| 可降解苯酚的产电芽孢杆菌WL027的分离筛选及其产电机制初探 |
微生物燃料电池(microbial fuel cell,MFC)是通过微生物体内的新陈代谢作用将易降解的物质降解并将其中的化学能转化为电能的装置[1]。产电微生物可附着在阳极上形成生物膜,使有机物氧化降解,同时将释放的电子传递[2]到阳极表面,形成电流。产电微生物因具有丰富的物种多样性和操作条件温和、易于控制等优势而被广泛研究和应用。
随着研究的深入,越来越多的产电菌被发现。目前,被报道的产电微生物种类呈现多元化趋势,主要包括细菌、古菌和酵母菌3大类,其中以细菌所占比例最多。在产电微生物中隶属于细菌的主要有变形菌门(Proteobacteria)、厚壁菌门(Firmicutes)、酸杆菌门(Acidobacteria)和放线菌门(Actinobacteria)。其中,以变形菌门中的数量最多,包括变形杆菌属(Proteus)[3]、欧文氏菌属(Erwinia)[4]、脱硫弧菌属(Desulfovibrio)[5]、红假单胞菌属(Rhodopseudomonas)[6]、气单胞菌属(Aeromonas)[7]、除硫单胞菌属(Desulfuromonas)[8]、红育菌属(Rhodoferax)[9]、地杆菌属(Geobacter)[10]、地缘嗜冷杆菌属(Geopsychrobacter)[11]、埃希菌属(Escherichia)[12]、脱硫球茎菌属(Desulfobulbus)[13]、副球菌属(Paracoccus)[14]、肠杆菌属(Enterobacter)[15]、苍白杆菌属(Ochrobactrum)[16]、嗜酸菌属(Acidiphilium)[17]、葡糖杆菌属(Gluconobacter)[18]、克雷伯氏菌属(Klebsiella)[19]、弓形杆菌属(Arcobacter)[20]、根微杆菌属(Rhizomicrobium)[21]、假单胞菌属(Pseudomonas)[22]、希瓦氏菌属(Shewanella)[23]及柠檬酸杆菌属(Citrobacter)[24]。此外,还有广古菌门(Euryarchaeota)[25]中的富盐菌属(Haloferax)和钠白菌属(Natrialba);酵母菌子囊菌门酵母菌亚门(Saccharomycotina)的毕赤酵母属(Pichia)[26]、酵母属(Saccharomyces)[27]及Arxula[28]。
苯酚因污染环境、使蛋白质凝结及危害人类健康等,被认为是应优先控制处理的水体污染物之一。目前,对含酚废水的处理主要有物理法[29](吸附法、溶剂萃取法和液膜法)、化学法[30](氧化法、沉淀法和光催化法)和生物法[31](活性污泥法、酶处理法和固定化微生物技术)。利用MFC处理苯酚废水时,MFC在降解苯酚的同时,还能产生电能,因而被越来越多的学者所关注。骆海萍等[32]利用葡萄糖和苯酚为底物构建MFC,发现苯酚降解率可达95%;范平等[33]通过对MFC不同驯化方式的研究表明,MFC对苯酚的降解率均可达90%以上。而筛选出同时具有高产电性和高降解性的菌株,对于提高MFC去除废水中的苯酚具有十分广泛的应用前景。
本文以含苯酚的生活污水为底物构建MFC,经过分离筛选,获得一株可降解苯酚的产电菌WL027,经鉴定为蜡样芽孢杆菌(Bacillus cereus),并对其产电性和苯酚降解情况进行了初步探讨;同时,利用该菌株构建MFC,测定该菌株的循环伏安曲线,以及用扫描电镜观察MFC阳极碳毡和测定菌株WL027的胞内外核黄素含量,探讨该菌株可能存在的电子传递机制。
1 材料与方法 1.1 材料 1.1.1 污水样品所用污水采自黑龙江省哈尔滨市文昌污水处理厂的厌氧-缺氧-好氧(A2/O)段曝气池和厌氧池。
1.1.2 培养基和缓冲液富集培养基:牛肉膏 5 g,蛋白胨 10 g,NaCl 5 g,苯酚500 mg/L,加蒸馏水配至1 000 mL,pH值调至7.2~7.4,121 ℃灭菌20 min。
分离和保存培养基:牛肉膏 5 g,蛋白胨 10 g,NaCl 5 g,琼脂18 g,苯酚500 mg/L,加蒸馏水配至1 000 mL,pH值调至7.2~7.4,121 ℃灭菌20 min。
无机盐培养基:KH2PO4 3 g/L,Na2HPO4 6 g/L,NaCl 0.5 g/L,酵母膏0.2 g/L,NH4Cl 1 g/L,CaCl2 0.011 g/L,FeSO4 0.025 g/L,MgSO4 0.24 g/L,苯酚 500 mg/L。
阴极缓冲液:Na2HPO4 2.75 g/L,NaH2PO4 4.22 g/L,KMnO4 200 mg/L。
阳极缓冲液:NH4Cl 1.5 g/L,MgCl2·6H2O 0.1 g/L,CaCl2 0.1 g/L,KH2PO4 0.1 g/L。
1.1.3 试验器材恒温培养箱、紫外-可见分光光度计、恒温水浴锅、超净工作台、高压蒸汽灭菌锅、电子分析天平、离心机、恒温加热磁力搅拌器、气浴恒温振荡器、电化学工作站(CHI600E)、扫描电镜(S-3400N)、荧光分光光度计(F-4500)等。
1.2 方法 1.2.1 获得菌株的MFC构建获得菌株的MFC是双室微生物燃料电池。电池双室的有效容积均为600 mL。阳极电极采用经氯化铁改性的碳毡(5 cm×5 cm,有效面积12.56 cm2)[34],阴极电极采用碳刷,使用前碳刷先用1 mol/L HCl和1 mol/L NaCl浸泡24 h。阴极室和阳极室之间用盐桥连接,接口处均做绝缘处理。电路中设有外接变阻箱(0~9 999 Ω),外电路电阻采用1 000 Ω。在试验过程中产生的电压通过双通道电压采集系统(PISO813)直接记录,每60 s测定一次。
电池启动前,阳极室通入20 min氮气,保证阳极室的厌氧环境。先将曝气池的污水与厌氧池的污水以体积比1∶1混合形成厌氧污水,厌氧污水与阳极液按体积比1∶5混合接入到阳极室,同时加入1 000 mg/L葡萄糖和500 mg/L苯酚。将整个电池阳极装置放在温度为30 ℃的恒温磁力搅拌器上进行搅拌,以维持阳极溶液的均匀。
1.2.2 菌株分离与纯化在MFC外界电压达到稳定后,将阳极室中的碳毡取出,放入到无菌生理盐水中,振荡,使粘在上面的菌体进入到生理盐水中,然后采用富集培养基于30 ℃、160 r/min对菌种富集培养24 h;将富集后的菌液用稀释涂布法涂布于分离和保存培养基平板上,于30 ℃恒温培养箱中培养48 h后,挑取较大的菌落进行逐步划线分离、纯化,最后将获得的纯菌株WL027接种于分离和保存培养基上,30 ℃培养48 h后,保存于4 ℃冰箱中。
1.2.3 菌株分类鉴定将分离筛选得到的菌株WL027送往中国普通微生物菌种保藏管理中心进行生理生化测定和16S rRNA测序;将测序结果提交至GenBank数据库中进行16S rDNA序列同源性比对,构建系统发育树。
1.2.4 苯酚降解率和库仑效率计算苯酚浓度使用4-氨基安替比林直接光度法进行测定[35]。
苯酚降解率/%=(空白样苯酚残余浓度-微生物作用后苯酚残余浓度)/空白样苯酚残余浓度×100。
库仑效率(Coulombic efficiency,CE)计算公式[36]:
| ${\rm{C}}{{\rm{E}}_{{\rm{Batch}}}}/\% = \frac{{M\sum\limits_{i = 0}^t {{I_i}\Delta {t_i}} }}{{{\rm{F}}b{\rm{V}}\left( {{\rm{CO}}{{\rm{D}}_{{\rm{in}}}} - {\rm{CO}}{{\rm{D}}_{{\rm{out}}}}} \right)}}$ |
式中:M为氧分子摩尔质量,32 g/mol;Ii为时间间隔Δti内电流的平均值,A;Δti为电流采样时间间隔,s;F为法拉第常数,86 485 C/mol;b为1 mol COD所产生的电子的摩尔数,4 mol/mol;V为阳极的有效容积,mL;CODin为初始化学需氧量,mg/L;CODout为反应后的化学需氧量,mg/L.
1.2.5 菌株WL027的产电特性初步探讨 1.2.5.1 菌株WL027的MFC构建采用双室微生物燃料电池装置(同1.2.1节),将处于对数中期的菌株培养液与阳极液按体积比1∶5混合后接入到阳极室,同时加入1 000 mg/L葡萄糖和500 mg/L苯酚。
1.2.5.2 产电循环伏安曲线绘制为研究菌株WL027是否具有电化学活性,将该菌株接种到无机盐培养基中,于30 ℃、160 r/min的摇床中分别培养7、18、31和52 h,形成菌悬液。取每株菌的菌悬液10 mL于三极系统中,以铟锡氧化物(indium tin-oxide,ITO)电极为工作电极,铂电极为辅助电极,Ag/AgCl为参比电极[36],扫描速率为100 mV/s,扫描范围为-0.8~0.8 V,扫描并绘制伏安曲线。
1.2.5.3 阳极碳毡扫描电镜观察将处于稳定期的MFC阳极碳毡取出,用叔丁醇和戊二醛法[1]对碳毡进行扫描电镜样品制备。具体操作:使用2.5%戊二醛固定碳毡,用0.1 mol/L磷酸缓冲溶液(pH=6.8)冲洗3次后,依次用50%、70%、80%、90%和100%的乙醇脱水,然后用叔丁醇进行置换,最后放至冷冻干燥器上干燥。将干燥样品喷金后,用扫描电镜在5.00 kV加速电压下进行表面形态观察。
1.2.5.4 菌株WL027细胞内外核黄素含量测定取菌株WL027的适量培养液离心15 min,取离心后的菌泥0.5 g和上清液10 mL放在不同的烧杯中,分别加入0.1 mol/L HCl 30 mL,在暗处静置20 min,再加入醋酸-醋酸钠缓冲液(pH=4.6)30 mL,用0.22 μm聚丙烯膜过滤3次,最后用100 mL容量瓶定容,置于暗处避光保存。用荧光分光光度计[37]测定胞内和胞外荧光强度,其中,激发波长600 nm,荧光发射波长449 nm,狭缝5 nm,扫描速度1 200 nm/min;对照标准曲线分别计算出菌体细胞内外的核黄素浓度。
2 结果与分析 2.1 获得菌株的MFC电压随时间变化的曲线从图 1可以看出:电压数据从开始记录到230 h的过程中上下波动幅度较大,经历大约6次电压迅速下降后又迅速回升,该过程通常被认为是产电微生物在电极表面富集形成生物膜的过程;电压在230 h后逐渐趋于平稳。MFC系统的最大输出电压为0.15 V,经计算,最大电流密度为25.9 mA/m2,最大功率密度为66.9 mW/m2。
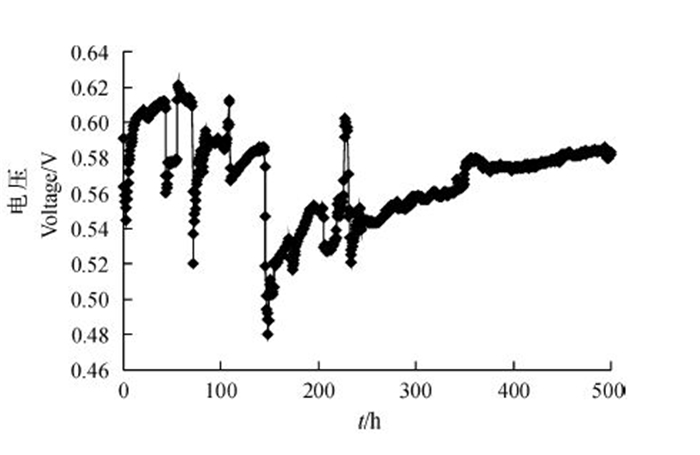 |
| 图1 MFC系统的产电曲线 Fig. 1 Electricity production curve of MFC |
本研究共分离出21株纯菌株,其斜面培养情况如图 2所示。各菌株菌落形态及产电性如表 1所示。在21株菌中只有WL013、WL024和WL027这3株菌具有产电性,且WL027的产电性最好;因此,选择菌株WL027作为优势菌株。
 |
| 图2 21株纯菌株的斜面培养情况 Fig. 2 Status of slant culture of 21 pure strains |
| 表1 纯菌株菌落形态及产电性 Table 1 Colony morphology and electrical property of 21 pure isolations |
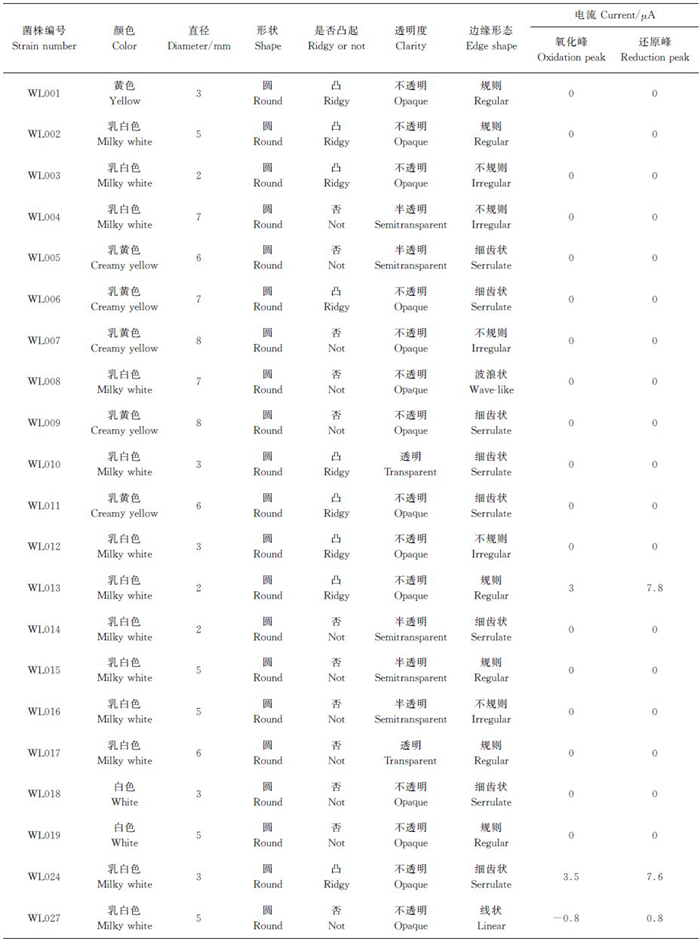 |
| 点击放大 |
菌株WL027为杆状的革兰氏阳性菌,菌落表面呈毛玻璃状,菌落直径达8~10 mm,其16S rRNA序列长度为1 384 bp。将其序列提交至GenBank,获得的序列号为BankIt1826037;将菌株WL027的测序结果与GenBank中的16S rDNA序列进行同源性比对,构建系统发育树(图 3)。从中可以看出,菌株WL027与蜡样芽孢杆菌(Bacillus cereus)聚在同一个分支中。同源性分析结果表明,该序列与蜡样芽孢杆菌属内各种之间有极高的同源性,达95%以上。结合菌体形态特征、生长条件、生理生化鉴定结果,确定菌株WL027属于芽孢杆菌属(Bacillus)中的蜡样芽孢杆菌(Bacillus cereus)。该菌种保藏在中国典型培养物保藏中心(CCTCC),编号为M 2015439。
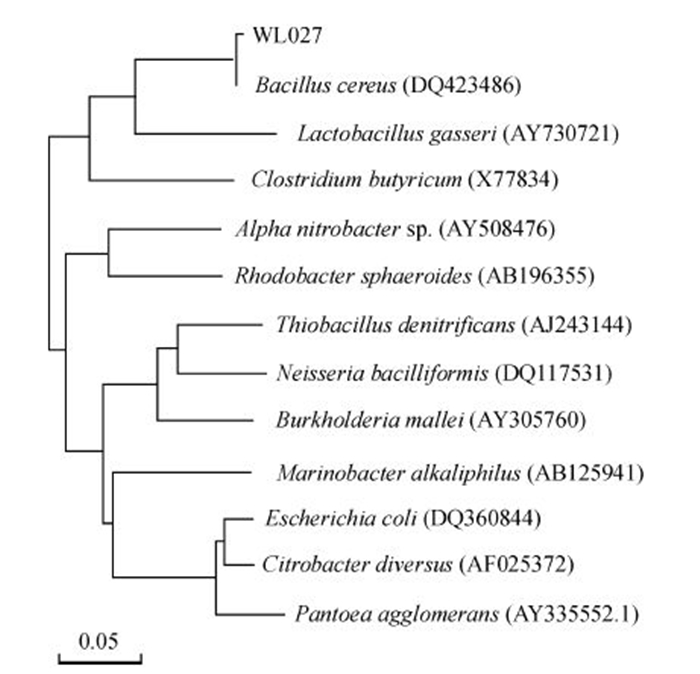 |
| 标尺表示系统发育树枝长所代表的遗传距离。 The ruler shows genetic distance represented by evolutionary branch length. 图3 菌株WL027的系统发育树 Fig. 3 Phylogenetic tree of strain WL027 |
以葡萄糖(1 000 mg/L)和苯酚(500 mg/L)作为基质,将菌株WL027接种到阳极,启动MFC,验证菌株的产电能力。从图 4可以看出:菌株WL027在 MFC中运行大致可以分为2个时期,分别为电压快速上升期和相对稳定期;启动电压为0.45 V,经历约8 h的电压快速增长后趋于平稳,在74 h处达到最大电压,较起始电压提高了65 mV;培养基更换后,电压快速增加,可能是由于阳极表面已经形成的生物膜在MFC中起重要作用;更换3次培养液,在340 h处达到电压相对稳定期,最大电压较起始电压增加了179 mV。随着电池的运行,电压在稳定期的数值与峰值行的差距逐渐缩小,这可能是由于菌体自身分泌氧化还原性物质,从而有效增加了电子的传递速率。
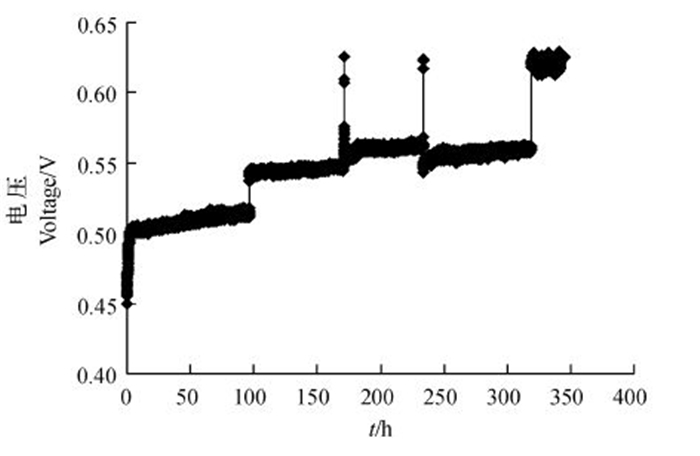 |
| 图4 利用菌株WL027构建的MFC产电曲线 Fig. 4 Electricity production curve of MFC constructed by strain WL027 |
丁巍巍等[38]研究表明,在35 ℃条件下用MFC处理0.5 g/L苯酚废水,对苯酚的去除效率为99.63%。陈少华等[39]研究发现,在MFC状态下,200 h可降解380 mg/L苯酚。本研究结果(图 5)表明:将菌株WL027接种到含500 mg/L苯酚的MFC中,苯酚降解率随着时间的延长而逐渐增大;在94 h时,苯酚降解率达到最大值68.62%,经计算,库仑效率达到64.25%。虽然菌株WL027利用MFC对苯酚的降解率低于已报道的其他菌株[38-39],但其库仑效率却高于其他以苯酚为燃料构建的MFC。而库仑效率是反映微生物燃料电池所降解的有机物用来产电部分所占的比例;库仑效率越大,电子回收率越高,也就是电池能量的转化率越高。骆海萍等[40]利用葡萄糖(1 000 mg/L)和苯酚(600 mg/L)作为燃料,MFC的库仑效率为2.3%;范平等[33]研究发现,直接驯化以600 mg/L苯酚为底物的MFC的库仑效率为1.47%;蒋胜韬等[41]以葡萄糖(500 mg/L)和苯酚(500 mg/L)为燃料构建MFC,库仑效率仅为0.48%。由此可以看出,在能量转化效率方面,利用菌株WL027构建的MFC具有很明显的优势。
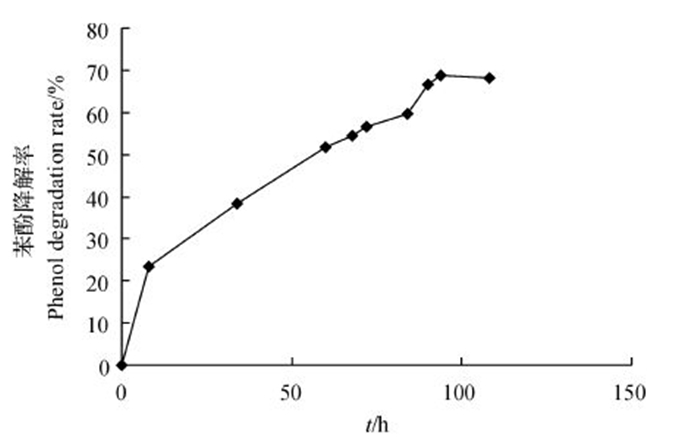 |
| 图5 利用菌株WL027构建的MFC的苯酚降解曲线 Fig. 5 Degradation curve of phenol in MFC constructed by strain WL027 |
菌种是否具有电化学活性可以使用循环伏安法进行测定。探究菌株不同生长时期的产电性,对菌株培养条件的优化具有十分重要的意义。由菌株在调整期(7 h)、对数期(18 h)、稳定期(31 h)和衰亡期(52 h)的循环伏安曲线(图 6)可见:未加菌液的空白培养基的循环伏安曲线是一条平滑曲线,无氧化还原峰;含有菌株WL027的曲线在稳定期有1对氧化峰和还原峰,其中,氧化峰位于-0.8~-0.4 V之间,还原峰在0.5~0.76 V附近。这说明该菌株具有产电活性,且在稳定期产电。
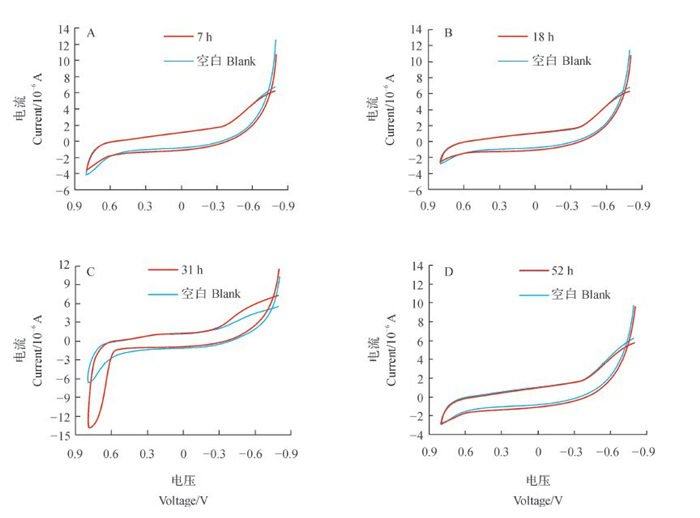 |
| 图6 菌株WL027在4个不同生长时期的循环伏安曲线 Fig. 6 Cyclic voltammetry curves of strain WL027 at four different growth stages |
利用扫描电镜观察到的生物膜形态如图 7所示。在MFC中阳极碳毡具有相互交叉的结构,便于菌株WL027的附着,从而易于形成生物膜。在图 7D中可观察到大量的杆状菌体细胞,说明菌株WL027可附着于电极表面。在阳极碳毡表面没有观察到有类似于希瓦氏菌的细菌纳米导线[42],WL027菌株只是与电极接触,猜测该菌株可能通过分泌某些氧化还原介体[43]促进电子在微生物中传递。
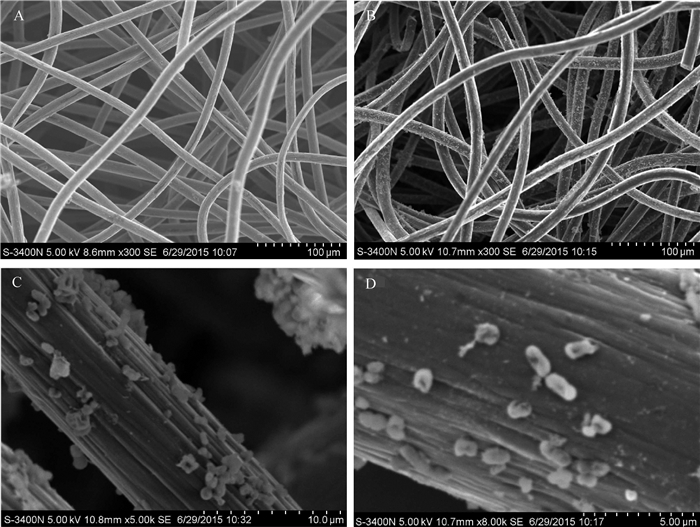 |
| A:空白阳极(×3 000倍);B:阳极生物膜(×3 000倍);C:阳极生物膜(×5 000倍);D:附着在单个碳纤维上的菌体(×8 000倍)。 A: Anode without biofilm in MFC (×3 000); B: Anode biofilm in MFC (×3 000); C: Anode biofilm in MFC (×5 000); D: Somatic cell adhered to carbon fiber (×8 000). 图7 MFC阳极生物膜的扫描电镜图 Fig. 7 Scanning electron microscope images of anode biofilm in MFC |
核黄素是微生物体内分泌的氧化还原性物质,可以作为介体传递电子。核黄素在波长为446~455 nm范围内有明显峰值。从图 8可以看出:菌株WL027在449 nm处出现明显峰值,说明菌液中含有核黄素,且胞外核黄素浓度明显高于胞内。对照标准曲线分别计算出菌体WL027细胞内外的核黄素质量浓度分别为6.10×10-3和1.32×10-2 mg/L。由此可知,核黄素主要在胞外累积,它可能在由胞内转移至胞外的过程中传递电子。
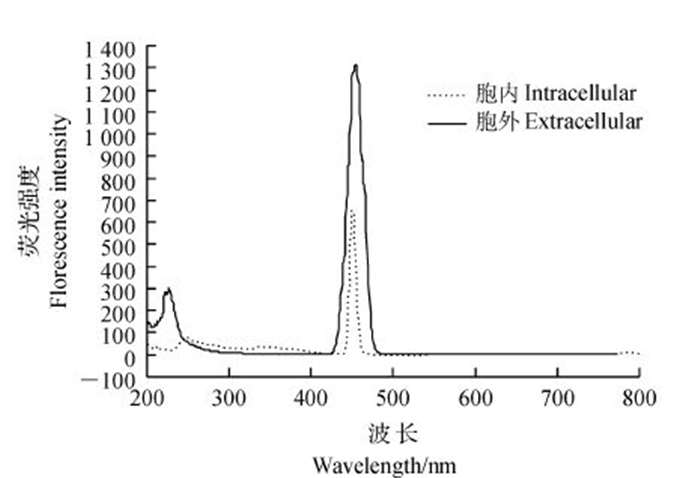 |
| 图8 菌株WL027胞内和胞外核黄素的发射光谱图 Fig. 8 Emission spectrogram of intracellular and extracellular riboflavin in strain WL027 |
为了进一步验证核黄素是否可以作为介体传递电子,在处于稳定期的MFC中加入核黄素,观察电压是否有明显变化。结果(图 9)表明:加入核黄素后电压快速上升,从0.526 V迅速上升到0.544 V,升高了18 mV。由此猜测,菌株WL027可能通过分泌核黄素促进电子在微生物中传递。
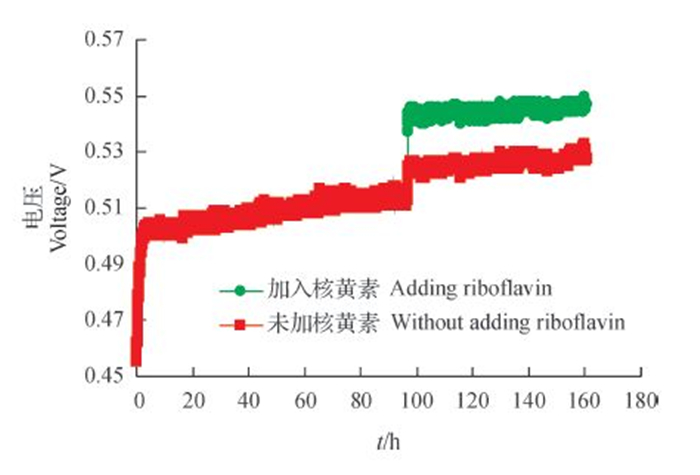 |
| 图9 MFC中添加和未添加核黄素的电压 Fig. 9 Voltage of MFC with or without adding riboflavin |
产电微生物的电子传递机制可分为生物膜机制和电子穿梭机制。微生物在电极表面附着,可形成生物膜。生物膜机制是指产电微生物利用细胞色素C或“纳米导线”将电子由胞内通过生物膜转移至电极的过程。目前,利用细胞色素C转移电子的产电微生物多为革兰氏阴性菌[44],因为其细胞壁中含有丰富的细胞色素C;已发现的有硫还原地杆菌(Geobacter sulfurreducens)[45],嗜水气单胞菌(Aeromonas hydrophila)[46]和铁还原红育菌(Rhodoferax ferrireducens)[47]等。在电子转移过程中,使用氧化还原介体在菌体与电极间传递电子的方式称为电子穿梭机制。氧化还原介体按其来源可分为外源性氧化还原介体和内源性氧化还原介体(菌体自分泌)。外源性氧化还原介体有中性红[48]、醌类衍生物[49]、亚甲蓝、硫堇、铁氰化钾[50]以及甲基紫精。内源性氧化还原介体可以由微生物厌氧呼吸的初级代谢产物、发酵的还原性代谢物和次级代谢中的低分子化合物组成。已经发现的由微生物产生的氧化还原介体包括吩嗪[51]、核黄素、醌类物质[52]、绿脓菌素[53]和黑色素[43]等。目前,核黄素作为氧化还原介体已在Shewanella sp.体内发现。VON CANSTEI等[54]研究得出,Shewanella细菌可利用核黄素来介导不溶性三价铁氧化物还原。MARSILI等[52]确证了Shewanella oneidensis可累积250~500 nmol/L核黄素,并且这些核黄素可作为氧化还原介体将胞外电子传递至电极;但是在其他微生物体内几乎很少报道。
蜡样芽孢杆菌是革兰氏阳性菌,在电子传递机制探讨过程中不存在利用细胞色素C来转移电子;在对MFC碳毡的电镜扫描中没有观察到“纳米导线”:说明菌株WL027不能利用生物膜机制进行电子转移。而菌株WL027胞外和胞内的核黄素质量浓度分别为1.32×10-2和6.10×10-3 mg/L;向处于稳定期的MFC中加入核黄素后电压快速上升。由此猜测,菌株WL027可能通过分泌核黄素促进电子在微生物中传递。而核黄素在菌株WL027电子传递过程中的传递机制,以及是否还有其他氧化还原介体的协同作用,尚需要进一步验证。
4 结论4.1 本文以500 mg/L苯酚为原料,从MFC阳极中分离出产电菌株WL027,鉴定结果为蜡样芽孢杆菌(Bacillus cereus)。该菌种保藏在CCTCC,编号为M 2015439。该研究结果丰富了可产电的菌种资源。
4.2 菌株WL027在生长稳定期产电。将菌株WL027接种到以苯酚(500 mg/L)和葡萄糖(1 000 mg/L)的MFC中,最大电压较起始电压增加179 mV,苯酚降解率为68.62%,产电菌的库仑效率为64.25%:表明利用该菌株构建的MFC不仅可高效降解苯酚,且在能量转化效率方面具有很明显的优势。
4.3 通过对菌株WL027产电机制的初步探讨,认为该菌株可能通过分泌核黄素来促进电子在微生物中传递。
| [1] |
ZHANG C P, CHEN S S, LIU G L, et al. Characterization of strain Pseudomonas sp. Q1 in microbial fuel cell for treatment of quinoline-contaminated water.
Pedosphere, 2012,22 (4):528–535. DOI: 10.1016/S1002-0160(12)60037-X. |
| [2] |
WU D, XING D F, MEI X X, et al. Electricity generation by Shewanella sp.
Electricity generation by Shewanella sp. HN-41 in microbial fuel cells. International Journal of Hydrogen Energy, 2013,38 :15568–15573. |
| [3] |
DELANEY G M, BENNETTO H P, MASON J R, et al. Electron-transfer coupling in microbial fuel cells: 2. Performance of fuel cells containing selected microorganism-mediator-substrate combinations.
Journal of Chemical Technology and Biotechnology B: Biotechnology, 1984,34 (1):13–27. |
| [4] |
VEGA C A, FERNNDEZ I. Mediating effect of ferric chelate compounds in microbial fuel cells with Lactobacillus plantarum, Streptococcus lactis, and Erwinia dissolvens.
Bioelectrochemistry and Bioenergetics, 1987,17 (2):217–222. DOI: 10.1016/0302-4598(87)80026-0. |
| [5] |
COONEY M J, ROSCHI E, MARISON I W, et al. Physiologic studies with the sulfate-reducing bacterium Desulfovibrio desulfuricans: Evaluation for use in a biofuel cell.
Enzyme and Microbial Technology, 1996,18 (5):358–365. DOI: 10.1016/0141-0229(95)00132-8. |
| [6] |
MCGRATH J E, HARFOOT C G. Reductive dehalogenation of halocarboxylic acids by the phototrophic genera Rhodospirillum and Rhodopseudomonas.
Applied and Environmental Microbiology, 1997,63 (8):3333–3335. |
| [7] |
CHOPRA A K, XU X J, RIBARDO D, et al. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages.
Infection and Immunity, 2000,68 (5):2808–2818. DOI: 10.1128/IAI.68.5.2808-2818.2000. |
| [8] |
BOND D R, HOLMES D E, TENDER L M, et al. Electrode-reducing microorganisms that harvest energy from marine sediments.
Science, 2002,295 (5554):483–485. DOI: 10.1126/science.1066771. |
| [9] |
FINNERAN K T, JOHNSEN C V, LOVLEY D R. Rhodoferax ferrireducens sp nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe (Ⅲ).
International Journal of Systematic and Evolutionary Microbiology, 2003,53 :669–673. DOI: 10.1099/ijs.0.02298-0. |
| [10] |
BOND D R, LOVLEY D R. Electricity production by Geobacter sulfurreducens attached to electrodes.
Applied and Environmental Microbiology, 2003,69 (3):1548–1555. DOI: 10.1128/AEM.69.3.1548-1555.2003. |
| [11] |
HOLMES D E, NICOLL J S, BOND D R, et al. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp nov., in electricity production by a marine sediment fuel cell.
Applied and Environmental Microbiology, 2004,70 (10):6023–6030. DOI: 10.1128/AEM.70.10.6023-6030.2004. |
| [12] |
MCKINLAY J B, ZEIKUS J G. Extracellular iron reduction is mediated in part by neutral red and hydrogenase in Escherichia coli.
Applied and Environmental Microbiology, 2004,70 :3467–3474. DOI: 10.1128/AEM.70.6.3467-3474.2004. |
| [13] |
HOLMES D E, BOND D R, LOVLEY D R. Electron transfer by Desulfobulbus propionicus to Fe (Ⅲ) and graphite electrodes.
Applied and Environmental Microbiology, 2004,70 (2):1234–1237. DOI: 10.1128/AEM.70.2.1234-1237.2004. |
| [14] |
RABAEY K, VAN DE SOMPEL K, MAIGNIEN L, et al. Microbial fuel cells for sulfide removal.
Environmental Science and Technology, 2006,40 (17):5218–5224. DOI: 10.1021/es060382u. |
| [15] |
ZHANG J T, ZHOU S G, ZHANG L X, et al. Mechanisms of bioelectricity generation in Enterobacter aerogenes-based microbial fuel cells.
Environmental Science, 2009,30 (4):1215–1220. |
| [16] |
ZUO Y, XING D, REGAN J M, et al. Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell.
Applied and Environmental Microbiology, 2008,74 (10):3130–3137. DOI: 10.1128/AEM.02732-07. |
| [17] |
BOROLE A P, O’NEILL H, TSOURIS C, et al. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum.
Biotechnology Letters, 2008,30 (8):1367–1372. DOI: 10.1007/s10529-008-9700-y. |
| [18] |
TOMASHEVSKAIA L G, ALFEROV S V, TOMASHEVSKII A A, et al. Power Characteristics of the Microbial Fuel Cell Based on Gluconobacter Cell Suspension and 2,6-Dichlorophe Nolindophenol as the Electron Transport Mediator.
Hauppauge, USA: Nova Science Publishers, Inc, 2008 :87–94. |
| [19] |
ZHANG L X, ZHOU S G, ZHUANG L, et al. Microbial fuel cell based on Klebsiella pneumoniae biofilm.
Electrochemistry Communications, 2008,10 (10):1641–1643. DOI: 10.1016/j.elecom.2008.08.030. |
| [20] |
FEDOROVICH V, KNIGHTON M C, PAGALING E, et al. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell.
Applied and Environmental Microbiology, 2009,75 (23):7326–7334. DOI: 10.1128/AEM.01345-09. |
| [21] |
KODAMA Y, WATANABE K. Rhizomicrobium electricum sp nov., a facultatively anaerobic, fermentative, prosthecate bacterium isolated from a cellulose-fed microbial fuel cell.
International Journal of Systematic and Evolutionary Microbiology, 2011,61 :1781–1785. DOI: 10.1099/ijs.0.023580-0. |
| [22] |
ZHANG T T, ZHANG L X, SU W T, et al. The direct electrocatalysis of phenazine-1-carboxylic acid excreted by Pseudomonas alcaliphila under alkaline condition in microbial fuel cells.
Bioresource Technology, 2011,102 (14):7099–7102. DOI: 10.1016/j.biortech.2011.04.093. |
| [23] |
BIFFINGER J C, FITZGERALD L A, RAY R, et al. The utility of Shewanella japonica for microbial fuel cells.
Bioresource Technology, 2011,102 (1):290–297. DOI: 10.1016/j.biortech.2010.06.078. |
| [24] |
XU S, LIU H. New exoelectrogen Citrobacter sp. SX-1 isolated from a microbial fuel cell.
Journal of Applied Microbiology, 2011,111 (5):1108–1115. DOI: 10.1111/jam.2011.111.issue-5. |
| [25] |
ABREVAYA X C, SACCO N, MAUAS P J D, et al. Archaea based microbial fuel cell operating at high ionic strength conditions.
Extremophiles, 2011,15 (6):633–642. DOI: 10.1007/s00792-011-0394-z. |
| [26] |
PRASAD D, ARUN S, MURUGESAN A, et al. Direct electron transfer with yeast cells and construction of a mediator less microbial fuel cell.
Biosensors and Bioelectronics, 2007,22 (11):2604–2610. DOI: 10.1016/j.bios.2006.10.028. |
| [27] |
HASLETT N D, RAWSON F J, BARRIRE F, et al. Characterisation of yeast microbial fuel cell with the yeast Arxula adeninivorans as the biocatalyst.
Biosensors and Bioelectronics, 2011,26 (9):3742–3747. DOI: 10.1016/j.bios.2011.02.011. |
| [28] |
SIU C P B, CHIAO M. A microfabricated PDMS microbial fuel cell.
Journal of Microelectromechanical Systems, 2008,17 (6):1329–1341. DOI: 10.1109/JMEMS.2008.2006816. |
| [29] |
EHTASH M, FOURNIER-SALAVN M C, DIMITROV K, et al. Phenol removal from aqueous media by pertraction using vegetable oil as a liquid membrane.
Chemical Engineering Journal, 2014,250 (15):42–47. |
| [30] |
NIKAZAR M, ALIZADEH M, LALAVI R, et al. The optimum conditions for synthesis of Fe3O4/ZnO core/shell magnetic nanoparticles for photodegradation of phenol.
Journal of Environmental Health Science and Engineering, 2014,12 (1):1–6. DOI: 10.1186/2052-336X-12-1. |
| [31] |
STEEVENSZ A, MADUR S, FENG W, et al. Crude soybean hull peroxidase treatment of phenol in synthetic and real wastewater: Enzyme economy enhanced by triton X-100.
Enzyme and Microbial Technology, 2014,55 :65–71. DOI: 10.1016/j.enzmictec.2013.12.005. |
| [32] |
骆海萍, 刘广立, 张仁铎, 等.高浓度苯酚的MFC降解及产电性能.
环境科学学报,2008,28 (11):2181–2185.
LUO H P, LIU G L, ZHANG R D, et al. Electricity generation by degradation of high concentration phenol using a microbial fuel cell. Acta Scientiae Circumstantiae, 2008,28 (11):2181–2185. (in Chinese with English abstract) |
| [33] |
范平, 宋天顺, 覃彪, 等.不同驯化方式对以苯酚为基质的微生物燃料电池产电性能的影响.
环境工程学报,2012,6 (11):3867–3872.
FAN P, SONG T S, QIN B, et al. Effect of different acclimation on performance of microbial fuel cells using phenol as substrate. Chinese Journal of Environmental Engineering, 2012,6 (11):3867–3872. (in Chinese with English abstract) |
| [34] |
薄晓, 李鹏, 赵晓峰, 等.废水微生物燃料电池阳极碳毡的产电特性的研究.
化工新型材料,2014,42 (12):126–129.
BO X, LI P, ZHAO X F, et al. Research on producing electricity of wastewater microbial fuel cell anode carbon felt. New Chemical Materials, 2014,42 (12):126–129. (in Chinese with English abstract) |
| [35] |
FAYIDH M A, KALLARY S, BABU P A S, et al. A rapid and miniaturized method for the selection of microbial phenol degraders using colourimetric microtitration.
Current Microbiology, 2015,70 :898–906. DOI: 10.1007/s00284-015-0809-7. |
| [36] |
陈姗姗, 张翠萍, 刘广立, 等.一株以喹啉为燃料的产电假单胞菌(Pseudomonas sp.) Q1的特性研究.
环境科学学报,2010,30 (6):1130–1137.
CHEN S S, ZHANG C P, LIU G L, et al. Electricity generation and quinoline degradation by an electrochemically active bacterium, Pseudomonas citronellolis strain Q1. Acta Scientiae Circumstantiae, 2010,30 (6):1130–1137. (in Chinese with English abstract) |
| [37] |
OSRIO M V, MARQUES S S, OLIVEIRA H M, et al. Fluorometric method based on molecular recognition solid-phase extraction for determination of riboflavin in milk and infant formula.
Journal of Food Composition and Analysis, 2016,45 :141–146. DOI: 10.1016/j.jfca.2015.10.007. |
| [38] |
丁巍巍, 汪家权, 吕剑, 等.微生物燃料电池处理苯酚废水.
合肥工业大学学报(自然科学版),2010,33 (1):94–96.
DING W W, WANG J Q, LV J, et al. Treatment of phenol wastewater with microbial fuel cells. Journal of Hefei University of Technology (Natural Science), 2010,33 (1):94–96. (in Chinese with English abstract) |
| [39] |
陈少华, 汪家权, 夏雪兰, 等.双室微生物燃料电池同时去除废水中的苯酚和硝酸盐.
环境工程学报,2012,6 (3):891–895.
CHEN S H, WANG J Q, XIA X L, et al. Simultaneous removal of phenol and nitrate from wastewater using a dual chamber microbial fuel cell. Chinese Journal of Environmental Engineering, 2012,6 (3):891–895. (in Chinese with English abstract) |
| [40] |
骆海萍, 张翠萍, 宋海红, 等.降解苯的微生物燃料电池产电性能研究.
中山大学学报(自然科学版),2010,49 (1):113–118.
LUO H P, ZHANG C P, SONG H H, et al. Electricity production of microbial fuel cell with biodegradation of benzene. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2010,49 (1):113–118. (in Chinese with English abstract) |
| [41] |
蒋胜韬, 管玉江, 白书立, 等.以苯酚为燃料的微生物燃料电池产电性能研究.
环境污染与防治,2013,35 (5):19–23.
JIANG S T, GUAN Y J, BAI S L, et al. Power generation from phenol degradation using microbial fuel cell. Environmental Pollution and Control, 2013,35 (5):19–23. (in Chinese with English abstract) |
| [42] |
马晨, 周顺桂, 庄莉, 等.微生物胞外呼吸电子传递机制研究进展.
生态学报,2011,31 (7):2008–2018.
MA C, ZHOU S G, ZHUANG L, et al. Electron transfer mechanism of extracellular respiration: A review. Acta Ecologica Sinica, 2011,31 (7):2008–2018. (in Chinese with English abstract) |
| [43] |
刘利丹, 肖勇, 吴义诚, 等.微生物电化学系统电子中介体.
化学进展,2014,26 (11):1859–1866.
LIU L D, XIAO Y, WU Y C, et al. Electron transfer mediators in microbial electrochemical systems. Progress in Chemistry, 2014,26 (11):1859–1866. (in Chinese with English abstract) |
| [44] |
刘敏, 邵军, 周奔, 等.微生物产电呼吸的最新研究进展.
应用与环境生物学报,2010,16 (3):445–452.
LIU M, SHAO J, ZHOU B, et al. Progress in research of microbial electricigenic respiration. Chinese Journal of Applied and Environmental Biology, 2010,16 (3):445–452. (in Chinese with English abstract) DOI: 10.3724/SP.J.1145.2010.00445. |
| [45] |
RICHTER H, NEVIN K P, JIA H F, et al. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type Ⅳ pili, and protons in extracellular electron transfer.
Energy and Environmental Science, 2009,2 (5):506–516. DOI: 10.1039/b816647a. |
| [46] |
WOŹNICA A, DZIRBA J, MAŃKA D, et al. Effects of electron transport inhibitors on iron reduction in Aeromonas hydrophila strain KB1.
Anaerobe, 2003,9 (3):125–130. DOI: 10.1016/S1075-9964(03)00059-3. |
| [47] |
FINNERAN K T, JOHNSEN C V, LOVLEY D R. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(Ⅲ).
International Journal of Systematic and Evolutionary Microbiology, 2003,53 (3):669–673. DOI: 10.1099/ijs.0.02298-0. |
| [48] |
PARK D H, ZEIKUS J G. Electricity generation in microbial fuel cells using neutral red as an electronophore.
Applied and Environmental Microbiology, 2000,66 (4):1292–1297. DOI: 10.1128/AEM.66.4.1292-1297.2000. |
| [49] |
TANG X H, DU Z W, LI H R. Anodic electron shuttle mechanism based on 1-hydroxy-4-aminoanthraquinone in microbial fuel cells.
Electrochemistry Communications, 2010,12 (8):1140–1143. DOI: 10.1016/j.elecom.2010.06.005. |
| [50] |
连静, 冯雅丽, 李浩然, 等.直接微生物燃料电池的构建及初步研究.
过程工程学报,2006,6 (3):408–412.
LIAN J, FENG Y L, LI H R, et al. Construction and preliminary studies on the direct microbial fuel cell. The Chinese Journal of Process Engineering, 2006,6 (3):408–412. (in Chinese with English abstract) |
| [51] |
RABAEY K, VERSTRAETE W. Microbial fuel cells: A novel biotechnology for energy generation.
Trends in Biotechnology, 2005,23 (6):291–298. DOI: 10.1016/j.tibtech.2005.04.008. |
| [52] |
MARSILI E, BARON D B, SHIKHARE I D, et al. Shewanella secretes flavins that mediate extracellular electron transfer.
Proceedings of the National Academy of Sciences of the USA, 2008,105 (10):3968–3973. DOI: 10.1073/pnas.0710525105. |
| [53] |
曹昌丽, 陈立香, 吴冉冉, 等.绿脓杆菌阳极的微生物燃料电池含溶解氧性能研究.
电化学,2014,20 (4):382–385.
CAO C L, CHEN L X, WU R R, et al. Effecting of dissolved oxygen on microbial fuel cells based on Pseudomonas Aeruginosa. Journal of Electrochemistry, 2014,20 (4):382–385. (in Chinese with English abstract) |
| [54] |
VON CANSTEI H, OGAWA J, SHIMIZU S, et al. Secretion of flavins by Shewanella species and their role in extracellular electron transfer.
Applied and Environmental Microbiology, 2008,74 (3):615–623. DOI: 10.1128/AEM.01387-07. |
 2016, Vol. 42
2016, Vol. 42


