistry, 2004, 279(24): 25813-25822. DOI:10.1074/jbc.M313538200 |
| [34] |
CASTAÑEDA-OVANDO A, DE LOURDES PACHECOHERNÁNDEZ M, PÁEZ-HERNÁNDEZ M E, et al. Chemical studies of anthocyanins: A review. Food Chemistry, 2009, 113(4): 859-871. DOI:10.1016/j.foodchem.2008.09.001 |
| [35] |
WOLUCKA B A, GOOSSENS A, INZÉ D. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. Journal of Experimental Botany, 2005, 56(419): 2527-2538. DOI:10.1093/jxb/eri246 |
| [36] |
GUO R F, YUAN G F, WANG Q M. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chemistry, 2011, 129(3): 1080-1087. DOI:10.1016/j.foodchem.2011.05.078 |
| [37] |
FLORES G, RUIZ DEL CASTILLO M L. Accumulation of anthocyanins and flavonols in black currants (Ribes nigrum L.) by pre-harvest methyl jasmonate treatments. Journal of the Science of Food and Agriculture, 2016, 96(12): 4026-4031. DOI:10.1002/jsfa.2016.96.issue-12 |
| [38] |
LORETI E, POVERO G, NOVI G, et al. Gibberellins, jasmonate and abscisic acid modulate the sucrose- induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytologist, 2008, 179(4): 1004-1016. DOI:10.1111/nph.2008.179.issue-4 |
| [39] |
BARRIENTOS CARVACHO H, PÉREZ C, ZÚÑIGA G, et al. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. Journal of the Science of Food and Agriculture, 2014, 94(12): 2555-2561. DOI:10.1002/jsfa.2014.94.issue-12 |
| [40] |
KIM H J, CHEN F, WANG X, et al. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). Journal of Agricultural and Food Chemistry, 2006, 54(19): 7263-7269. DOI:10.1021/jf060568c |
 2018, Vol. 44
2018, Vol. 44


