| Description of two new records of genus Longidorus (Nematoda: Dorylaimida) in China |
The genus Longidorus established by Micoletzky in 1922, consist of a number of migratory ectoparasitic nematodes that are polyphagous by nature[1]. In general, Longidorid species are in cosmopolitan distribution, and several species in the genus are important as vector of viruses and considered species specific for types of virus that can transmit[2-3]. The distribution and host association of increasing numbers of described species are precisely determined, which gradually received a great attention in the taxonomic community around the world[4]. Several studies of Longidorus with regards to distribution, occurrence and their associated type hosts had been published worldwide. It was described three new species from beech and spruce forest in Slovakia[5], three species recorded from fruit orchards of Bohemia and South Moravia in Czech Republic[6] and 158 Longidorus species from forest nurseries and riparian habitat in Bulgaria[7-8].
In China, 13 Longidorus species have already been recorded or described[9], including L. hangzhouensis Zheng et al. 2001, L. camelliae Zheng et al. 2000, L. fangi Xu & Cheng 1991, L. henanus Xu & Cheng 1992, L. litchii Xu & Cheng 1992, L. jiangsuensis Xu & Hooper 1990, L. fursti Heyns et al. 1987, L. jonesi Siddiqi 1962, L. pawneensis Luc & Coomans 1988, L. macromucronatus Siddiqi 1962, L. martini Merny 1966 and L. moniloides Heyns 1966, L. pisi Edward et al. 1964.
During the nematode survey conducted from natural vegetation in China, two unrecorded Longidorus species were detected in various deciduous trees associated with aralia and apricot in Hangzhou and Wuhan described herein as L. pius and L. diadecturus, respectively. This paper will lay emphasis on the first record of two Longidorus species occurring in China. Characterization based on morphology, rDNA sequences and phylogenetic relationships of L. pius and L. diadecturus are presented for the first time in Chinese populations.
1 Materials and methods 1.1 Extraction and morphological examinationNematodes were extracted from rhizosphere soil of host plants by decanting and sieving technique[10], heat killed at 55-60 ℃ for 2 min and fixed with V(formaldehyde) : V(glycerin)=4: 1 solution[11]. Specimens were processed and mounted temporarily on a slide for further morphometric assessment and photomicrography. Photographs were taken using Leica DM5000B compound microscope operated with a digital camera, while morphological measurements were made using a LAS tool provided from Leica software package. All measurements were presented in micrometers (μm), except for body length (L) in mm, and data were expressed as mean standard ± deviation.
1.2 Total DNA extractionExtraction of nematode DNA was prepared from single adult female and carefully transferred into a glass slide containing 13 μL distilled H2O, and the nematode was cut into fragments using a sterilized scalpel. The nematode pieces were pipetted up to 10 μL and placed to small Eppendorf tubes, added with 8 μL Mg2+ free buffer and 2 μL proteinase K[11]. Samples were briefly centrifuged at 13 000 r/min for 3 min and immediately frozen at -70 ℃ for at least 30 min to overnight or stored in liquid nitrogen for 1 min. Each tube was incubated at 58 ℃ for 3 h, afterwards added with 2 μL proteinase K and incubated again at 95 ℃ for 10 min. Finally, the DNA suspensions were cooled down at 4 ℃ and stored at -20 ℃[12].
1.3 Polymerase chain reaction (PCR) and sequencingFragments of ITS1 and 28S region were amplified using two sets of primers V1 (TTGATTACGTCCCTG CCCTTT) [13] and 5.8S (ACGAGCCGAGTGATCCAC CG) [14], D2A (5′-ACAAGTACCGTGAGGGAAAGT TG-3′) and D3B (5′-TCGGAAGGAACCAGCTAC TA-3′) [15], respectively. PCR thermal protocol was carried out under the following conditions[16]: one cycle of 94 ℃ for 2 min, followed by 35 cycles of 94 ℃ for 30 s, annealing temperature of 57 ℃ for 45 s, extension of 72 ℃ for 3 min and a final extension of 72 ℃ for 10 min. After DNA amplification, 2.5 μL aliquots of PCR products were analyzed by gel electrophoresis in 1% agarose gel (100 V, 400 mA, 30 min) stained with DuRed 10 000× stain (Cat#D009-500) and the DNA was visualized under UV illumination. The amplified DNA was purified according to TaKaRa DNA fragment purification kit version 4.0 (catalogue No. 9761) from TaKaRa Clontech Bio Inc., China. The purified DNA was ligated to pUCM-T vector and transformed into DH 5alpha competent cells, and the transformants were screened on an ampicillin agar LB plates at 37 ℃ overnight. White colonies were selected and transferred to 5 mL LB containing 100 μg/μL ampicillin and incubated at 37 ℃ for 16-24 h. PCR amplification was further confirmed with the primer insertion and expected band. Sequencing was carried out in Shanghai Sangon Biotechnology Co. Ltd. The obtained sequences were submitted to GenBank for further comparison of closely related species. The sequences were analyzed and aligned using ClustalW program of the MEGA 5.0[17-18].
1.4 Phylogenetic analysisPhylogenetic reconstruction was made from closely related Longidorus sequences available from GenBank. Nematode species and GenBank accession numbers were listed for each taxon in phylogenetic trees. The newly obtained and published sequences for each gene were aligned using ClustalW software with default parameter values. Phylogenetic analysis of the sequencing data sets were performed by maximum likelihood method using 1 000 bootstraps utilized in MEGA 5.0 software and visualized in Tree Explorer.
2 Results 2.1 Systematics 2.1.1 Longidorus pius Barti & Lamberti, 2001Its morphometric characters and photomicrographs are shown in Table 1 and Fig. 1.
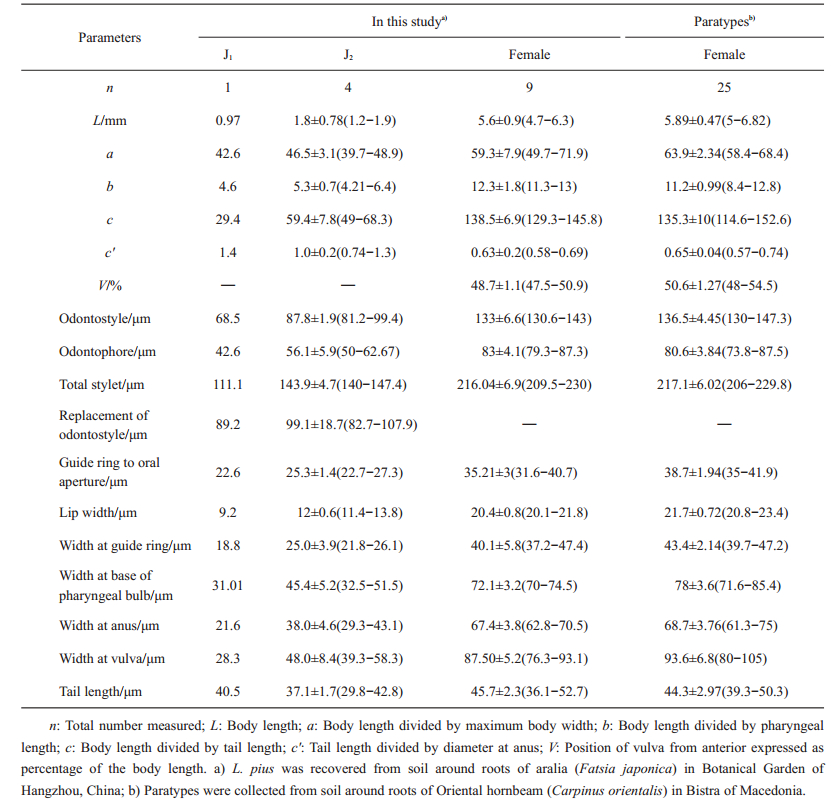
| Table 1 Morphometric comparisons of Longidorus pius from Hangzhou of China with the paratype specimens from Macedonia |
 |
| 点击放大 |
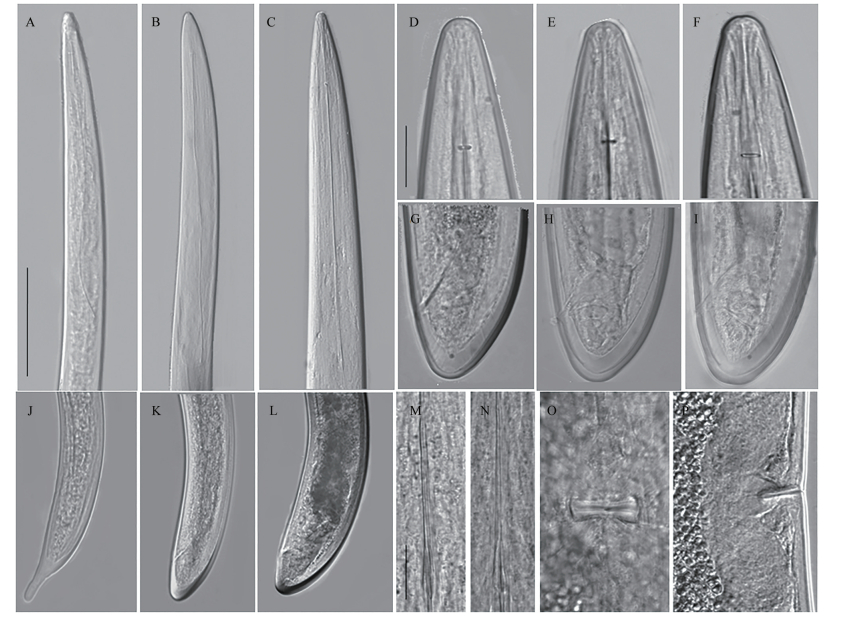 |
| (A-C): Head regions of J1, J2 and female, respectively; (D-F): Lip area and guide ring position of female; (G-I): Tail of female; (J-L): Tail shape of J1, J2 and female, respectively; (M-N): Odontophore; O: Vulva in ventral position; P: Vulva in sub-lateral position. Scale bars: A-C, JL=20 μm; D-I, M-P=5 μm. Fig. 1 Photomicrograph of Longidorus pius recovered from aralia tree (Fatsia japonica) in Hangzhou, Zhejiang Province |
Habitus ventrally curves at posterior. Body robust, medium length (4.7-6.3 mm), gradually tapering towards head apex. Cuticle smooth sometimes with fine transverse striae usually visible at the tail terminus. Lip area continuous with the rest of the body tapering anteriorly (Fig. 1 D-F). Odontostyle 130-143 μm while odontophore 79-87 μm long. Guiding ring 31-40 μm from oral aperture (Fig. 1E). Pharynx measures 112-138 μm long and 30-35 μm wide. Three glandular nuclei present. Oesophago-intestinal valve cylindrical. Vulva a transverse slit, positioned utmost mid-body, vagina extending 40%-60% of corresponding body diameter. Genital branch amphidelphic are equally the same length and structure, and ovaries reflexed. Tail bluntly conoid with terminus rounded to almost hemispherical (Fig. 1 G-I), and two pairs of caudal pores visible. The code for identifying the species from the polytomous key[19] is A56-B4-C3-D1-E1-F3-G1-H1-I1.
2.1.1.2 JuvenileJuveniles are separated into four stages, but only two developmental stages are recovered. First stage (J1) in short body length (> 1 mm) with digitate tail (Fig. 1J). Replacement of odontostyle (RoS) position in J1 is embedded at the basal wall of odontophore, while J2 gradually positions far from the odontophore base, and the length of RoS becomes longer along with body size at each successive stage. Tail shape varies from digitate (J1) to conoid or rounded-conoid until reaching adult (Fig. 1 J-L).
2.1.1.3 Locality and hostJuveniles and adult female of L. pius were recovered from soil around roots of aralia (Fatsia japonica) cultivated in Hangzhou, Zhejiang Province (22°2′14″ N and 110°18′32″ E).
2.1.1.4 Differential diagnosisL. pius is a medium size nematode. Lip area continuous and rounded, odontostyle long. Tail short and rounded. The species has four juvenile stages, where J1 has digitate tail. The code for identifying the species based in polytomous key is A56-B4-C3-D1-E1-F3-G1-H1-I1. L. pius is most similar to L. picenus Roca et al. 1985 and L. nevesi Macara 1986, but differs in the pocket shape of amphid without basal lobes. L. pius has lower a (49-71 vs 80-90) and c values (129-145 vs 155-201) compared to L. picenus. Furthermore, L. pius has shorter body length (4.7-6.3 mm vs 8 mm), lower a ratio (49-71 vs 72-100) and almost hemispherical or pointed lip compared to L. nevesi.
2.1.2 Longidorus diadecturus Eveleigh & Allen, 1982Its morphometric characters and photomicrographs are shown in Table 2 and Fig. 2.
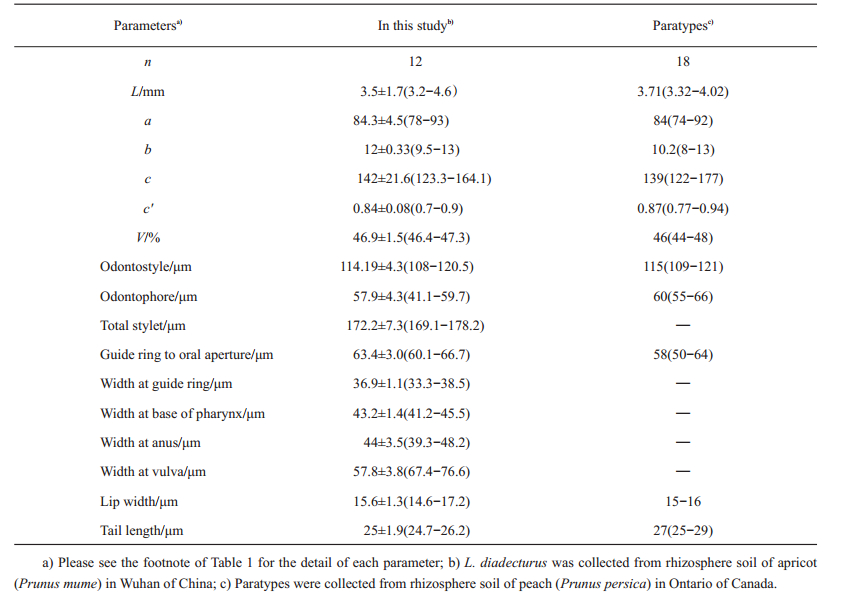
| Table 2 Morphometric comparisons of Longidorus diadecturus from Wuhan of China with the paratype specimens from Canada |
 |
| 点击放大 |
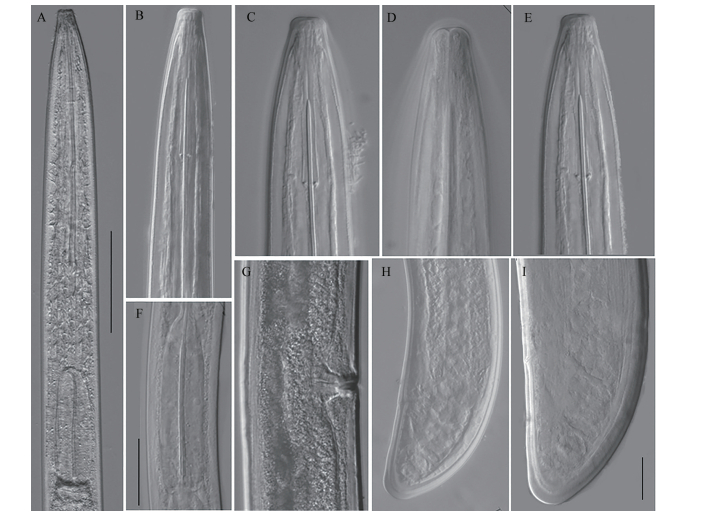 |
| (A-B): Female anterior region; (C-E): Lip area and position of guide ring; F: Pharyngeal bulb; G: Vulva; (H-I): Tail of female. Scale bars: A=50 μm; B, F, H=20 μm; C-E, G, I=10 μm. Fig. 2 Photomicrographs of Longidorus diadecturus recovered from apricot tree (Prunus mume) in Wuhan, Hubei Province |
Body ventrally arcuate varying in degree of curvature at posterior region upon heat relaxation. Cuticle in two distinct layers, inner layer thickened towards tail terminus. Lip region round (13-14 μm), head expanded and set off from body by a slight depression (Fig. 2 C-E). Odontostyle slender with the length of 108-120 μm, and odontophore 41-59 μm long. Stylet guiding ring 60-66 μm positioned posterior from head apex (Fig. 2E). Anterior part of pharynx looped and bulb hemispherical about 368 μm long, and basal bulb occupying about 20% of the total length (Fig. 2F). Vulva transverse slit with thickened labia (Fig. 2G). Ovaries paired and reflexed extending 230 μm anterior and 221 μm posterior to vulva. Tail dorsally convex (24-26 μm) with bluntly rounded terminus (Fig. 2 H-I) and visible pairs of caudal pores. The codes for identifying the species from the polytomous key is A4-B23-C5-D3-F2-G2-H1-I1.
2.1.2.2 Locality and hostAdult females of L. diadecturus were recovered from rhizosphere soil of apricot tree (Prunus mume) in Wuhan, Hubei Province (30°35′09″ N and 120°37′ 60.3″ E).
2.1.2.3 Differential diagnosisL. diadecturus is distinguishable from other Longidorus species by having a posterior position of guiding ring (60-66 μm) from oral aperture. L. diadecturus showed closest similarity to L. macromucronatus Siddiqi 1962, L. jonesi Siddiqi 1962, L. martini Merney 1966, and can be differentiated from L. macromucronatus by shorter length of corpal mucro (1-2 μm vs 5 μm), shape of mucro (sagittate vs conoid), length of odontophore (41-59 μm vs 67-77 μm), and smaller c value (123-164 vs 190-230). L. diadecturus differs from its head slightly sets off compared to continuous in L. jonesi, longer odontostyle (108-120 μm vs 100 μm) and more anteriad position of vulva than L. martini.
2.2 Molecular characterization and phylogenyRibosomal products of ITS1 and 28S region indicated a fragment length of approximately 1 474 bp and 789 bp in L. pius and 1 188 bp and 776 bp in L. diadecturus, respectively. Amplicon of ITS1 region of L. pius from China showed 99% sequence similarity with type population L. pius (AM743178) and several type clones (AM743180-AM743184) from Macedonia. Similarly, L. diadecturus from China showed 99% sequence similarity with L. diadecturus type population (AF511416) from Canada. Meanwhile, 28S region of L. diadecturus from China showed 99% sequence similarity with L. diadecturus (AY601584) from Canada, while L. pius showed 99% resemblance to L. raskii (AJ549983-AJ549984) and Longidorus sp. FDL-2011 (FR775757-FR7757560). Phylogenetic relationship of L. diadecturus from China as inferred using 28S (Fig. 3) and ITS1 regions (Fig. 4) showed a well supported clade (99%) with L. diadecturus type specimens (AF511416, AY601584). Alternatively, L. pius from China was clustered in the same clade with L. pius type specimen AM743178 and other type clones AM743180-AM743184 with 99% clade support in ITS1 region, but noticeably varied in 28S region which clustered to L. raskii (AJ549983-AJ549984) and Longidorus sp. FDL-2011 (FR775757-FR775760).
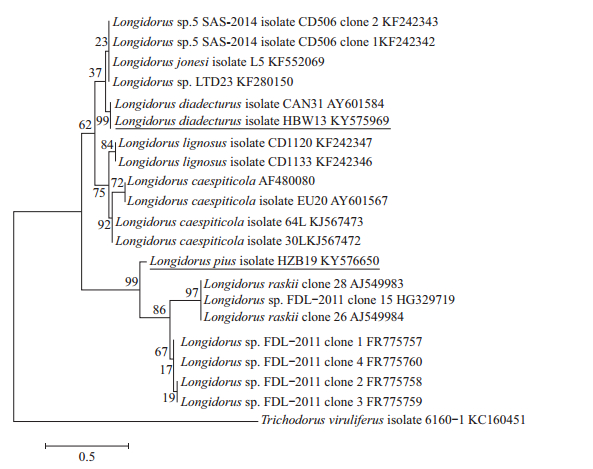 |
| The evolutionary distances were computed using maximum composite likelihood method, and the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1 000 replicates) was shown above the branches. Fig. 3 Phylogenetic relationships of L. pius and L. diadecturus from China within genus Longidorus as inferred from D2D3 expansion segments of 28S region |
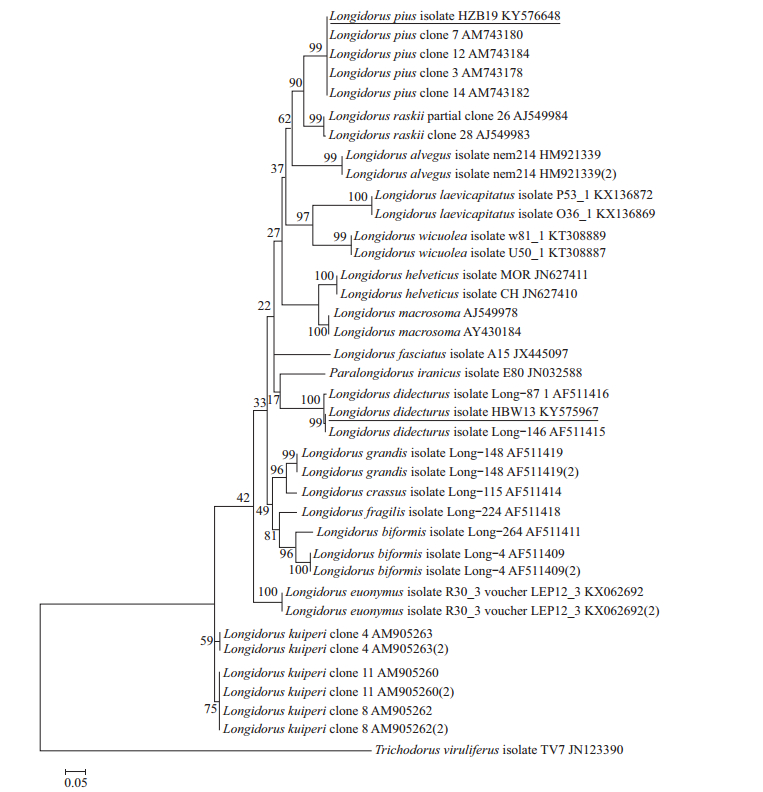 |
| The evolutionary distances were computed using maximum composite likelihood method, and the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1 000 replicates) was shown above the branches. Fig. 4 Phylogenetic relationships of Longidorus pius and L. diadecturus from China within genus Longidorus as inferred from ITS1 region |
Records of occurrence of Longidorus species in China had been increased in the past several years. Six species has been described as new species, including L. hangzhouensis Zheng et al. 2001, L. camelliae Zheng et al. 2000, L. fangi Xu & Cheng 1991, L. henanus Xu & Cheng 1991, L. litchii Xu & Cheng 1992, and L. jiangsuensis Xu & Hooper 1990. While seven others are reported as new records, including L. fursti, L. jonesi, L. pawneensis, L. macromucronatus, L. martini, L. pisi and L. moniloides[20-22]. The species described herein as L. pius and L. diadecturus were reported for the first time occurring in China. Morphologically, L. pius from China is in complete agreement with that of type population L. pius Barsi & Lamberti 2001 with codes A56-B4-C3-D1-E1-F3-G1-H1-I1 and A56-B45-C34-D1-E1-F3-G1-H1-I1, respectively. The only difference is in codes B (lip width) and C (position of guide ring) in which type specimen has code B4, 5 and C3, 4 that explain the minor difference of morphometric in lip diameter (20.1-21.8 μm vs 20.8-23.4 μm) and guiding ring position (31.6-40.7 μm vs 35-41.9 μm). The similarity of morphological characters of L. pius can be seen in L. picenus and L. nevesi, but can be distinguished by shorter body length (4.7-6.3 mm), lower a (49-71) and c (129-145) values. In contrast, L. diadecturus has a short body length (3.2-4.6 mm) and can be distinguished by a posterior guide ring. The code of L. diadecturus from China is A4-B23-C5-D3-F2-G2-H1-F1, and the type population L. diadecturus Eveleigh & Allen 1981 is A4-B23-C45-D3-F2-G2-H1-I1. An obvious difference is in code C (position of guide ring) in which L. pius from China has a more posterior location of guide ring (code C5=60.1-66.7 μm) compared to type population (code C45=50-64 μm). The closest resemblance of L. diadecturus is also evident in L. macromucronatus, L. jonesi and L. martini but differs in lower c value (123-164), longer odontostyle (108-120 μm) and anteriorly positioned vulva (46%-47%), respectively. Phylogenetic relationship of L. diadecturus Chinese population is well clustered in both ITS1 and 28S region to L. diadecturus type populations AF511416 and AY601584, showing 99%-100% clade support. The small difference of nucleotide base pairs of L. diadecturus Chinese population (963/963 bp) in ITS1 and (773/776 bp) in 28S suggested that the species was confirmed conspecific population of L. diadecturus as supported from its morphometry and phylogeny. Meanwhile, L. pius showed a well supported clade (99%) to L. pius holotype (AM743178) and other L. pius clones (AM743180-AM743184) in ITS1 region; however, molecular phylogeny in 28S region of L. pius can not be established in the meantime, because 28S rDNA gene was not provided from original description and lack of available sequences in the GenBank. However, in comparison to the closest sequence, the identity of L. pius was phylogenetically constructed and revealed a subclade clustering to L. raskii and Longidorus sp. FDL-2011, and the sequence variation of 11.3% suggested that a similarity of genetic structure but significant variation on morphological characters were evident, for example, L. pius has shorter body length (3.2-4.6 μm vs 6.5-8.1 μm vs 6.1-8.1 μm) and far posterior guiding ring (60-66 μm vs 32-38 μm vs 30-37 μm) compared to latter species. To date, the taxonomy and identification of Longidorus species in China are of great importance especially that nematode dispersal are brought upon the imported planting materials. Quarantine ports in China have administered subsequent actions and are strictly implementing regulations to avoid nematode dissemination during quarantine shipment. It is important to pay attention that L. diadecturus is originally described as virus vector species of peach in southwestern Ontario, Canada. Whether L. diadecturus Chinese population has the ability to transmit virus should be confirmed in future studies. The Longidorus identification and maintaining records of occurrence especially with quarantined species are very essential to avoid substantial distribution of harmful species that may cause potential problem in the future.
4 ConclusionsMeasurements and morphological data of juvenile and female specimens of two Longidorus species are described in this study, which are provided for the first time in Chinese populations and conformed well in the original description of type populations. The results are useful as an additional data to the distribution records of Longidorus species in China.
| [1] | TAYLOR C E, BROWN D J F. Nematode Vectors of Plant Viruses. Wallingford, UK: CAB International , 1997 : 130 -396. |
| [2] |
DECRAEMER W, ROBBINS R T. The who, the what and the where of Longidoridae and Trichodoridae.
Journal of Nematology, 2007,39 (4):295–297. |
| [3] |
TAYLOR C E, BROWN D J F. Nematode-virus interactions.
Plant Parasitic Nematodes, 1981 (3):281–301. |
| [4] |
XU J H, HOOPER D J. Observation of some species of Longidorus (Nematoda: Longidoridae) from Jiangsu Province, China, with a description of Longidorus jiangsuensis n. sp.
Revue de Nématologie, 1990,13 (3):323–330. |
| [5] |
LIZKOVA M, ROBBINS R T, BROWN D J F. Description of three new Longidorus species from Slovakia (Nemata: Longidoridae).
Journal of Nematology, 1997,29 (3):336–348. |
| [6] |
KUMARI S, DECRAEMER W. The genus Longidorus (Nematoda: Longidoridae) from Bohemia and South Moravia in the rhizosphere of fruit orchards and vineyards.
Helminthologia, 2007,44 (4):193–203. |
| [7] |
PENEVA V, CHOLEVA B. Nematodes of the family Longidoridae from forest nurseries in Bulgaria.
Helminthologia, 1992,32 :35–45. |
| [8] |
PENEVA V K, LAZAROVA S S, DE LUCA F, et al. Description of Longidorus cholevae sp. n. (Nematoda, Dorylaimida) from a riparian habitat in the Rila Mountains, Bulgaria.
Zookeys, 2013,330 :1–26. DOI: 10.3897/zookeys.330.5750. |
| [9] |
GUO K, SHI H L, LIU K, et al. Past and present distribution and hosts of Longidorus (Nematoda: Dorylaimida) in mainland China.
Zootaxa, 2011,3088 :27–38. |
| [10] |
BROWN D J F, BOAG B. An examination of methods used to extract virus-vector nematodes (Nematoda: Longidoridae and Trichodoridae) from soil samples.
Nematologia Mediterranea, 1988,16 :93–99. |
| [11] |
YE W M, SZALANSKI A L, ROBBINS R T. Phylogenetic relationship and genetic variation in Longidorus and Xiphinema species (Nematoda: Longidoridae) using ITS1 sequences of nuclear ribosomal DNA.
Journal of Nematology, 2004,36 (1):14–19. |
| [12] |
GUTIÉRREZ-GUTIÉRREZ C, RIUS J E P, CANTALAPIEDRA-NAVARRETE C, et al. Prevalence, polyphasic identification, and molecular phylogeny of dagger and needle nematodes infesting vineyards in southern Spain.
European Journal of Plant Pathology, 2011,129 (3):427–453. DOI: 10.1007/s10658-010-9705-y. |
| [13] |
VRAIN T C, WAKARCHUK D A, LEVESQUE A C, et al. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group.
Fundamental Applied Nematology, 1992,15 (6):563–573. |
| [14] |
WANG X R, BOSSELUT N, CASTAGNONE C, et al. Multiplex polymerase chain reaction identification of single individuals of the Longidorid nematodes Xiphinema index, X. diversicaudatum, X. vuittenezi, and X. italiae using specific primers from ribosomal genes.
Phytopathology, 2003,93 (2):160–166. DOI: 10.1094/PHYTO.2003.93.2.160. |
| [15] |
DE LEY P, FELIX M A, FRISSE L M, et al. Molecular and morphological characterization of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae).
Nematology, 1999,1 (6):591–612. DOI: 10.1163/156854199508559. |
| [16] |
NEILSON R, YE W, OLIVEIRA C M G, et al. Phylogenetic relationships of selected species of Longidoridae species (Nematoda: Dorylaimida) from North America inferred from 18S rDNA gene sequence data.
Helminthologia, 2004,41 (4):209–215. |
| [17] |
THOMPSON J D, HIGGINS D G, GIBSON T J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionspecific gap penalties and weight matrix choice.
Nucleic Acids Research, 1994,22 (22):4673–4680. DOI: 10.1093/nar/22.22.4673. |
| [18] |
TAMURA K, PETERSON D, PETERSON N, et al. MEGA 5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods.
Molecular Biology and Evolution, 2001,28 (10):2731–2739. |
| [19] |
CHEN Q W, HOOPER D J, LOOF P A A, et al. A revised polytomous key for identification of the genus Longidorus Micoletzky, 1922.
Fundamental and Applied Nematology, 1997,20 (1):15–28. |
| [20] |
WANG S, CHIU W F, YU C, et al. The occurrence and geographical distribution of Longidorids and Trichodorids nematodes associated with vineyards and orchards in China.
Russian Journal of Nematology, 1996 (4):145–153. |
| [21] |
徐建华, 程瑚瑞.长针属线虫一新种.
南京农业大学学报,1991,14 (1):38–42.
XU J H, CHENG H R. A new species of Longidorus (Nematoda: Longidoridae). Journal of Nanjing Agricultural University, 1991,14 (1):38–42. (in Chinese with English abstract) |
| [22] |
张绍升, 陈永宝.杨梅线虫研究.
福建农业大学学报,1994,23 (2):172–177.
ZHANG S S, CHEN Y B. Studies on nematode of bayberry (Myrica rubra). Journal of Fujian Agricultural University, 1994,23 (2):172–177. (in Chinese with English abstract) |
 2018, Vol. 44
2018, Vol. 44


