| 柑橘链格孢褐斑病菌对4种新型杀菌剂敏感性评价 |
2. 浙江大学生物技术研究所, 杭州 310058
2. Institute of Biotechnology, Zhejiang University, Hangzhou 310058, China
柑橘是世界上第一大水果, 也是我国最重要的水果之一。我国是世界上最大的柑橘生产和消费国, 种植面积和产量均居世界第一。我国生产的柑橘以鲜食为主, 主要包括芸香科柑橘属中的宽皮柑橘(Citrus reticulate), 甜橙(C. sinensis)、柚(C. grandis)、柠檬(C. limon)和金橘属中的金橘(Fortunella margarita)等, 其中以宽皮柑橘所占的比重最大。柑橘链格孢褐斑病(Alternaria brown spot, ABS)由交链格孢菌橘致病型(Alternaria alternata pathotype tangerine也称A. alternata pv. citri)引起, 主要为害一些宽皮柑橘, 以及这些宽皮柑橘和葡萄柚及以和橙的杂交柑橘, 引起落叶、落果、枯梢, 不仅严重影响果实产量, 带病果实还因果面病斑而难以鲜销[1]。
链格孢褐斑病在南美洲、北美洲、澳洲、地中海国家和欧洲, 以及非洲等各大柑橘产区均有发生, 是这些国家一些地区柑橘生产中的一大限制因素[1-3]。然而, 在我国以及东南亚柑橘产区, 虽然ABS的最早记载可追溯到1995年[4], 但直到最近几年才在局部地区的一些宽皮橘上爆发, 损失严重, 从而引起关注[5-9]。已知柑橘对链格孢褐斑病的抗性是由单个隐性遗传位点控制的, 遗传位点也已获得定位, 但尚未培育出抗病品种[10]。目前链格孢褐斑病的防治主要还是依赖于化学药剂, 对一些化学防治也很难奏效的高感品种, 不得不逐渐淘汰。国外用于褐斑病防治的主要药剂有铜制剂类(如氢氧化铜等)、甲氧基丙烯酸酯类(如嘧菌酯等)、二甲酰亚胺类(如异菌脲等)和二硫代氨基甲酸盐(如代森锰锌等)类杀菌剂等[1, 11-13]。甲氧基丙烯酸酯类和二甲酰亚胺类杀菌剂由于作用位点的单一, 推广后不久, 田间即出现抗药菌系, 导致这些药剂的防效下降, 甚至失效[11, 14]。最近, 烟酰胺类杀菌剂啶酰菌胺已在美国登记用于该病害的防治[15]。
由于ABS在国内爆发成灾的历史不长, 尚未有针对该病害防治的技术储备, 也无药剂登记用于该病害的防治。因此, 开展我国柑橘褐斑病种群对常用杀菌剂的敏感性离体的评价, 对进一步研究杀菌剂的田间防效和有效防治技术的取得和推广, 以及杀菌剂抗性监测和治理的意义重大。
1 材料与方法 1.1 菌株、药剂和培养基本研究使用的54株菌株为本实验室在2010—2012年, 从有褐斑病发生的6个省(市)、自治区所采集的发病材料上分离获得[16], 菌株信息(包括地点和柑橘品种)见表 1。病料的采集是随机的, 每树只采1个样品, 被采样的病树间至少间隔10 m。分离菌株单孢纯化后, 接种于PDA(马铃薯葡萄糖琼脂培养基)斜面中, 4 ℃保存备用。
本研究所用的4种烟酰胺类杀菌剂:84.39%啶酰菌胺, 由德国巴斯夫股份有限公司提供; 98%氟酰胺, 由泰州百力化学股份有限公司提供; 89%噻氟酰胺, 由浙江博仕达作物科技有限公司提供; 98%氟啶胺原药由浙江禾田化工有限公司提供。实验所用的显色剂刃天青(resazurin, RZ)购自Sigma公司(Sigma-Aldrich, St. Louis, USA), 使用时先配置成5 mmol/L的母液, 在4 ℃冰箱中保存, 使用时稀释到400 μmol/L。4种杀菌剂的原药分别溶于丙酮, 配成0.01 mg/mL的母液, 4 ℃保存, 备用。
实验所用培养基为完全培养基(CM), 其配方(1 000 mL)为胰蛋白胨10 g, 酵母浸膏5 g, K2HPO4 3 g, 葡萄糖1 g, 蒸馏水1 000 mL, 调整pH至7;V8培养基(果汁200 g, CaCO3 3.0 g, 琼脂20.0 g, 蒸馏水1 000 mL, pH 5.8)。
1.2 分生孢子的诱导和孢子悬浮液配制将菌丝块从PDA斜面中转移到含有V8培养基的平板上, 25 ℃、16 h光照和8 h的黑暗条件下培养7~10 d, 诱导分生孢子的形成。待产生足够的孢子后, 在培养皿中加适量的无菌水, 并用消毒后的三角棒轻轻刮擦菌落, 促使孢子的脱落, 孢子悬浮液经4层纱布过滤, 以去除菌丝。所获得孢子悬浮液, 经离心浓缩和加无菌水稀释, 并通过血球计数器调整到孢子数105个/mL。
1.3 孢子萌发对药剂的敏感性实验在96孔细胞培养板(Corning Inc. USA)中进行, 根据之前的实验, 筛选明确最优的反应条件是:培养基为CM培养基, 显色剂RZ至最终为不超过40 μmol/L, 孢子数为105/mL。在培养板的每孔中, 首先加入已经添加不同浓度药剂的100 μL的CM培养基(培养基中药剂的最终质量浓度分别为0.001 μg/mL、0.01 μg/mL、0.05 μg/mL、0.1 μg/mL、0.5 μg/mL、1 μg/mL和10 μg/mL), 然后添加80 μL的105个/mL孢子悬浮液, 最后添加20 μL的400 μmol/L的RZ染料, 使得RZ的最终浓度达到40 μmol/L。每一处理重复3次。对照孔以添加无菌水代替孢子悬浮液, 其他成分不变。添加好的培养板先用保鲜膜覆盖, 再盖上培养板盖子, 在温度为28 ℃、转速为400 r/min的摇床中振荡培养24 h。然后采用酶标仪(Bio-Tek, Instruments, Inc. USA)在570 nm和600 nm波长下测量光吸收值。整个实验重复2次。
1.4 数据处理数据处理主要参考Vega,等[13]的方法进行。RZ还原值根据以下公式进行计算:
| $ \text{R}{{\text{Z}}_{\text{reduction}}}=\frac{{{\text{ }\!\!\varepsilon\!\!\text{ }}_{\text{ox}}}{{\text{ }\!\!\lambda\!\!\text{ }}_{2}}D{{\text{ }\!\!\lambda\!\!\text{ }}_{1}}-{{\text{ }\!\!\varepsilon\!\!\text{ }}_{\text{ox}}}{{\text{ }\!\!\lambda\!\!\text{ }}_{1}}D{{\text{ }\!\!\lambda\!\!\text{ }}_{2}}}{{{\text{ }\!\!\varepsilon\!\!\text{ }}_{\text{red}}}{{\text{ }\!\!\lambda\!\!\text{ }}_{\text{1}}}{D}'{{\text{ }\!\!\lambda\!\!\text{ }}_{\text{2}}}-{{\text{ }\!\!\varepsilon\!\!\text{ }}_{\text{red}}}{{\text{ }\!\!\lambda\!\!\text{ }}_{\text{2}}}{D}'{{\text{ }\!\!\lambda\!\!\text{ }}_{\text{1}}}}\times 100. $ |
其中:εox=染料在氧化过程中的相关系数(蓝色:570 nm=80 568;600 nm=117 216);εred=染料在还原过程中的相关系数(粉红色:570 nm=155 677;600 nm=14 652);D=处理中光吸收值, D′=阴性对照的光吸收值; λ1=570 nm; λ2=600 nm。
由于RZ显色反映了真菌生长代谢活力, 所以染料的还原值可以和真菌的呼吸速率对应。以药剂浓度为lc, 染料还原值为RZlc, 根据SigmaPlot软件公式: RZlc=ae-b×lc计算染料还原值。其中:a为lc的初始值, b为斜率, a、b值均可由软件得出。有效中浓度EC50为还原值在对照50%情况下的杀菌剂浓度。以EC50对数值为横坐标, 菌株分布频率为纵坐标, 绘制频率分布柱状图。在杀菌剂-病菌的组合中, EC50对数值的分类间隔值(柱形图中单根柱的宽度)按Scott的公式:HN=3.49SN-1/3, 其中:H为分类间隔值(bin width); S为EC50对数值的标准偏差(standard deviation); N为测定的菌株数量。求取分类间隔值后, 依据EC50对数值的分布范围确定杀菌剂-病菌的组合中柱形图的柱体数量[17]。
用SPSS 16.0软件对EC50值作正态分布检验。当P>0.05, 视为符合正态分布;反之则视为非正态分布。根据野生敏感型病原群体对药剂敏感性为正态分布的原理, 当EC50值处于正态分布时, 孢子萌发速率测定法得到的各自EC50值, 即分别为它们对药剂的敏感基线。根据建立的敏感性分布曲线确定病原菌对4种药剂的平均EC50值, 明确抗性指数(抗性指数=供试菌株EC50值/菌株的平均EC50值)。
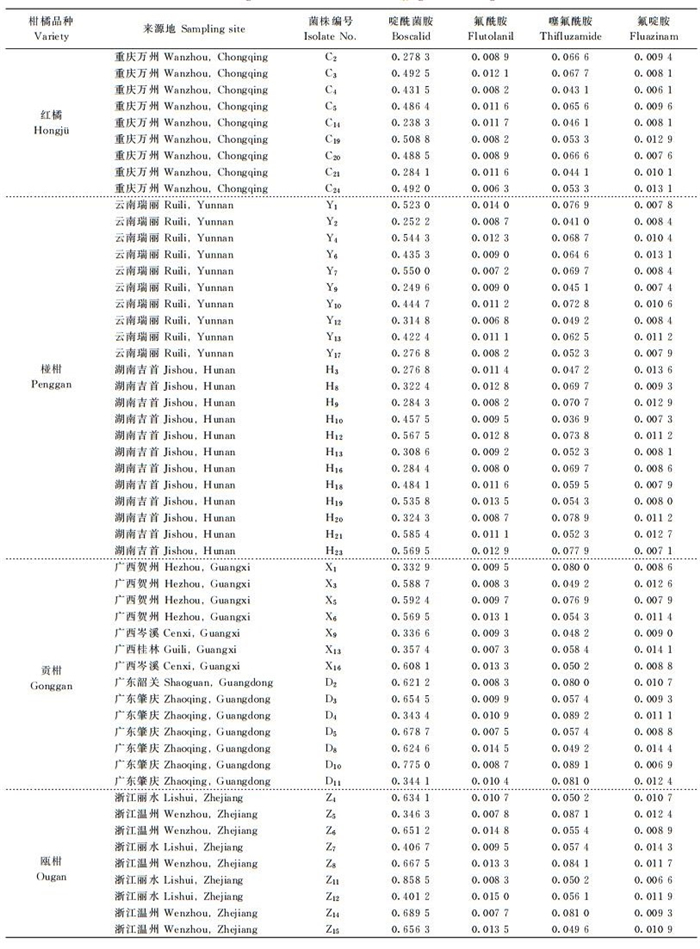
2 结果与分析 2.1 对啶酰菌胺的敏感性54株链格孢褐斑病菌菌株孢子萌发和幼菌丝生长对啶酰菌胺的敏感性(EC50值)测定结果(表 1)可知, 最不敏感, 即EC50值最大的菌株(Z11)来自浙江瓯柑, 其EC50值为0.858 5 μg/mL, 最小的菌株为0.238 3 μg/mL,来自重庆的红橘(C14), 其平均值为(0.467 7±0.149 0) μg/mL, 抗性指数的分布范围为0.509 5~1.835 6。经SPSS软件的W法正态检测得W=0.952, P=0.061>0.05, 表明这54个菌株对啶酰菌胺的敏感性频次分布符合正态分布, 呈连续的单峰曲线(图 1A), 我国链格孢褐斑病菌的群体为啶酰菌胺野生敏感种群, 实验所得54株菌株的平均EC50值(0.467 7 μg/mL)可作为褐斑病菌对啶酰菌胺的敏感基线。
 |
| 柱的高度代表该区间的菌株总数, 柱宽度(分类间隔值, 啶酰菌胺为0.134 8、氟酰胺为0.089 6、噻氟酰胺为0.089 6、氟啶胺为0.089 6)按Scott公式计算获得。 Bar height indicates the total number of isolates within each bin, and bin width (boscalid is 0.134 8, flutolanil is 0.089 6, thifluzamide is 0.089 6, fluazinam is 0.089 6) was based on Scott's method. 图1 柑橘链格孢褐斑病菌种群孢子萌发对啶酰菌胺(A)、氟酰胺(B)、噻氟酰胺(C)和氟啶胺(D)的敏感性频次分布 Fig. 1 Frequency distribution of median effective concentration (EC50) of boscalid (A), flutolanil (B), thifluzamide (C), fluazinam (D) to inhibit spore germination and young mycelia of Alternaria alternata from citrus in China |
氟酰胺抑制各褐斑病菌菌株孢子萌发和幼菌丝生长的中浓度EC50值见表 1。EC50值最高的菌株来自浙江的瓯柑(Z12), 其EC50值为0.015 0 μg/mL, 最小为0.006 3 μg/mL, 来自重庆的红橘(C24), 平均值为(0.010 3±0.002 3) μg/mL, 抗性指数范围为0.611 7~1.456 3。经SPSS软件的W法正态检测得W=0.966, 0.123=P>0.05, 说明这54个菌株对氟酰胺的敏感性频次分布符合正态分布, 呈连续的单峰曲线(图 1B), 实验测得54个株菌株的平均EC50值(0.010 3 μg/mL)可作为褐斑病菌对氟酰胺的敏感基线。
| 表1 菌株来源及各菌株孢子萌发对4种杀菌剂的EC50值 Table 1 Origins of the isolates and their EC50 against four fungicides |
 |
| 点击放大 |
54株链格孢褐斑病菌孢子萌发和幼菌丝生长对噻氟酰胺的EC50值范围为0.036 9~0.089 2 μg/mL, 最敏感菌株(EC50值最小)来自湖南的椪柑(H10), 最不敏感的菌株来自广东的贡柑(D4), 平均值为(0.061 9±0.013 8) μg/mL, 抗性指数范围为0.596 1~1.441 0。经SPSS软件的W法正态检测得W=0.967, 0.138=P>0.05, 说明这54个菌株对噻氟酰胺的不同敏感性频次分布也符合正态分布, 分布呈连续的单峰曲线(图 1C), 其平均EC50值(0.061 9 μg/mL)可作为我国柑橘褐斑病菌对噻氟酰胺的敏感基线。
2.4 对氟啶胺的敏感性54株柑橘褐斑病菌菌株对氟啶胺的EC50值最大为0.014 4 μg/mL, 来自广东贡柑(D8)最小为0.006 1 μg/mL, 来自重庆红橘(C4)平均值为(0.010 0±0.002 2) μg/mL, 抗性指数范围为0.610 0~1.440 0 (表 1)。经SPSS软件的W法正态检测得W=0.966, 0.128=P>0.05, 说明我国柑橘褐斑病菌种群对氟啶胺敏感性频次分布符合正态分布, 呈连续单峰曲线(图 1D), 种群为氟啶胺敏感的野生种群, 其平均EC50值(0.010 0 μg/mL)可作为我国柑橘褐斑病菌对氟啶胺的敏感基线。
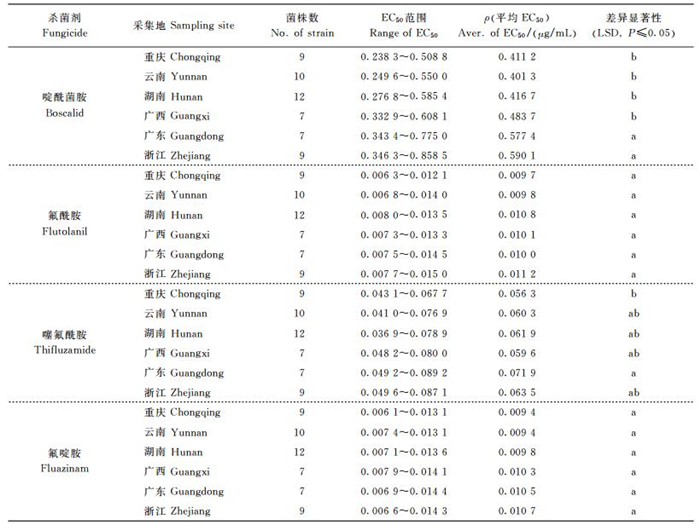
2.5 柑橘褐斑病菌对4种药剂的敏感性比较本研究测定的4种杀菌剂中, 以氟啶胺和氟酰胺抑制柑橘褐斑病菌孢子萌发的效果最佳, 其EC50值分别为0.010 0 μg/mL和0.010 3 μg/mL;其次是噻氟酰胺, EC50值为0.061 9 μg/mL;而啶酰菌胺的抑制效果最差, EC50值为0.467 7 μg/mL。
2.6 菌株地理来源与药剂敏感性的关系柑橘褐斑病菌对啶酰菌胺的敏感性与地理来源相关, 广东和浙江菌株的敏感性较差, 而重庆、湖南、云南和广西菌株对啶酰菌胺较敏感(表 2)。重庆菌株较其他地理来源的菌株对噻氟酰胺更敏感。菌株对氟酰胺和氟啶胺的敏感性与地理来源无关(表 2)。
| 表2 不同地区柑橘褐斑病菌对4种药剂的敏感性比较 Table 2 Susceptibility of Alternaria alternata from citrus in different provinces of China to four fungicides |
 |
| 点击放大 |
菌丝生长和孢子萌发抑制法是常用的杀菌剂敏感性离体测定方法, 通常工作量大, 测定受人为因素影响较大。刃天青是一种稳定的水溶性染料, 对细胞无毒, 当进入细胞后, 在线粒体酶作用下可发生氧化还原反应, 颜色从蓝色(氧化态)转变成粉红色(还原态), 其颜色变化程度可反映病菌细胞代谢活性强弱和增殖状况, 而颜色变化可通过定性或者定量荧光方法检测获得。因此, 刃天青显色法已被广泛用于医学上的结核杆菌和部分真菌对药剂敏感性评价[18-19]。VEGA,等[13]建立了基于刃天青显色的柑橘褐斑病菌种群对嘧菌酯的敏感性快速评价方法, 本文应用体系测定了我国柑橘褐斑病菌种群对4种新型杀菌剂的敏感性。结果表明我国柑橘褐斑病菌种群均是新型烟酰胺类杀菌剂啶酰菌胺、氟酰胺和噻氟酰胺的野生敏感群体, 其抑制孢子萌发和幼菌丝生长的EC50值分别为0.467 7 μg/mL、0.010 3 μg/mL和0.061 9 μg/mL, 来自浙江和广东的菌株对啶酰菌胺敏感性较来自其他地区的低, 而对氟酰胺和氟啶胺的敏感性则与菌株的来源无关。本研究还证明我国柑橘褐斑病菌种群也是氟啶胺的敏感种群, 其平均EC50值为0.010 0 μg/mL, 菌株对氟啶胺的敏感性与地理来源无关。本实验所选择的4种新型药剂有望作为防治柑橘褐斑病的候选药剂, 可对其开展使用技术的研究, 继而推广应用。
啶酰菌胺、氟酰胺和噻氟酰胺均属于新型烟酰胺类杀菌剂, 其靶标位点为病原菌线粒体呼吸电子传递链上的蛋白复合体Ⅱ, 即琥珀酸脱氢酶(succinate dehydrogenase, SDH)或琥珀酸-泛醌还原酶(succinate ubiquinone reductase, SQR), 通过杀菌剂与辅酶Q位点的结合, 阻断电子由铁硫中心向辅酶Q的传递, 从而干扰真菌的呼吸作用, 阻碍其能量代谢, 抑制病原菌的生长, 导致其死亡[20]。新型烟酰胺类杀菌剂的杀菌谱较广, 对白粉病、灰霉病、褐腐病和菌核病等多种作物真菌性病害非常有效。由于其作用机制的独特, 这类杀菌剂与常见的杀菌剂无交互抗性, 已广泛用于果树、蔬菜和大田作物等病害的防治[21]。在美国, 啶酰菌胺已被登记用于柑橘褐斑病的防治[15]。然而, 柑橘褐斑病菌种群对氟酰胺和噻氟酰胺的敏感性尚无研究报道。氟啶胺属于吡啶胺类的一种保护性杀菌剂, 已有研究表明其杀菌谱广、持效期长、耐雨水冲刷, 低环境风险, 对葡萄孢霉属真菌引起的病害具有良好的防治效果, 广泛用于防治多种作物的灰霉病[22]。目前国内外尚无柑橘褐斑病菌对氟啶胺敏感性的研究报道。柑橘链格孢褐斑病最近在我国一些重要柑橘品种上爆发成灾, 造成严重损失, 而且发生面积在逐年扩大, 已成为我国柑橘植保中的新问题而引起关注[5-9, 23]。本研究评价我国褐斑病菌种群对这4种药剂的敏感性, 为有选择性地开展田间药效实验, 以及应用后定期监测我国柑橘褐斑病菌种群对啶酰菌胺、氟酰胺和噻氟酰胺的抗性提供基础研究。
值得重视的是, 由于作用位点的单一, 病菌对新烟酰胺类杀菌剂极易产生抗性, 国内外的应用实践也证明了这个事实, 抗性分子机制是编码琥珀酸脱氢酶基因的某个亚基基因发生了点突变[20, 24-29]。因此, 在使用这些药剂时应重视其抗性风险, 提倡与其他作用机制的杀菌剂混用、轮用, 避免连续使用, 并限制每个生长季节的使用次数。同时, 定时监测抗药性群体的变化动态, 以便及时调整用药方案。
| [1] | TIMMER L W, PEEVER T L, SOLEL Z, et al. Alternaria diseases of citrus: Novel pathosystems. Phytopathologia Mediterranea, 2003, 42(2): 99-112. |
| [2] | PEEVER T L, IBANEZ A, AKIMITSU K, et al. Worldwide phylogeography of the citrus brown spot pathogen, Alternaria alternata. Phytopathology, 2002, 92(7): 794-802. DOI:10.1094/PHYTO.2002.92.7.794 |
| [3] | PEEVER T L, SU G, CARPENTER-BOGGS L, et al. Molecular systematics of citrus-associated Alternaria species. Mycologia, 2004, 96(1): 119-134. DOI:10.2307/3761993 |
| [4] |
俞立达, 崔伯发. 柑橘病虫害彩色图谱. 北京: 中国农业出版社, 1995: 15. YU L D, CUI B F. The Color Map of Plant Diseases and Insect Pests on Citrus. Beijing: China Agriculture Press, 1995: 15. (in Chinese with English abstract) |
| [5] | WANG X F, LI Z A, TANG K Z, et al. First report of Alternaria brown spot of citrus caused by Alternaria alternata in Yunnan Province, China. Plant Disease, 2010, 94(3): 375-375. |
| [6] |
陈昌胜, 黄峰, 程兰, 等. 红橘褐斑病病原鉴定. 植物病理学报, 2011, 41(5): 449-455. CHEN C S, HUANG F, CHENG L, et al. Identification of the pathogenic fungus causing brown spot on tangerine (Citrus reticulata cv. Hongjü). Acta Phytopathologica Sinica, 2011, 41(5): 449-455. (in Chinese with English abstract) |
| [7] |
黄峰, 朱丽, 侯欣, 等. 瓯柑褐斑病病原鉴定. 浙江农业科学, 2012(9): 1281-1282. HUANG F, ZHU L, HOU X, et al. Identification of the pathogenic fungus causing brown spot on tangerine (Citrus reticulata cv. Ougan). Journal of Zhejiang Agricultural Sciences, 2012(9): 1281-1282. (in Chinese with English abstract) |
| [8] |
阳廷密, 邓明学, 王明召, 等. 贡柑(皇帝柑)疑似"急性性炭疽病"的病原鉴定. 南方园艺, 2011, 22(5): 30-32. YANG T M, DENG M X, WANG M Z, et al. Identification of the pathogenic fungus causing suspected acute anthracnose on Gongan. Southern Horticulture, 2011, 22(5): 30-32. (in Chinese with English abstract) |
| [9] |
赵圆, 王玲杰, 王雪峰, 等. 杂柑褐斑病的病原鉴定. 果树学报, 2014, 31(2): 292-295. ZHAO Y, WANG L J, WANG X F, et al. Identification of the pathogenic fungus causing brown spot in two tangerine hybrid varieties. Journal of Fruit Science, 2014, 31(2): 292-295. (in Chinese with English abstract) |
| [10] | CUENCA J, ALEZA P, VICENT A, et al. Genetically based location from triploid populations and gene ontology of a 3.3-Mb genome region linked to Alternaria brown spot resistance in citrus reveal clusters of resistance genes. PLoS ONE, 2013, 8(10): e76755. DOI:10.1371/journal.pone.0076755 |
| [11] | SOLEL Z, TIMMER L W, KIMCHI M. Iprodione resistance of Alternaria alternate pv. citri from Minneola tangelo in Israel and Florida. Plant Disease, 1996, 80(3): 291-293. DOI:10.1094/PD-80-0291 |
| [12] | SOLEL Z, OREN Y, KIMCHI M. Control of Alternaria brown spot of Minneola tangelo with fungicides. Crop Protection, 1997, 16(7): 659-664. DOI:10.1016/S0261-2194(97)00042-2 |
| [13] | VEGA B, LIBERTI D, HARMON P F, et al. A rapid resazurin-based microtiter assay to evaluate QoI sensitivity for Alternaria alternata isolates and their molecular characterization. Plant Disease, 2012, 96(9): 1262-1270. DOI:10.1094/PDIS-12-11-1037-RE |
| [14] | VEGA B, DEWDNEY M M. Distribution of QoI resistance in populations of tangerine-infecting Alternaria alternata in Florida. Plant Disease, 2014, 98(1): 67-76. DOI:10.1094/PDIS-04-13-0449-RE |
| [15] | VEGA B, DEWDNEY M M. Sensitivity of Alternaria alternata from citrus to boscalid and polymorphism in the iron-sulfur and in anchored membrane subunits of succinate dehydrogenase. Plant Disease, 2015, 99(2): 231-239. DOI:10.1094/PDIS-04-14-0374-RE |
| [16] | HUANG F, FU Y S, NIE D N, et al. Identification of a novel phylogenetic lineage of Alternaria alternata causing citrus brown spot in China. Fungal Biology, 2015, 119(5): 320-330. DOI:10.1016/j.funbio.2014.09.006 |
| [17] | SCOTT D W. On optimal and data-based histograms. Biometrika, 1979, 66(3): 605-610. DOI:10.1093/biomet/66.3.605 |
| [18] | RAMPERSAD S N, TEELUCKSINGH L D. Differential responses of Colletotrichum gloeosporioides and C. truncatum isolates from different hosts to multiple fungicides based on two assays. Plant Disease, 2012, 96(10): 1526-1536. DOI:10.1094/PDIS-10-11-0906-RE |
| [19] | FAI P B, GRANT A. A rapid resazurin bioassay for assessing the toxicity of fungicides. Chemosphere, 2009, 74: 1165-1170. DOI:10.1016/j.chemosphere.2008.11.078 |
| [20] | AVENOT H F, MICHAILIDESI T J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Protection, 2010, 29(7): 643-651. DOI:10.1016/j.cropro.2010.02.019 |
| [21] |
李良孔, 袁善奎, 潘洪玉, 等. 琥珀酸脱氢酶抑制剂类(SDHIs)杀菌剂及其抗性研究进展. 农药, 2011, 50(3): 165-169. LI L K, YUAN S K, PAN H Y, et al. Progress in research on SDHIs fungicides and its resistance. Agrochemicals, 2011, 50(3): 165-169. (in Chinese with English abstract) |
| [22] |
晓岚. 新杀菌剂氟啶胺的生物学活性. 世界农药(原名农药译丛), 1996, 18(1): 43-48. XIAO L. The biological activity of new fungicide fluazinam. World Pesticides, 1996, 18(1): 43-48. (in Chinese with English abstract) |
| [23] |
李鸿筠, 姚廷山, 王联英, 等. 重庆万州红桔褐斑病田间防治药剂的筛选. 中国南方果树, 2013, 42(3): 52-54. LI H J, YAO T S, WANG L Y, et al. The field fungicides screening on controlling Alternaria brown spot of Wanzhou, Chongqing. South China Fruits, 2013, 42(3): 52-54. (in Chinese with English abstract) |
| [24] | AVENOT H F, MICHAILIDES T J. Resistance to boscalid fungicide in Alternaria alternata isolates from pistachio in California. Plant Disease, 2007, 91(10): 1345-1350. DOI:10.1094/PDIS-91-10-1345 |
| [25] | AVENOT H F, SELLAM A, KARAOGLANIDIS G, et al. Characterization of mutations in the iron-sulphur subunit of succinate dehydrogenase correlating with boscalid resistance in Alternaria alternata from California pistachio. Phytopathology, 2008, 98(6): 736-742. DOI:10.1094/PHYTO-98-6-0736 |
| [26] | ISHII H, FOUNTAINE J, MIYAMOTO T, et al. Occurrence of a mutation in the succinate dehydrogenase gene found in some isolates of cucumber Corynespora leaf spot fungus resistant to boscalid. Annals of the Phytopathological Society of Japan, 2008, 74: 38-39. |
| [27] | MIYAMOTO T, ISHII H, STAMMLER G, et al. Distribution and molecular characterization of Corynespora cassiicola isolates resistant to boscalid. Plant Pathology, 2010, 59: 873-881. DOI:10.1111/ppa.2010.59.issue-5 |
| [28] | STEVENSON K L, LANGSTON D B, SANDERS F. Baseline sensitivity and evidence of resistance to boscalid in Didymella bryoniae. Phytopathology, 2008, 98: S151. |
| [29] | MIAZZI M M, MCGRATH M T. Sensitivity of Podosphaera xanthii to registered fungicides and experimentals in GA and NY, USA, in 2007. Journal of Plant Pathology, 2008, 90: 90. |
 2016, Vol. 42
2016, Vol. 42


