| 2株乳酸菌的生长和潜在益生菌特性 |
2. 浙江大学生物系统工程与食品科学学院,杭州 310058
2. College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou 310058, China
益生菌是指当摄入一定数量后,能以活菌状态到达胃肠道的单一或特定微生物的混合物。作为促进机体健康的有益菌[1],益生菌具有增强人体免疫力、调节肠道健康、促进营养物质消化吸收、降低胆固醇、缓解肥胖、抑制癌细胞生长、减肥等功能[2-6]。为了发挥其在人体中的有益作用,益生菌必需可以耐受胃中的酸性环境,抵抗小肠中存在的胆盐和胰酶,从而顺利到达人体肠道,黏附并定殖在肠道内,进而发挥其益生作用[7-8]。目前,一些应用于益生菌产品的菌株由于较弱的耐酸、耐胆盐能力,从而影响了其在机体肠道内发挥益生作用[9]。
本实验室(农业部水产品贮藏保鲜质量安全风险评估重点实验室)从传统发酵食品——东北泡菜和臭豆子中分离出优势菌株,并通过16s RNA测序鉴定,分别为植物乳杆菌D28(Lactobacillus plantarum D28)和粪肠球菌B21(Enterococcus faecium B21)。为了更好地开发和利用这2个菌株,我们对其生长特性、部分益生特性(耐酸、耐NaCl和耐胆盐特性,耐受人工胃液和人工肠液特性,体外抗氧化和胆盐水解酶活性)进行了初步探索。
1 材料与方法 1.1 材料 1.1.1 菌株植物乳杆菌D28(L. plantarum D28)分离自东北泡菜,粪肠球菌B21(E. faecium B21)分离自臭豆子。在MRS(de Man-Rogosa-Sharpe)固体培养基上生长48 h的菌落形态如图 1所示:L. plantarum D28菌落凸起,呈圆形,表面光滑,细密,呈黄色;E. faecium B21菌落凸起,呈圆形,表面光滑,细密,色白。革兰氏染色结果均为阳性。
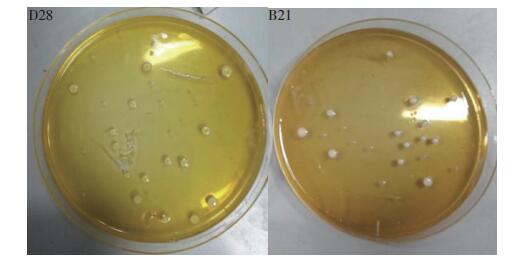 |
| D28: L. plantarum D28;B21: E. faecium B21. 图1 植物乳杆菌D28和粪肠球菌B21在MRS培养基上的菌落形态 Fig. 1 Colony morphology of L. plantarum D28 and E. faecium B21 on MRS agar plate |
MRS固体和液体培养基(北京陆桥技术有限公司);胃蛋白酶(活性为1:3 000)、胰酶(1:250)(上海阿拉丁生化科技股份有限公司);牛胆盐和琼脂(国药集团化学试剂有限公司);氯化钠和溴甲酚紫(上海生工生物工程股份有限公司);DPPH(2, 2⁃ diphenyl⁃1⁃picrylhydrazyl,二苯基苦基苯肼)(Sigma公司,美国)。
1.1.3 仪器与设备多功能酶标仪(BioTek公司,美国);TGL-16C型高速离心机(上海安亭科学仪器厂);PL2002电子天平(Mettler-Toledo公司,瑞士);PHX-DHS-X型隔水式电热恒温培养箱(上海一恒科技有限公司);pHS-3C pH仪(上海雷磁仪器厂);LDZX-30FA型立式压力蒸汽式灭菌锅(上海申安医疗器械厂);Bioruptor® UCD200超声波破碎仪(Diagenode公司,比利时)。
1.2 菌株培养将待测试的乳酸菌菌株以1%的接种量接入MRS液体培养基中,置于37 ℃厌氧培养箱中培养至对数期,连续培养2代,得到新鲜培养物,备用。
1.3 生长曲线测定将经过2次传代培养18 h至对数期的新鲜培养物按0.1%(体积分数)接种量接入MRS培养基中,每隔2 h取样测定D600 nm值;3次重复。
1.4 起始pH值对生长的影响将经过2次传代培养的新鲜乳酸菌培养物在6 000 r/min下离心10 min,收集菌体,用无菌磷酸盐缓冲溶液(phosphate-buffered saline,PBS)(pH 7.4)离心洗涤3次(避免带入接种物中的酸性物质改变pH),然后按照接种率1%(体积分数)接种到不同起始pH值的MRS液体培养基中,在37 ℃下静置培养18 h,测定培养物在600 nm波长处的吸光度值(D600 nm),以判断起始pH值对生长的影响;3次重复。
1.5 耐受性测定将经过2次传代培养至对数期的新鲜乳酸菌培养物在4 000 r/min下离心10 min,收集菌体,用无菌PBS(pH 7.4)离心洗涤3次后,用等量无菌PBS悬浮,备用。
1.5.1 对NaCl和胆盐的耐受性按体积分数1%的接种量分别将菌株接入到含有不同浓度NaCl或胆盐的MRS液体培养基(添加溴甲酚紫)中,在37 ℃下静置培养18 h,观察培养物的产酸变色情况,从而判断测试菌株对NaCl和胆盐的耐受性;8次重复。
1.5.2 对人工胃液的耐受性参照CHARTERIS等[9]的方法,将0.2 mL细胞悬液(1×108 CFU/mL)接种于膜过滤除菌(0.22 μm)的1.0 mL人工胃液(pH 2.0)和0.3 mL NaCl中,充分混匀,并在37 ℃恒温水浴槽中处理,分别于人工胃液处理1、90和180 min后取样,梯度稀释,以标准平板法计数(稀释倍数为1×10-10);3次重复。
1.5.3 对人工肠液的耐受性参照CHARTERIS等[9]的方法,将0.2 mL细胞悬液(1×108 CFU/mL)接种于膜过滤除菌(0.22 μm)的人工小肠液(pH 8.0)和0.3 mL NaCl中,充分混匀,并在37 ℃恒温水浴槽中处理,分别于人工小肠液处理1和240 min后取样,梯度稀释,以标准平板法计数(稀释倍数为1×10-10);3次重复。
1.6 清除DPPH自由基的能力测定参考LIN等[10]的方法,略微改动。将保存的乳酸菌菌株在MRS培养基中2次传代培养活化,然后按1%(体积分数)接种于MRS肉汤培养基中,在37 ℃下培养16~18 h,得到乳酸菌培养物;取1 mL细胞培养物于6 000 r/min、4 ℃下离心10 min,收集菌体细胞沉淀,并用PBS(pH 7.4)洗涤2次,并重新悬浮调整细胞终浓度为1×109 CFU/mL(D600 nm=1.1),即为乳酸菌的完整细胞(intact cells, IC)。将获得的完整细胞用超声波破碎仪破碎细胞(在功率300 W条件下,冰浴超声处理5 s,间隔5 s,持续时间10 min),然后以4 ℃、8 000 r/min离心10 min,弃沉淀得到的上清液即为乳酸菌的无细胞提取物(intracellular cell-free extracts, CFE)。
参照LIN等[11]的方法,略微改动。分别取800 μL样品与1 mL新配制的DPPH溶液(含0.2 mmol/L无水乙醇)于2 mL棕色离心管中混合均匀,置于室温下避光反应30 min,然后将反应物以6 000 r/min离心10 min,取上清液,测定样品在波长517 nm处的吸光度值(As),对照组以等体积蒸馏水代替样品,其吸光度值记为Ab。对DPPH自由基清除率的计算公式如下:DPPH清除率=(Ab-As)/Ab×100%。
1.7 胆盐水解酶活性测定乳酸菌菌株无细胞提取物(CFE)的胆盐水解酶(bile salt hydrolase, BSH)活性可以通过测定胆盐酰胺键水解产生的氨基酸的量来确定[12-13]。参考LIONG等[12]的方法,稍做修改,用于测定菌体胞内BSH活性。步骤如下:将过夜培养的细胞离心分离(4 ℃,8 000 r/min,10 min),用0.1 mol/L PBS(pH 7.0)洗涤2次,然后再悬浮于PBS(pH 7.0)中,以调整菌体的D600 nm为3~5;加入二硫苏糖醇10 mmol/L用以减少酶的氧化,随后用超声波破碎仪破碎细胞,功率调整到H位置,时间10 min,超声处理为5 s的开/关(on/ off)循环交替,将样品置于水冷却器内保持在4 ℃下超声处理,在4 ℃、8 000 r/min下离心10 min后,弃沉淀得到的上清液即为CFE,储存在-20 ℃冰箱中。取10 μL CFE样品,加入180 μL PBS(0.1 mol/L,pH 6.0)、10 μL牛磺酸胆盐(10 mmol/L)和10 μL液体石蜡,混匀后置于37 ℃孵育30 min,然后立即加入200 μL质量体积分数为15%的三氯乙酸终止反应(以反应后加入牛磺酸胆盐的样品作为对照组),混合均匀,离心10 min(离心机最大转速,4 ℃),得到上清液;取100 μL上清液(样品/对照)与1.9 mL茚三酮试剂[0.5 mL茚三酮按1%(质量体积分数)的比例溶解于pH 5.5的0.5 mol/L柠檬酸钠缓冲液中]充分混合,并与1.2 mL甘油和0.2 mL 0.5 mol/L柠檬酸钠缓冲液(pH 5.5)混合,振荡混匀,沸水浴14 min,然后在自来水中冷却3 min,测定在570 nm处的吸光度值。标准曲线用甘氨酸制备。BSH的酶活单位表示在单位时间内、单位质量总蛋白质中的粗酶使牛磺胆酸水解产生氨基酸的物质的量。蛋白质浓度用Bradford蛋白测定试剂盒(上海生工生物工程股份有限公司)测定,并用牛血清白蛋白制作标准曲线。
1.8 数据分析采用SPSS 20.0统计软件对试验所得数据进行统计分析,结果用平均值±标准差表示,并用GraphPad Prism 5.0软件作图。
2 结果与分析 2.1 菌株生长曲线测定结果从图 2可以看出:L. plantarum D28在培养最初的4 h内生长缓慢,培养6 h后进入对数生长期,培养18 h时D600 nm达到最大,为1.591,随后进入稳定期;E. faecium B21在培养最初的6 h内生长缓慢,培养8 h后进入对数生长期,在培养16 h时D600 nm达到最大,为0.872,随后进入稳定期;同时,在相同培养条件下,L. plantarum D28的生长速度显著高于E. faecium B21。
 |
| 图2 植物乳杆菌D28和粪肠球菌B21在液体MRS培养基中的生长曲线 Fig. 2 Growth curve of L. plantarum D28 and E. faecium B21 in MRS medium |
分别将L. plantarum D28和E. faecium B21接种到不同起始pH(2.0~7.0)的MRS液体培养基中,结果(图 3)表明:L. plantarum D28的最适pH为5.5~ 7.0,E. faecium B21的最适pH为6.0~7.0;菌株在低pH值环境下生长受到抑制,但仍有一定的存活量,且在pH 4.0~5.5时,L. plantarum D28的生长明显优于E. faecium B21。
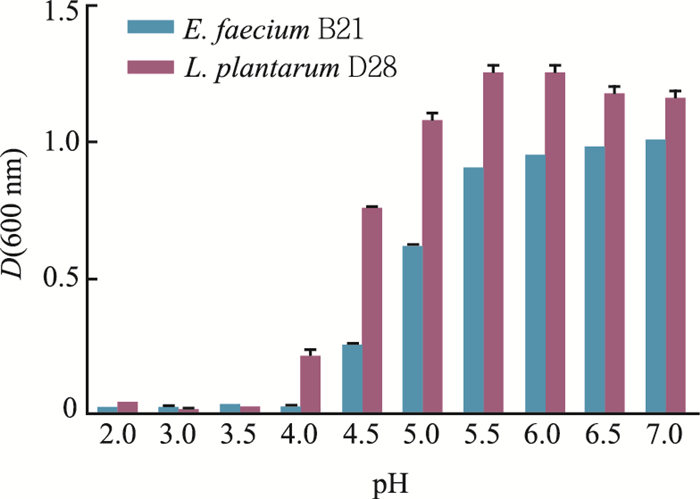 |
| 图3 不同起始pH值对植物乳杆菌D28和粪肠球菌B21生长的影响 Fig. 3 Effects of different initial pH on the growth of L. plantarum D28 and E. faecium B21 |
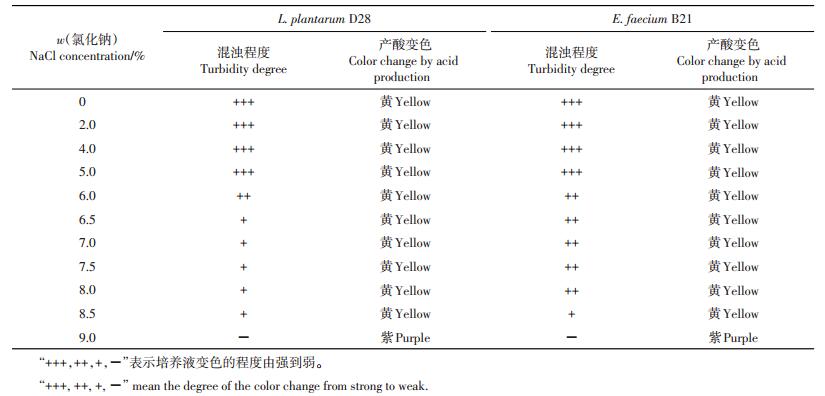
由表 1可知:L. plantarum D28和E. faecium B21在含0%~8.5% NaCl的MRS培养基中均可生长,但随着NaCl含量的升高,2株菌的生长逐渐受到抑制;2株菌均可以耐受5%及以下的NaCl,而菌株B21在6.5%~8% NaCl中的耐受性高于D28。
| 表1 植物乳杆菌D28和粪肠球菌B21对不同质量分数NaCl的耐受性 Table 1 Tolerance of L. plantarum D28 and E. faecium B21 against NaCl |
 |
| 点击放大 |
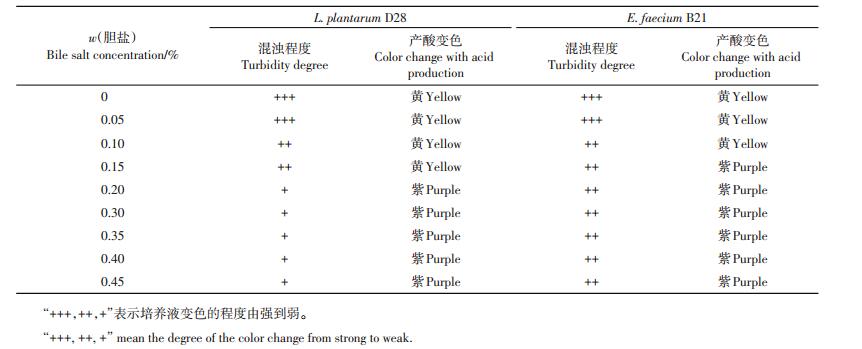
从表 2可知:L. plantarum D28和E. faecium B21在含牛胆盐0%~0.45%的MRS培养基中均可以生长;随着胆盐含量的升高,L. plantarum D28和E. faecium B21的生长逐渐受到抑制,且菌株D28在高含量胆盐环境中的耐受性明显低于B21。
| 表2 植物乳杆菌D28和粪肠球菌B21对不同质量分数胆盐的耐受性 Table 2 Tolerance of L. plantarum D28 and E. faecium B21 against bile salt |
 |
| 点击放大 |
由表 3可知:与对照相比,在人工胃液(pH 2.0)处理开始阶段,L. plantarum D28和E. faecium B21的数量没有明显差别;当人工胃液处理90 min后,L. plantarum D28和E. faecium B21的存活率降低,分别为176.00 lg (CFU/mL) 和102.33 lg (CFU/mL);而当处理180 min后,L. plantarum D28和E. faecium B21的存活率锐减,但菌株D28的存活量高于B21,分别为41.33 lg (CFU/mL) 和8.33 lg (CFU/mL)。
| 表3 植物乳杆菌D28和粪肠球菌B21对人工胃液的耐受能力 Table 3 Tolerance of L. plantarum D28 and E. faecium B21 against artificial gastric juice |
 |
| 点击放大 |
由表 4可知:在人工肠液(pH 8.0)处理开始阶段,L. plantarum D28和E. faecium B21的数量没有明显差别;当人工肠液处理240 min后,L. plantarum D28和E. faecium B21的存活量降低,分别为36.33 lg (CFU/mL) 和18.67 lg (CFU/mL),且前者对人工肠液的耐受性高于后者。
| 表4 植物乳杆菌D28和粪肠球菌B21对人工肠液的耐受能力 Table 4 Tolerance of L. plantarum D28 and E. faecium B21 against artificial gastric juice |
 |
| 点击放大 |
图 4显示,2株试验菌完整细胞(IC)对DPPH自由基的清除能力均低于无细胞提取物(CFE),且L. plantarum D28(34.86%)无细胞提取物对DPPH自由基的清除能力显著高于E. faecium B21(25.32%)(P<0.01),但其无细胞提取物的DPPH自由基清除能力仍小于50%。
 |
| IC:完整细胞;CFE:无细胞提取物。短栅上的“*”或“**”分别表示菌株之间在P<0.05或P<0.01水平差异有统计学意义;n=3。 IC: Intact cells; CFE: Intracellular cell⁃free extracts. Single asterisk (*) and double asterisks (**) above the bars represent statistically significant differences between strains at the 0.05 or 0.01 probability level, respectively; n=3. 图4 植物乳杆菌D28和粪肠球菌B21对DPPH自由基的清除能力 Fig. 4 DPPH radical scavenging capacity of L. plantarum D28 and E. faecium B21 |
图 5表明,2株试验菌均具有较好的BSH活性,其中E. faecium B21的BSH活性为0.415 U/mg,显著高于L. plantarum D28(0.114 U/mg)(P<0.01)。
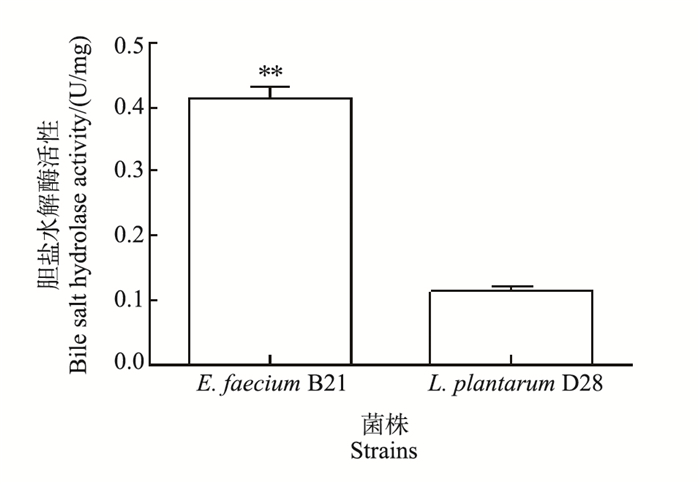 |
| 短栅上的“**”表示菌株之间在P<0.01水平差异有统计学意义;n=3。 Double asterisks (**) above the bar represent statistically significant differences between strains at the 0.01 probability level; n=3. 图5 植物乳杆菌D28和粪肠球菌B21的胆盐水解酶活性 Fig. 5 Bile salt hydrolase activity of L. plantarum D28 and E. faecium B21 |
微生物生长曲线分为适应期、对数期、稳定期和衰亡期4个时期,在一般微生物试验中,通常采用微生物生长对数期中后期和稳定期的菌液。在本研究中,L. plantarum D28的生长速度高于E. faecium B21,在MRS培养基中分别培养18 h和16 h后进入稳定期;培养基的起始pH对菌株的影响很大,在pH 4.0及以下环境下2株菌的生长受到抑制,但还是有一定的存活量。
胆盐是由肝细胞分泌的胆汁酸与甘氨酸或牛磺酸结合而形成的钠盐或钾盐。目前,耐受胆盐被认为是筛选潜在益生菌菌株的基本标准[14]。前期研究表明,乳酸菌如果能够在胃肠道中存活和增殖,必须能够耐受5%左右的NaCl和0.3%的胆盐环境[15]。在本试验中,L. plantarum D28可以耐受6%的NaCl,而E. faecium B21可以耐受8%的NaCl;但0.20%的胆盐即可抑制L. plantarum D28的生长,而E. faecium B21可以耐受0.45%的胆盐。
人体只有摄入含有1×107 CFU/g以上的乳酸杆菌对其身体健康才会有益[14]。但是人体内的胃和肠道环境都具有明显的抗菌特性,大多数菌株经消化道进入胃肠道以后是否能够抵抗人体内恶劣的胃和小肠环境,是乳酸菌在胃肠道中能否存活和正常发挥生理作用的先决条件[16]。在本试验中,L. plantarum D28和E. faecium B21均具有一定的抗氧化活性,且前者显著高于后者。
DPPH法简单、快捷、灵敏并且重复性好,是评估菌株抗氧化能力最被广泛使用的方法。在本试验中,L. plantarum D28和E. faecium B21均具有一定的抗氧化活性,且L. plantarum D28的活性显著高于E. faecium B21。
研究表明,L. plantarum 91的BSH活性为0.299 U/mg[17],且乳酸菌的BSH活性与降低胆固醇水平成正比。在本试验中,E. faecium B21表现出了显著的牛磺胆酸水解能力,能够产生BSH,具有较高的BSH酶比活力(0.415 U/mg),显著高于L. plantarum D28。
4 结论从发酵食品中筛选出的2株菌(L. plantarum D28和E. faecium B21)具有不同的生长特性,其生长速度受培养基起始pH值的影响,且在相同培养条件下,L. plantarum D28的生长速度快于E. faecium B21;2株菌均具有NaCl和胆盐耐受性,以及对人工胃液和人工肠液的耐受性,两者均具有一定的益生菌特性;2株菌均具有较好的体外抗氧化活性和BSH活性,且L. plantarum D28可作为抗氧化菌株进行相关研究。此外,由于这2株菌均有较好的BSH活性,可以作为降低胆固醇的菌株进行相关研究。
| [1] | DALIRI E B M, LEE B H. New perspectives on probiotics in health and disease. Food Science and Human Wellness, 2015, 4(2): 56-65. DOI:10.1016/j.fshw.2015.06.002 |
| [2] | LEE Y K. Effect of diet on gut microbiota profile and the implications for health and disease. Bioscience of Microbiota, Food and Health, 2013, 32(1): 1-12. DOI:10.12938/bmfh.32.1 |
| [3] | FEANZ C M P, HUCH M, MATHARA J M. African fermented foods and probiotics. International Journal of Food Microbiology, 2014, 190(3): 84-96. |
| [4] | WANG X, WU Q L, DENG K, et al. A novel method for screening of potential probiotics for high adhesion capacity. Journal of Dairy Science, 2015, 98(7): 4310-4317. DOI:10.3168/jds.2015-9356 |
| [5] | TANIDA M, SHEN J, MAEDA K, et al. High-fat-diet-induced obesity is attenuated by probiotic strain Lactobacillu sparacasei ST11 (NCC2461) in rats. Obesity Research & Clinical Practice, 2008, 2(3): 159-169. |
| [6] | RATHER S A, POTHURAJU R, SHARMA R K, et al. Antiobesity effect of feeding probiotic dahi containing Lactobacillus casei NCDC 19 in high fat diet-induced obese mice. Society of Dairy Technology, 2014, 67(4): 504-509. DOI:10.1111/idt.2014.67.issue-4 |
| [7] | IYE H S, RAHMAT-ALI G R, Liong M T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. International Dairy Journal, 2010, 20(3): 169-175. DOI:10.1016/j.idairyj.2009.10.003 |
| [8] |
郭志华, 杨洪. 分离自藏灵菇的乳酸菌的益生特性. 食品与发酵工业, 2013, 39(1): 151-154. GUO Z H, YANG H. Study on the probiotic of lactic acid bacteria from Tibet kefir. Food & Fermentation Industry, 2013, 39(1): 151-154. (in Chinese with English abstract) |
| [9] | CHARTERIS W P, KELLY P M, MORELLI L, et al. Development and application of an in vitro methodology todetermine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upperhuman gastrointestinal tract. Journal of Application Microbiology, 1998, 84(5): 759-768. DOI:10.1046/j.1365-2672.1998.00407.x |
| [10] | LIN M Y, YEN C L. Antioxidative ability of lactic acid bacteria. Journal of Agriculture and Food Chemistry, 1999, 47(4): 1460-1466. DOI:10.1021/jf981149l |
| [11] | LIN M Y, CHANG F J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Digestive Diseases & Sciences, 2000, 45(8): 1617-1622. |
| [12] | LIONG M T, SHAH N P. Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. International Dairy Journal, 2005, 15(4): 391-398. DOI:10.1016/j.idairyj.2004.08.007 |
| [13] | DONG Z, ZHANG J, LEN B, et al. A bile salt hydrolase gene of Lactobacillus plantarum BBE7 with high cholesterol-removing activity. European Food Research & Technology, 2012, 235(3): 419-427. |
| [14] |
陈臣, 郭本恒, 陈卫, 等. 3株益生菌黏附性质和机理的初步研究. 中国微生态学杂志, 2007, 19(6): 492-495. CHEN C, GUO B H, CHEN W, et al. Preliminary study on the adhesive properties and mechanisms of three probiotic strains. Chinese Journal of Microbiology, 2007, 19(6): 492-495. (in Chinese with English abstract) |
| [15] |
赵瑞香, 李云瑞, 孙俊良, 等. 嗜酸乳杆菌在模拟胃肠环境中抗性的研究. 微生物学报, 2002, 29(2): 35-38. ZHAO R X, LI Y R, SUN J L, et al. Studies on the antagonistic properties of Lactobacillus acidophilus in the imitative gastroenteric environment. Microbiology, 2002, 29(2): 35-38. (in Chinese with English abstract) |
| [16] | ROSS G R, GUSILES C, OLISZEWSKI R, et al. Effects of probiotic administration in swine. Journal of Bioscience and Bioengineering, 2010, 109(6): 545-549. DOI:10.1016/j.jbiosc.2009.11.007 |
| [17] | KUMAR M, NAGPAL R, KUMAR R, et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Experimental Diabetes Research, 2012, 2012: 902-917. |
 2017, Vol. 43
2017, Vol. 43
