| 类固醇雌激素的环境暴露及其迁移转化 |
2. 北京市土肥工作站,北京 100029;
3. 中国科学院南京土壤研究所,土壤环境与污染修复重点实验室,南京 210008
2. Beijing Soil and Fertilizer Extension Service Station, Beijing 100029, China;
3. Key Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China)
内分泌干扰物(endocrine disrupting chemicals, EDCs)污染已成为继气候变暖和臭氧层破坏之后的另一重大环境问题。内分泌干扰物进入人或动物体内通过模拟生物体自身激素效应,能阻断、刺激或抑制生物体内激素的合成、转运等,进而影响其自身平衡、代谢、免疫、生殖、发育等过程,对机体的生殖、神经和免疫系统造成危害[1]。类固醇雌激素(steroid estrogens, SEs)是一类内分泌干扰物,常见的有17α-雌二醇、17β-雌二醇、雌三醇、雌酮等[2]。随着分析检测技术的进步,近年来,国内外屡有在水体、土壤、河流底泥及畜禽粪便中检出类固醇雌激素的报道[3-4]。
作为一类低浓度、高毒性的污染物,几ng/L类固醇雌激素就可以危害生物机体的正常运作。如17β-雌二醇在河水中的质量浓度为1 ng/L时就会造成雄鱼的雌性化[5]。这类污染物的环境暴露已对生态系统构成了安全隐患。因此,对环境中类固醇雌激素进行研究显得尤为重要。
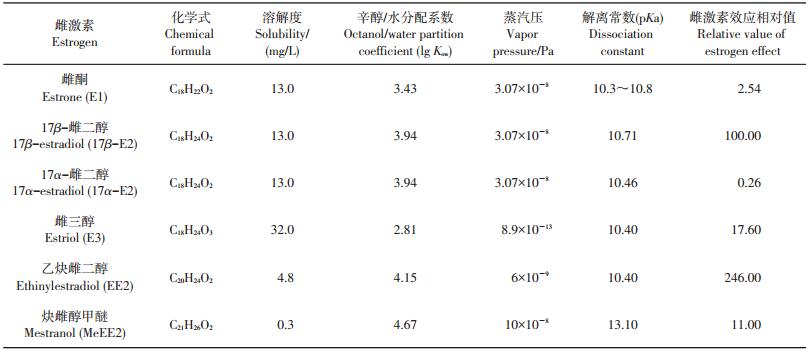
1 类固醇雌激素的种类及性质环境中的类固醇雌激素主要可以分为天然雌激素和人工合成雌激素。天然雌激素包括雌酮(estrone, E1)、17α-雌二醇(17α-estradiol, 17α-E2)、17β-雌二醇(17β-estradiol, 17β-E2)和雌三醇(estriol, E3),人工合成雌激素包括乙炔雌二醇(ethinylestradiol, EE2)和炔雌醇甲醚(mestranol, MeEE2)等[6],它们的结构及物理化学性质如表 1所示。这几种雌激素的辛醇/水分配系数范围为2.81~4.67,具有较强疏水性,在水中的溶解度较低,其中天然雌激素的溶解度普遍大于人工合成雌激素。从图 1中可以看出,它们具有类似的分子结构,均具有4个碳环,其差异在于不同位置的碳结合了不同的官能团。E1在C-17位上是碳基而非羟基,E3在C-16位和C-17位上均有羟基,所以具有4种异构体。17β-E2和17α-E2虽然具有相同的物理化学性质,但其中C-17位所连接的羧基具有不同的空间结构,两者的雌激素效应相差500倍,相对值分别为100和0.26。可见,不同的结构将极大地影响雌激素效应。上述几种类固醇雌激素的雌激素效应从大到小排序依次为:EE2>17β-E2>E3>MeEE2>E1>17α-E2(表 1)。
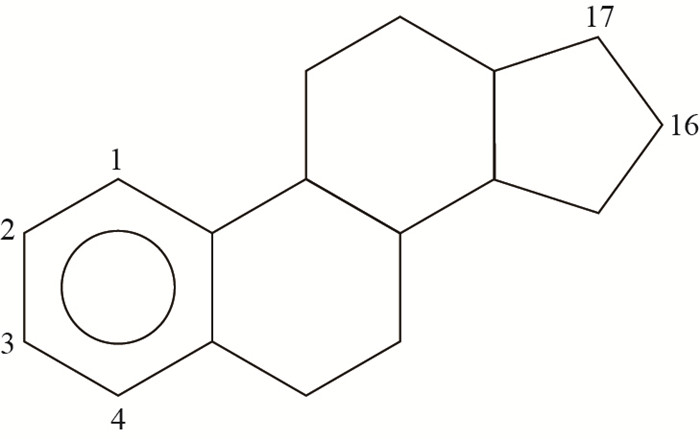 |
| 图1 通用结构式C位置编号 Fig. 1 Basic steroid estrogen structure with carbon labeled |
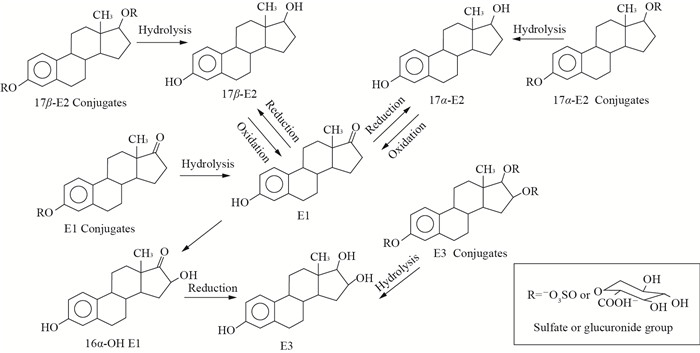
E1、17β-E2、17α-E2、E3这几种天然雌激素之间可以互相转化,其中17α-E2与17β-E2在环境中会被氧化为E1,而E1可以通过还原反应转化为17α-E2与17β-E2,17α-E2也可以通过外消旋化作用转化为17β-E2[7],E1也能降解为E3。环境中的类固醇雌激素分为结合态和游离态,其中,结合态雌激素主要通过人类和畜禽的尿液排放,而游离态雌激素则大多存在于粪便中[8]。根据HUTCHINS等[9]提出的雌激素转化路径(图 2),雌激素以结合态排出,这种共轭体本身没有生物活性,但水解后生成有活性的游离雌激素,游离态的雌激素也能与葡糖苷酸和硫酸盐酯化形成结合态雌激素。
2 类固醇雌激素的危害外源性雌激素进入生物体内可模拟细胞内雌激素作用或改变其雌激素活性,从而影响人和动物性分化以及体内正常代谢功能,干扰生物正常的生长、发育、生殖等过程,甚至产生致死、致癌和致畸等作用。已有一些雌激素环境残留报道,如:BUXTON等[15]对美国30个州的139处地表水进行调查取样表明,40%的水体受到了不同程度的雌激素污染;TABATA等[16]对日本100余条河流中的雌激素含量调查结果显示,近80%的河水样品被检测出雌激素。雌激素在几ng/L时就显现出高危害性,诱导雄鱼产生卵黄蛋白原进而使其变性[5]。
近年来,针对环境中雌激素对水生生物发育和生殖毒性的研究不断增多,分别在分子水平、细胞水平、组织水平及表型层面上进行了不同的研究。BRION等[17]和张续等[18]在对雄性成年斑马鱼的研究中发现,低质量浓度的17β-E2不能引起斑马鱼体长、体质量变化,但10 μg/L 17β-E2可引起成年雄性斑马鱼肥满度指数显著升高。ZHA等[19]报道了稀有鲍鲫在5.0和25.0 ng/L EE2处理组暴露28 d后,出现肝细胞细胞核增加和肝细胞肥大等现象。李国超[20]通过实验发现,17β-E2能使斑马鱼发生畸形(心包水肿、体轴弯曲和色素沉着)甚至死亡,其畸形程度、死亡率和畸形率随17β-E2浓度的升高呈现明显的剂量-依赖效应。雌激素除了影响生物机体的心血管功能、骨代谢、脑功能等,还对性分化和生殖功能具有重要的调节效应[21]。17β-E2能延迟斑马鱼胚胎孵化,导致性腺中精子细胞比例下降,性腺发育迟缓[17, 22]。黑头鱼暴露在EE2质量浓度大于4.0 ng/L的水环境中会全部发育成雌性[23]。此外,雌激素还能直接影响雄性动物精子水平。如:17β-E2可以明显提高成熟的雄性金黄地鼠精子的运动参数,包括活动力、直线运动速率、曲线运动速率、尾部鞭打频率、头部侧向位移的平均振幅[24];对精子活力较低病人的精子也可产生直接的刺激作用[25]。
研究发现,由于雌激素暴露导致雄性个体出现卵黄蛋白原或雌性化器官现象[26],可能会使生物种群结构发生变化。目前,在鱼类、两栖类和鸟类中都发现了由于雌激素暴露导致群落中雌性个体占优势的生物种群现象[27]。鱼类是水生环境中受雌激素影响最严重的生物种群[28],17β-E2暴露可以使雄性虹鳄鱼的斑纹转变为雌性斑纹,同时,雄鱼的生殖活动也向雌性特点转变[29]。KIDD等[30]通过7年的实验观察发现,长期暴露于含有5~6 ng/L EE2水体中的黑头鲤鱼,其性腺发育异常,进而导致其种群出现严重退化。由此可见,环境雌激素对人和动物的繁殖和种群结构有着潜在威胁。
3 类固醇雌激素的源及环境暴露近年来,环境中类固醇雌激素含量分布状况备受关注。城市污水处理厂是环境中雌激素的一个源头,而畜禽养殖废弃物对环境中雌激素的贡献更应引起重视。一方面是养殖过程中产生的废水、粪便等直接排放使激素污染物进入到河流、湖泊中,另一方面是大量畜禽粪便进行土地利用时,各种激素污染物随粪肥进入土壤,在农田土壤中也被报道出越来越多的残留问题。
3.1 污水处理厂源污水处理厂是收纳和处理生活污水的主要场所,近年来在污水处理过程中雌激素的降解及浓度变化相继被报道。北京市朝阳区某污水处理厂进水中E1、17β-E2、E3、EE2的最高质量浓度分别为110、140、760、330 ng/L,出水中相应激素的去除率超过90%[31]。武汉市某污水处理厂进出水中E1、17β-E2、E3的质量浓度分别是38.6、21.4、53.9 ng/ L,出水中E1、17β-E2质量浓度分别是7.2~31.5、0.5~8.6 ng/L,但未检测到E3[32]。上述研究反映出污水处理厂进水中雌激素浓度较高,这跟我国城市人口数量多、人均用水量少有关。经过处理后,其中大部分雌激素得以去除,某些雌激素浓度甚至低于检出限,但是绝大多数雌激素依然保持在几到几十ng/L水平。和我国情况类似,日本东京的某污水处理厂进水和出水经连续取样测定显示,其进水中E1、17β-E2、E3的质量浓度分别是30~200、10~30、80~300 ng/L,均处于较高水平,经过处理后,其出水中E1、17β-E2、E3的质量浓度降至2.8~110、0.49~16.7、0~0.84 ng/L[33]。与我国和日本相比,伊朗德黑兰的污水处理厂进水中E1、17β-E2、EE2质量浓度分别是11.4、3.0和6.2 ng/L,经过处理后分别有72.6%、83.2%和90%的污染物被去除[34]。同样,德国的一家污水处理厂对雌激素的去除也取得较好效果,进水中雌激素质量浓度为几十ng/L,经过厌氧-缺氧-好氧工艺处理后,出水中雌激素的质量浓度降低到<1 ng/L[35]。综上可以看出:不同地区污水处理厂进、出水中雌激素的检出种类和浓度水平存在较大差异,污水中E1和17β-E2的检出率高,与当地的人口特征(如性别、年龄、体格等)、产业结构、用水特点和污水收集工艺等有关;污染物的去除效率也存在差异,这和污水处理工艺及运行状况等因素相关。如HAMID等[36]发现,E1、17β-E2和EE2在3种氧化还原条件(有氧、缺氧和厌氧)下都可生物降解,但在有氧条件下的降解效果最佳。
一般而言,污泥吸附和生物降解过程是污水处理厂中类固醇雌激素的主要去除机制。由于类固醇雌激素具有相对较低的辛醇/水分配系数(表 1),在水中溶解度低,有利于被污泥所吸附[13],且吸附过程是自发、快速和放热的,能够在1 h以内达到平衡[37];类固醇雌激素吸附到污泥表面后能够被微生物降解为完全无害的物质[38]。对比前文数据可以看出,由于污水处理技术、标准等差距,我国污水处理厂出水中雌激素含量仍然处于较高的水平,对地表水体的污染风险应引起足够的关注。
3.2 畜禽养殖场源随着集约化养殖业的发展,畜禽养殖所带来的环境污染日益引起人们的关注。据RAMAN等[39]估算,畜禽养殖产生的雌激素排放量约是污水处理厂的10倍;英国畜禽养殖场每年产生的17β-E2总量大约是人产生的4倍[40]。由此看来,畜禽养殖业已成为环境中雌激素的一个重要来源。
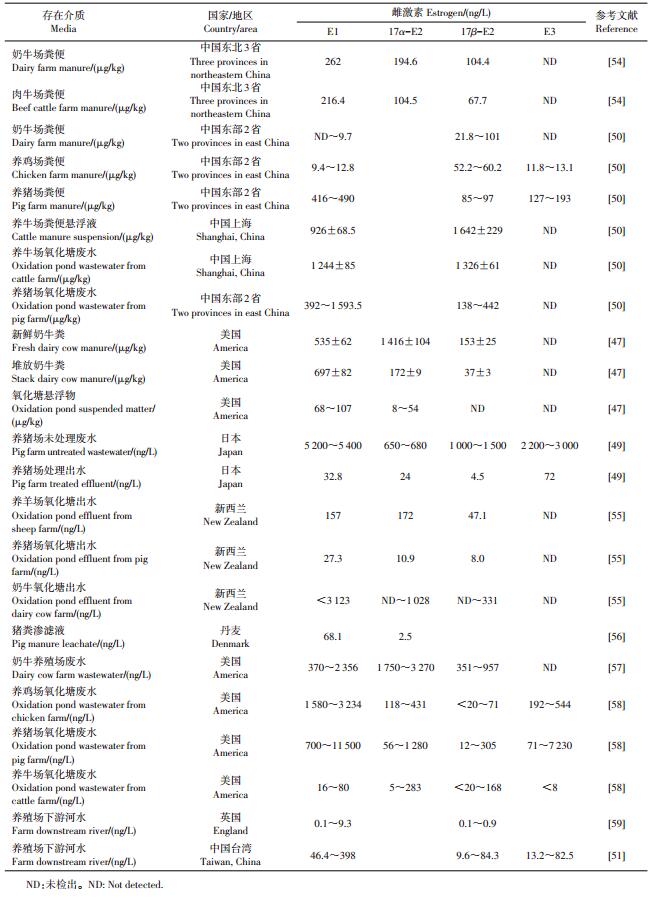
养殖场粪污中雌激素含量如表 2所示。畜禽粪污中的雌激素质量浓度显著高于污水处理厂的污水,例如美国、日本的养殖场废水中4种污染物都达到数千ng/L。这些报道中的数据之间差异较大,可能与不同地区的畜禽种类、养殖规模、粪便采样方式、检测方法等因素有关。另外,不同畜禽类型,其雌激素的主要排放方式、种类和数量也不尽相同。牛、羊主要通过粪便排出雌激素,而猪通过尿液排泄的雌激素可以达到90%[41-42]。牛、羊雌激素排放量分别为299~540和23~25 μg/d,以E1和E2为主;猪和家禽的雌激素排放量分别为120~2 300和2.5~6.0 μg/d,主要含有E1、E2和E3[41-42]。我国畜禽养殖业发达,每年动物粪便和尿液的产量可以达到40亿t[43]。如此大量的畜禽粪便如果不经有效处理直接进入土壤或水体环境,将会对环境构成严重的威胁。
| 表2 类固醇雌激素在养殖场中的含量 Table 2 Concentration of steroid estrogens from animal feeding operations |
 |
| 点击放大 |
关于类固醇雌激素的环境暴露风险,我国已有一些研究报道。有研究[44-46]统计分析了我国1998— 2008年间畜禽养殖雌激素产生量的特征,结果表明,各省之间畜禽粪便雌激素排放水平表现出显著差异,主要原因是各省的畜禽养殖数量以及养殖结构比重的不同:河南、山东、四川和东北3省是雌激素的产生大省,呈现显著增长的趋势;河南省排放的雌激素总量最大,2008年达到7 040.9 kg,是当年上海产生量的49倍;雌激素产生总量增速最快的是吉林省,10年增加了1.2倍;相反,由于政府对养殖数量及养殖业结构的调整,北京、上海、山西、河北、江西和安徽等省市的雌激素总产生量呈下降趋势。总体来看,西北等省份畜禽粪便雌激素的产生量呈增长趋势,而直辖市及沿海发达城市变化不明显。
对于畜禽养殖业产生的废水可以通过氧化塘、厌氧-好氧工艺等生化方法进行处理。ZHENG等[47]研究了氧化塘对养牛场废水中雌激素的去除效果,经连续式氧化塘处理后,E1、17α-E2和17β-E2质量浓度从250~3 000 ng/L均降低到10 ng/L以下。通过湿地处理与氧化塘结合使用,可以降解掉养猪场废液中83%~93%的雌激素[48]。日本某养猪场废水经过上流式厌氧污泥床工艺处理后,雌激素的质量浓度也都减少到10 ng/L水平[49]。但并不是所有的生化处理方法都能有效去除雌激素,如有研究报道,厌氧-好氧工艺处理无明显的雌激素去除能力[48]。对于固体粪便,堆肥处理是有效去除雌激素的方法。牛粪经2个月堆腐后,70.7%的雌激素被降解,并且随着堆腐持续时间增加而增加[50]。上述研究可以看出,养殖场废水、粪便经处理后,雌激素的含量可以大幅度降低。但是表 2中数据显示,养殖场处理后出水中的雌激素仍然处于较高的水平,排放到湖泊、河流等水体势必会增加环境风险。如中国台湾南部某养殖场下游河流检测到较高质量浓度的雌激素水平,E1最高可达398 ng/L[51]。因此,开展针对养殖场排放的类固醇雌激素的环境行为规律及高效处理技术研究显得尤为重要。
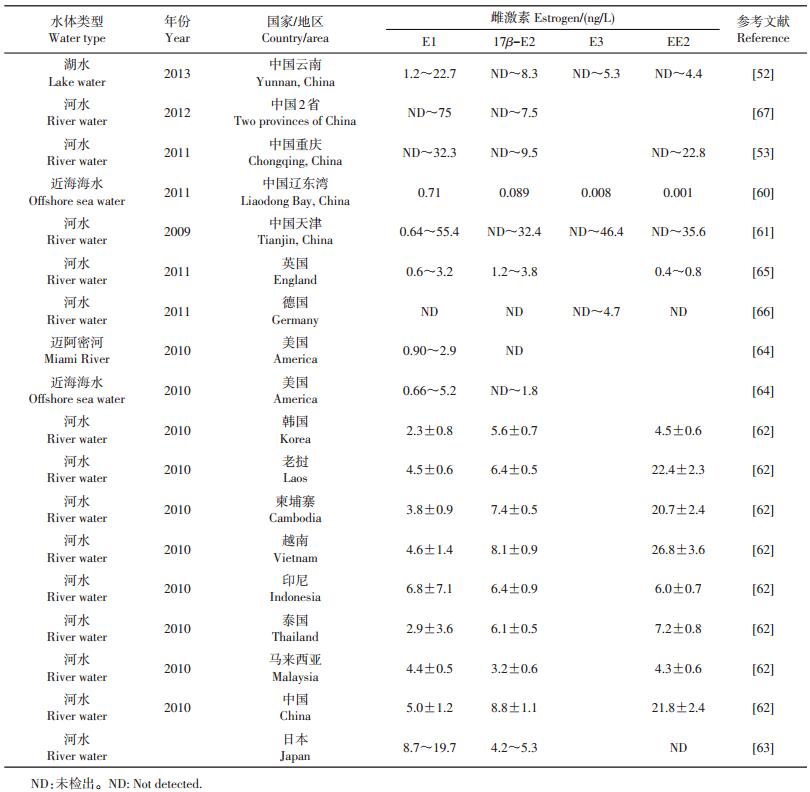
3.3 地表水体中雌激素的暴露水平地表水中类固醇雌激素暴露问题是最早引起关注的,国内外不断有关于水体中雌激素残留水平的报道(表 3)。我国研究者采集了滇池的水样,检测出E1、17β-E2、E3、EE2质量浓度范围分别为1.2~ 22.7、ND~8.3、ND~5.3、ND~4.4 ng/L[52]。对嘉陵江磁器口江段分别于2009年和2010年进行2次取样发现,E1、17β-E2、EE2的最高质量浓度分别达到了32.3、9.5和22.8 ng/L[53]。辽东湾近海海水中也有雌激素的检出,但其质量浓度水平相对较低,其中E1为0.71 ng/L,17β-E2、E3、EE2均小于0.1 ng/L[60]。而天津地区河流中E1、17β-E2、E3、EE2的质量浓度范围分别为0.64~55.4、ND~32.4、ND~46.4、ND~ 35.6 ng/L[61],最高质量浓度明显高于相关的报道。以上研究反映出我国各类水体中均有类固醇雌激素检出,且均处于低质量浓度水平,但各地区污染物水平差异显著,其中靠近城市的河流、湖泊中往往较高,而近海海水中质量浓度相对较低。国外的水体中也有关于雌激素的大量检出报道。DUONG等[62]报道表明,亚洲一些国家河水中含有较高浓度的E1、17β-E2和EE2(表 3)。在日本的多摩川河中检测出19.7 ng/L E1和5.3 ng/L 17β-E2[63]。在美国迈阿密河和佛罗里达港湾取样发现,E1质量浓度范围分别为0.90~2.9和0.66~5.2 ng/L[64]。相比亚洲国家和美国,欧洲一些国家的检出水平相对略低:英国某地河流样品中E1、17β-E2、EE2的质量浓度范围为0.6~ 3.2、1.2~3.8、0.4~0.8 ng/L[65],德国Saale河只被检测出了E3[66]。由此可以看出,世界上很多国家地表水体均受到了不同程度的雌激素污染,但其质量浓度水平差异较大,这可能与各国经济发展、产业结构、污水排放标准等因素差异有关。此外,通过比较可发现,各个国家或地区地表水(河水和湖水)中雌激素的检出浓度大于近海区域,说明人口密集的内陆地区受人类活动影响水体受雌激素污染的风险更大,而近海区域水流量大,稀释作用较强,从而检出浓度较低。
| 表3 类固醇雌激素在地表水体中的质量浓度 Table 3 Concentration of steroid estrogens in surface water |
 |
| 点击放大 |
目前,类固醇雌激素环境暴露研究多集中于河流、湖泊等地表水体,在土壤中的残留报道较少。设施菜地因为有机肥使用量较大,极有可能存在雌激素污染问题。ZHANG等[68]开展了调查并证实,中国北方最大的蔬菜种植基地寿光因长期大量施用畜禽粪便已出现温室土壤中类固醇激素暴露残留。雨水冲刷土壤会使地表径流中雌激素浓度升高[69]。当农田中猪粪施用量为5 000 kg/hm2时,地表径流中17β-E2质量浓度可达3 500 ng/L[70],部分吸附于矿物质颗粒和有机胶体上的激素污染物会随径流迁移,进而威胁土壤周围的水生环境[68]。
迄今为止,有关雌激素的土壤环境行为研究大多采用室内模拟实验,普遍认为其进入农田后会迅速被土壤吸附[71],并且在几小时至几天内即被微生物所降解[72],但由于室内模拟实验与实际环境的差异,并不能反映畜禽粪便施用于土壤后雌激素的吸附降解情况。FINLAY-MOORE等[73]研究发现,施用4 500 kg肉鸡粪便4 d后,土壤中17β-E2质量分数从55 ng/kg增加到675 ng/kg。KJAER等[56]测定了经畜禽粪便改良3个月后的壤质土,发现土壤浸出液中E1和17β-E2的质量浓度仍然达到68.1和2.5 ng/L,说明雌激素在实际土壤环境中并不会在几天内被彻底降解去除。SCHUH等[74]对一次性施用猪粪120 m3/hm2的土壤进行了长时间的跟踪测定,发现土壤中17β-E2的平均质量分数从施用猪粪前的0.9 ng/kg增加至1年后的202.55 ng/kg,如此大的残留被认为是土壤组分吸附的雌激素随雨水再次释放所致。相比于国外,我国有关土壤中雌激素的研究更为缺乏。韩伟[75]对北京某养殖场附近农田土壤中雌激素的分析显示,E1、17α-E2和17β-E2质量分数范围分别为ND~6.51,ND~6.60和ND~6.38 ng/g,但是在氧化塘地下水中检出高达31.6 ng/L的E1。王代懿[76]的研究显示,在30个施用畜禽粪便的大棚土壤样品中均能检出E(1 2 103 ng/kg)、17α-E(2 323 ng/kg)、17β-E2(87 ng/kg)、E3(15 ng/kg)。国内外的研究均表明,农田土壤在施用畜禽粪便之后,其中的雌激素含量会明显增加并残留在土壤中。因此,长期大量施用畜禽粪便带来的激素污染将是设施蔬菜生产中一个不安全因素。
许多学者对有效地降低畜禽粪便中雌激素的残留和污染风险也做了许多探究。DERBY等[77]研究表明,通过堆肥方式可以将雌激素减少74%。DELAUNE等[78]用十二水硫酸铝钾处理畜禽粪便也取得了不错的效果:添加20%十二水硫酸铝钾的鸡粪其降雨径流中17β-E2减少了57%,说明十二水硫酸铝钾遇水形成的胶体显著降低了17β-E2的溶解性。另外,ZHANG等[79]通过模拟实验也发现,添加生物质炭的土壤可以较好地吸附固定雌激素,而且有机质含量越低的土壤效果越显著。综上,通过对畜禽粪便进行预处理,可以降低其中的雌激素对土壤的污染风险。上述结果为今后有效降低土壤中雌激素污染研究提供了思路。
4 类固醇雌激素在环境中的迁移转化类固醇雌激素通过污水处理厂或养殖场废弃物进入环境后,在水体、土壤系统中将发生吸附、迁移、降解和转化等行为。雌激素在土壤介质中有明显的淋溶迁移行为,如:英国的一项研究发现,河流15 cm处底泥中E1含量是表层的9倍[80],表明雌激素具有从底泥表层向深层转移的特征;土壤施用猪粪3个月后,距地面1 m深的地下水中E1和17β-E2质量浓度分别达到68.1与2.5 ng/L[81];THOMPSON等[82]在更深层的地下水(23 m)中也检测到质量浓度相当可观的17β-E2(16~100 ng/L);ARNON等[83]甚至在某奶牛场氧化塘地下32 m处仍然检测出了雌激素的存在。由此可见,类固醇雌激素不仅能发生水平迁移,还有向土壤深层迁移的趋势,但是现有的对流、扩散和吸附等模型都无法很好地解释雌激素向下迁移的机制。FAN等[84]研究表明,17β-E2在厌氧条件下的矿化率远远低于有氧条件,雌激素向土壤和沉积物深层迁移会延长其环境残留时间,增大环境风险。
进入环境中的类固醇雌激素会发生多种物理化学作用,其中吸附是最重要的行为。水体中富含大量的胶体、悬浮物及底泥沉积物,类固醇雌激素可以在固相-液相界面上不断进行吸附、解吸过程。土壤中的黏土矿物、有机组分易于吸附雌激素。类固醇雌激素在悬浮物、沉积物和土壤上的吸附过程包括初期快速吸附和后期缓慢吸附2个阶段,很快达到吸附平衡[85]。CLARA等[86]研究了17β-E2与EE2在活性污泥上的吸附过程,结果表明,吸附符合Freundlich吸附等温线,而且吸附行为与pH值有关。研究还显示,17β-E2在河流沉积物、潮土、黑土皆表现为非线性,其中黑土吸附的非线性最强,说明土壤/沉积物吸附17β-E2的能力与有机质含量相关[87]。针对EE2的土壤吸附实验也得出类似的结论,Freundlich模型和Dubinin-Astakhov模型均能较好的拟合EE2的等温吸附线,且后者拟合效果更好,EE2的饱和吸附容量随土壤有机质含量增大而增大[85]。此外,有机质的性质也会影响土壤对EE2的吸附能力,其吸附能力依次为碳黑>非水解性有机质>腐殖酸,说明有机质成熟度越高,吸附能力越强[88]。因此,类固醇雌激素在环境介质中的吸附行为不仅受自身理化性质的影响,也受到土壤类型、环境pH、有机质种类和含量等多种因素的影响。此外,研究还发现黏土矿物对E2的吸附能力顺序为蒙脱石>高岭石>伊利石≥针铁矿[89],而共存的表面活性剂将抑制土壤对17β-E2的吸附[90]。这些研究均表明,水体沉积物和土壤中的一些黏土矿物、共存污染物等因素都会影响类固醇雌激素的吸附行为。
类固醇雌激素在环境中的降解包括光降解、化学降解和微生物降解。由表 1可知,雌激素蒸汽压较低,不易挥发,在水体中的挥发行为可以忽略不计。由于E1、17β-E2和EE2具有感光的酚类结构,因此光解对其具有一定的去除效果。当这3种雌激素同时存在时,168 μW/cm2紫外光辐照强度下的E1、17β-E2和EE2均能在50 min内发生不同程度的光解,去除率分别为88.7%、26.6%和23.0%[91]。雌激素的降解受含水率、温度、pH、养分、微生物数量等环境条件的影响。XUAN等[92]研究发现,土壤微生物数量、含水量和温度会影响E2的降解,其中:未灭菌土的比例由0%增加至33%时,17β-E2的半衰期由29 d明显降低为0.54 d;土壤含水量由10%提高到20%时,17β-E2的半衰期由1.3 d降到0.69 d;温度由15 ℃升高到25 ℃时,17β-E2的半衰期由4.9 d降到0.92 d。其他研究也有类似的结果,在5~ 30 ℃范围内,17α-E2、EE2降解速率随温度升高而增加[73]。此外,雌激素的降解还受共存污染物的影响。在17β-E2单一污染体系中,溶解性有机质(dissolved organic matter,DOM)能明显促进17β-E2降解,其降解速率常数由0.062 d-1提高到0.430 d-1[93];而在Cu(Ⅱ)-E2复合污染体系中,17β-E2降解速率常数由0.006 d-1提高到0.030 d-1。分析认为,由于DOM与Cu(Ⅱ)的络合改变了Cu(Ⅱ)的沉淀形态,降低了Cu(Ⅱ)对微生物的抑制作用,进而影响了对雌激素的降解速率[93]。XUAN等[92]研究发现,向含有17β-E2的土壤中分别添加40和200 μmol/kg磺胺二甲嘧啶,17β-E2的半衰期较空白对照分别延长了0.48和1.68 d,说明抗生素的存在会抑制17β-E2的降解。而目前各种微量元素和抗生素作为添加剂或药物广泛用于畜禽养殖,这意味着大量存在的微量元素和抗生素可能会改变畜禽粪便中微生物对雌激素的降解,从而增大雌激素在环境中的残留时间和危害。然而,复合污染往往存在复杂的相互作用机制,目前仍缺乏详细的机制性研究。因此,研究多种污染物共存下雌激素的降解机制也将是今后重点关注的方向。
5 展望类固醇雌激素广泛分布在环境介质中,虽然浓度较低但危害巨大。畜禽养殖业和城市污水厂是环境雌激素的主要排放源。根据现有的研究,畜禽粪便的土地利用应该成为关注的重点,其持续大量的使用,不但会造成土壤中雌激素含量升高,同样会对人和动物造成潜在风险,对于环境行为的研究及控制技术的开发显得尤为重要。综上,对今后有关类固醇雌激素的研究展望及建议如下:
1) 国内外对于类固醇雌激素的吸附研究大多基于室内的模拟实验,而针对实际污染场地的研究鲜有报道。室内理想条件下的模拟实验往往进行一些表观性描述,虽然得出的结论具有一定的代表性,但是并不符合复杂污染场地的实际情况,所以开展实际污染场地尤其是农田系统的研究更有实际价值,例如设施菜地土壤中畜禽粪便使用量较大,由此带来的雌激素污染问题更值得关注。
2) 对于雌激素在土壤、水体等环境介质中的行为研究,尤其伴随畜禽粪便或城市污泥等介质进入土壤中的雌激素,其吸附、降解、迁移以及环境介质相态间的迁移转化研究仍然不足。针对多相态体系开展研究,对掌握激素污染源的环境行为特征具有重要意义。
3) 在实际情况下,土壤或各种水体中的污染总是呈现出多样性,多种污染物造成的复合污染更复杂,研究难度更大。以往的研究大多是探讨某种或几种特定雌激素的环境行为,但实际环境中雌激素往往与其他污染物共存,它们之间会相互作用从而影响其环境效应,因此,建议关注雌激素与其他污染物共存情况下的环境行为及作用机制,以期更有效地判断类固醇雌激素的污染风险及生态效应。
| [1] | SWEENEY M F, HASAN N, SOTO A M, et al. Environmental endocrine disruptors: Effects on the human male reproductive system. Reviews in Endocrine and Metabolic Disorders, 2015, 16(4): 341-357. DOI:10.1007/s11154-016-9337-4 |
| [2] |
李艳霞, 韩伟, 林春野, 等. 畜禽养殖过程中雌激素的排放及其环境行为. 生态学报, 2010, 30(4): 1058-1065. LI Y X, HAN W, LIN C Y, et al. Excretion of estrogens in the livestock and poultry production and their environmental behaviors. Acta Ecologica Sinica, 2010, 30(4): 1058-1065. (in Chinese with English abstract) |
| [3] | BARTELTHUNT S, SNOW D D, DAMONPOWELL T, et al. Occurrence of steroid hormones and antibiotics in groundwater impacted by livestock waste control facilities. Journal of Contaminant Hydrology, 2011, 123(3/4): 94-103. |
| [4] | WANG D P, LUO Z X, ZHANG X, et al. Occurrence, distribution and risk assessment of estrogenic compounds for three source water types in Ningbo City, China. Environmental Earth Sciences, 2015, 74(7): 5961-5969. DOI:10.1007/s12665-015-4619-9 |
| [5] | HANSEN P D, DIZER H, HOCK B, et al. Vitellogenin: A biomarker for endocrine disruptors. Trends in Analytical Chemistry, 1998, 17(7): 448-451. DOI:10.1016/S0165-9936(98)00020-X |
| [6] | YIN G G, KOOKANA R S, RU Y J. Occurrence and fate of hormone steroids in the environment. Environment International, 2002, 28(6): 545-551. DOI:10.1016/S0160-4120(02)00075-2 |
| [7] | KOLOK A S, SELLIN M K. The environmental impact of growthpromoting compounds employed by the United States beef cattle industry: History, current knowledge, and future directions. New York, USA: Springer, 2008: 1-30. |
| [8] |
林建德, 邵坚, 冯成洪, 等. 天然类固醇雌激素源解析、环境行为及其污染控制. 环境科学与技术, 2012, 35(5): 60-64. LIN J D, SHAO J, FENG C H, et al. Source, environmental behavior and pollution control of natural estrogen in environment. Environment Science & Technology, 2012, 35(5): 60-64. (in Chinese with English abstract) |
| [9] | HUTCHINS S R, WHITE M V, HUDSON F M, et al. Analysis of lagoon samples from different concentrated animal feeding operations for estrogens and estrogen conjugates. Environmental Science & Technology, 2007, 41(3): 738-744. |
| [10] | COMBALBERT S, HERNANDEZ-RAQUET G. Occurrence, fate, and biodegradation of estrogens in sewage and manure. Applied Microbiology and Biotechnology, 2010, 86(6): 1671-1692. DOI:10.1007/s00253-010-2547-x |
| [11] | BOVEE T F H, HELSDINGEN R J R, RIETJENS I M C M, et al. Rapid yeast estrogen bioassays stably expressing human estrogen receptors α and β, and green fluorescent protein: A comparison of different compounds with both receptor types. The Journal of Steroid Biochemistry and Molecular Biology, 2004, 91(3): 99-109. DOI:10.1016/j.jsbmb.2004.03.118 |
| [12] | KIM D G, JIANG S F, JEONG K, et al. Removal of 17α-ethinylestradiol by biogenic manganese oxides produced by the Pseudomonas putida strain MnB1. Water, Air, & Soil Pollution, 2012, 223(2): 837-846. |
| [13] | LAI K M, JOHNSON K L, SCRIMSHAW M D, et al. Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environmental Science & Technology, 2000, 34(18): 3890-3894. |
| [14] | QUINTANA J B, CARPINTEIRO J, RODRGUEZ I, et al. Determination of natural and synthetic estrogens in water by gas chromatography with mass spectrometric detection. Journal of Chromatography A, 2004, 1024(1/2): 177-185. |
| [15] | BUXTON H T, KOLPIN D W. Pharmaceuticals, Hormones, and other Organic Wastewater Contaminants in U.S. Streams. New York, USA: John Wiley & Sons, Inc., 2005: 1202-1211. |
| [16] | TABATA A, KASHIWADA S, OHNISHI Y, et al. Estrogenic influences of estradiol-17 beta, p-nonylphenol and bis-phenol-A on Japanese medaka (Oryzias latipes) at detected environmental concentrations. Water Science & Technology: A Journal of the International Association on Water Pollution Research, 2001, 43(2): 109-116. |
| [17] | BRION F, TYLER C R, PALAZZI X, et al. Impacts of 17β-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile-and adult-life stages in zebrafish (Danio rerio). Aquatic Toxicology, 2004, 68(3): 193-217. DOI:10.1016/j.aquatox.2004.01.022 |
| [18] |
张续, 黎娟, 刘雅思, 等. 三-(2, 3-二溴丙基)异氰脲酸酯和17β-雌二醇复合暴露对雄性斑马鱼的毒性效应. 环境科学学报, 2012, 32(2): 450-456. ZHANG X, LI J, LIU Y S, et al. Toxic effects on adult male zebrafish (Danio rerio) following co-exposure to tris-(2, 3-dibromopropyl) isocyanurate and 17β-estradiol. Acta Scientiae Circumstantiae, 2012, 32(2): 450-456. (in Chinese with English abstract) |
| [19] | ZHA J M, WANG Z J, WANG N, et al. Histological alternation and vitellogenin induction in adult rare minnow (Gobiocypris rarus) after exposure to ethynylestradiol and nonylphenol. Chemosphere, 2007, 66(3): 488-495. DOI:10.1016/j.chemosphere.2006.05.071 |
| [20] |
李国超. 17β-雌二醇对斑马鱼的毒性研究. 北京: 中国农业科学院, 2015: 11-13. LI G C. Toxicity of 17 beta-estradiol for zebrafish. Beijing: Chinese Academy of Agricultural Sciences, 2015: 11-13. (in Chinese with English abstract) http://d.wanfangdata.com.cn/Thesis_Y2787675.aspx |
| [21] | GRUBER C J, TSCHUGGUEL W, SCHNEEBERGER C, et al. Production and actions of estrogens. New England Journal of Medicine, 2002, 346(5): 340-352. DOI:10.1056/NEJMra000471 |
| [22] | HILL R L Jr, JANZ D M. Developmental estrogenic exposure in zebrafish (Danio rerio): Ⅰ. Effects on sex ratio and breeding success. Aquatic Toxicology, 2003, 63(4): 417-429. DOI:10.1016/S0166-445X(02)00207-2 |
| [23] | LANGE R, HUTCHINSON T H, CROUDACE C P, et al. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 2001, 20(6): 1216-1227. DOI:10.1002/etc.v20:6 |
| [24] | JIN W Z, ARAI K Y, WATANABE G, et al. The stimulatory role of estrogen on sperm motility in the male golden hamster (Mesocricetus auratus). Journal of Andrology, 2005, 26(4): 478-484. |
| [25] | MISAO R, NIWA K, MORISHITA S, et al. Immunohistochemical detection of estrogen and progesterone receptors in spermatozoa of infertile men. International Journal of Fertility and Womens Medicine, 1997, 42(6): 421-425. |
| [26] | GABET V, MIEGE C, BADOS P, et al. Analysis of estrogens in environmental matrices. Trends in Analytical Chemistry, 2007, 26(11): 1113-1131. DOI:10.1016/j.trac.2007.10.003 |
| [27] | ANGUS R A, STANKO J, JENKINS R L, et al. Effects of 17α-ethynylestradiol on sexual development of male western mosquitofish (Gambusia affinis). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2005, 140(3/4): 330-339. |
| [28] | NIMROD A C, BENSON W H. Reproduction and development of Japanese medaka following an early life stage exposure to xenoestrogens. Aquatic Toxicology, 1998, 44(1/2): 141-156. |
| [29] | BIEGEL L B, FLAWS J A, HIRSHFIELD A N, et al. 90-day feeding and one-generation reproduction study in Crl: CD BR rats with 17β-estradiol. Toxicological Sciences, 1998, 44(2): 116-142. |
| [30] | KIDD K A, BLANCHFIELD P J, MILLS K H, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences of the USA, 2007, 104(21): 8897-8901. DOI:10.1073/pnas.0609568104 |
| [31] | ZHOU Y Q, ZHA J M, XU Y P, et al. Occurrences of six steroid estrogens from different effluents in Beijing, China. Environmental Monitoring & Assessment, 2012, 184(3): 1719-1729. |
| [32] | JIN S W, YANG F X, LIAO T, et al. Seasonal variations of estrogenic compounds and their estrogenicities in influent and effluent from a municipal sewage treatment plant in China. Environmental Toxicology and Chemistry, 2008, 27(1): 146-153. DOI:10.1897/07-072.1 |
| [33] | NAKADA N, TANISHIMA T, SHINOHARA H, et al. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Research, 2006, 40(17): 3297-3303. DOI:10.1016/j.watres.2006.06.039 |
| [34] | MOHAGHEGHIAN A, NABIZADEH R, MESDGHINIA A, et al. Distribution of estrogenic steroids in municipal wastewater treatment plants in Tehran, Iran. Journal of Environmental Health Science and Engineering, 2014, 12: 97. DOI:10.1186/2052-336X-12-97 |
| [35] | ANDERSEN H, SIEGRIST H, HALLING-SRENSEN B, et al. Fate of estrogens in a municipal sewage treatment plant. Environmental Science & Technology, 2003, 37(18): 4021-4026. |
| [36] | HAMID H, ESKICIOGLU C. Fate of estrogenic hormones in wastewater and sludge treatment: A review of properties and analytical detection techniques in sludge matrix. Water Research, 2012, 46(18): 5813-5833. DOI:10.1016/j.watres.2012.08.002 |
| [37] | RACZ L, GOEL R K. Fate and removal of estrogens in municipal wastewater. Journal of Environmental Monitoring, 2010, 12(1): 58-70. DOI:10.1039/B917298J |
| [38] | KHANAL S K, XIE B, THOMPSON M L, et al. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Cheminform, 2007, 38(1): 6537-6546. |
| [39] | RAMAN D R, WILLIAMS E L, LAYTON A C, et al. Estrogen content of dairy and swine wastes. Environmental Science & Technology, 2004, 38(13): 3567-3573. |
| [40] | JOHNSON A C, WILLIAMS R J, MATTHIESSEN P. The potential steroid hormone contribution of farm animals to freshwaters, the United Kingdom as a case study. Science of the Total Environment, 2006, 362(1/2/3): 166-178. |
| [41] | HANSELMAN T A, GRAETZ D A, WILKIE A C. Manure-borne estrogens as potential environmental contaminants: A review. Environmental Science & Technology, 2003, 37(24): 5471-5478. |
| [42] | SHEMESH M, SHORE L S. Naturally produced steroid hormones and their release into the environment. Pure and Applied Chemistry, 2003, 75(11/12): 1859-1871. |
| [43] | ZHANG F S, LI Y X, YANG M, et al. Copper residue in animal manures and the potential pollution risk in northeast China. Journal of Resources and Ecology, 2012, 2(1): 91-96. |
| [44] | LI Y X, GAO S Y, LIU S F, et al. Excretion of manure-borne estrogens and androgens and their potential risk estimation in the Yangtze River Basin. Journal of Environmental Sciences, 2015, 37(11): 110-117. |
| [45] |
李艳霞, 刘姝芳, 张雪莲, 等. 我国直辖市畜禽养殖排放类固醇激素特征及其潜在污染风险. 环境科学学报, 2013, 33(8): 2314-2323. LI Y X, LIU S F, ZHANG X L, et al. The excretion features of manure-borne steroid hormones and their potential risk in the municipalities of China. Acta Scientiae Circumstantiae, 2013, 33(8): 2314-2323. (in Chinese with English abstract) |
| [46] |
刘姝芳, 李艳霞, 张雪莲, 等. 东北三省畜禽养殖类固醇激素排放及其潜在污染风险. 环境科学, 2013, 34(8): 3180-3187. LIU S F, LI Y X, ZHANG X L, et al. Excretion of manure-borne steroid hormones and their potential risk in the three northeast provinces of China. Environmental Science, 2013, 34(8): 3180-3187. (in Chinese with English abstract) |
| [47] | ZHENG W, YATES S R, BRADFORD S A. Analysis of steroid hormones in a typical dairy waste disposal system. Environmental Science & Technology, 2008, 42(2): 530-535. |
| [48] | TANG X J, NAVEEDULLAH, HASHMI M Z, et al. A preliminary study on the occurrence and dissipation of estrogen in livestock wastewater. Bulletin of Environmental Contamination and Toxicology, 2013, 90(4): 391-396. DOI:10.1007/s00128-012-0912-4 |
| [49] | FURUICHI T, KANNAN K, SUZUKI K, et al. Occurrence of estrogenic compounds and removal by a swine farm waste treatment plant. Environmental Science & Technology, 2006, 40(24): 7896-7902. |
| [50] | ZHANG H, SHI J H, LIU X W, et al. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China. Water Research, 2014, 58(7): 248-257. |
| [51] | CHEN T S, CHEN T C, YEH K J, et al. High estrogen concentrations in receiving river discharge from a concentrated livestock feedlot. The Science of the Total Environment, 2010, 408(16): 3223-3230. DOI:10.1016/j.scitotenv.2010.03.054 |
| [52] | HUANG B, WANG B, REN D, et al. Occurrence, removal and bioaccumulation of steroid estrogens in Dianchi Lake catchment, China. Environment International, 2013, 59(3): 262-273. |
| [53] |
胡碧波, 阳春, 张智, 等. 嘉陵江典型城市江段的类固醇雌激素分布特性. 中国给水排水, 2011, 27(21): 54-58. HU B B, YANG C, ZHANG Z, et al. Distribution characteristics of steroid estrogens in a typical urban section of Jialing River. China Water & Wastewater, 2011, 27(21): 54-58. (in Chinese with English abstract) |
| [54] | HAN W, LI Y X, YANG M, et al. Presence and determination of manure-borne estrogens from dairy and beef cattle feeding operations in northeast China. Bulletin of Environmental Contamination and Toxicology, 2011, 86(5): 465-469. DOI:10.1007/s00128-011-0247-6 |
| [55] | SARMAH A K, NORTHCOTT G L, LEUSCH F D, et al. A survey of endocrine disrupting chemicals (EDCs) in municipal sewage and animal waste effluents in the Waikato region of New Zealand. Science of the Total Environment, 2006, 355(1/2/3): 135-144. |
| [56] | KJAER J, OLSEN P, BACH K, et al. Leaching of estrogenic hormones from manure-treated structured soils. Environmental Science & Technology, 2007, 41(11): 3911-3917. |
| [57] | JIN X L, JIANG G B, HUANG G L, et al. Determination of 4-tertoctylphenol, 4-nonylphenol and bisphenol A in surface waters from the Haihe River in Tianjin by gas chromatography-mass spectrometry with selected ion monitoring. Chemosphere, 2004, 56(11): 1113-1119. DOI:10.1016/j.chemosphere.2004.04.052 |
| [58] | HUTCHINS S R, WHITE M V, HUDSON F M, et al. Analysis of lagoon samples from different concentrated animal feeding operations for estrogens and estrogen conjugates. Environmental Science & Technology, 2007, 41(3): 738-744. |
| [59] | MATTHIESSEN P, ARNOLD D, JOHNSON A C, et al. Contamination of headwater streams in the United Kingdom by oestrogenic hormones from livestock farms. Science of the Total Environment, 2006, 367(2/3): 616-630. |
| [60] |
吴世闽, 贾瑗, 彭辉, 等. 辽东湾海水中甾体雌激素的检测及生态风险评价. 中国环境科学, 2011, 31(11): 1904-1909. WU S M, JIA A, PENG H, et al. Determination and risk assessment of sterodal estrogens in Liaodong Bay, China. China Environmental Science, 2011, 31(11): 1904-1909. (in Chinese with English abstract) |
| [61] | LEI B L, HUANG S B, ZHOU Y Q, et al. Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere, 2009, 76(1): 36-42. DOI:10.1016/j.chemosphere.2009.02.035 |
| [62] | DUONG C N, RA J S, CHO J, et al. Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere, 2010, 78(3): 286-293. DOI:10.1016/j.chemosphere.2009.10.048 |
| [63] | KAWAGUCHI M, ISH Ⅱ Y, SAKUI N, et al. Stir bar sorptive extraction with in situ derivatization and thermal desorption-gas chromatography-mass spectrometry in the multi-shot mode for determination of estrogens in river water samples. Journal of Chromatography A, 2004, 1049(1/2): 1-8. |
| [64] | SINGH S P, AZUA A, CHAUDHARY A, et al. Occurrence and distribution of steroids, hormones and selected pharmaceuticals in South Florida coastal environments. Ecotoxicology, 2010, 19(2): 338-350. DOI:10.1007/s10646-009-0416-0 |
| [65] | GROVER D P, ZHOU J L, FRICKERS P E, et al. Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: Impact on receiving river water. Journal of Hazardous Materials, 2011, 185(2/3): 1005-1011. |
| [66] | PRIETO A, VALLEJO A, ZULOAGA O, et al. Selective determination of estrogenic compounds in water by microextraction by packed sorbents and a molecularly imprinted polymer coupled with large volume injection-in-port-derivatization gas chromatography-mass spectrometry. Analytica Chimica Acta, 2011, 703(1): 41-51. DOI:10.1016/j.aca.2011.07.007 |
| [67] | WANG L, YING G G, CHEN F, et al. Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools. Environmental Pollution, 2012, 165(6): 241-249. |
| [68] | ZHANG F S, XIE Y F, LI X W, et al. Accumulation of steroid hormones in soil and its adjacent aquatic environment from a typical intensive vegetable cultivation of North China. Science of the Total Environment, 2015, 538: 423-430. DOI:10.1016/j.scitotenv.2015.08.067 |
| [69] | MANSELL D S, BRYSON R J, HARTER T, et al. Fate of endogenous steroid hormones in steer feedlots under simulated rainfall-induced runoff. Environmental Science & Technology, 2011, 45(20): 8811-8818. |
| [70] | NICHOLS D J, DANIEL T C, EDWARDS D R, et al. Use of grass filter strips to reduce 17 beta-estradiol in runoff from fescue-applied poultry litter. Journal of Soil & Water Conservation, 1998, 53(1): 74-77. |
| [71] | SARMAH A K, NORTHCOTT G L, SCHERR F F. Retention of estrogenic steroid hormones by selected New Zealand soils. Environment International, 2008, 34(6): 749-755. DOI:10.1016/j.envint.2007.12.017 |
| [72] | COLUCCI M S, TOPP E. Persistence of estrogenic hormones in agricultural soils: Ⅱ. 17(Alpha)-ethynylestradiol. Journal of Environmental Quality, 2001, 30(6): 2077-2080. DOI:10.2134/jeq2001.2077 |
| [73] | FINLAY-MOORE O, HARTEL P G, CABRERA M L. 17β -estradiol and testosterone in soil and runoff from grasslands amended with broiler litter. Journal of Environmental Quality, 2000, 29(5): 1604-1611. |
| [74] | SCHUH M C, CASEY F X, HAKK H, et al. Effects of fieldmanure applications on stratified 17β -estradiol concentrations. Journal of Hazardous Materials, 2011, 192(2): 748-752. DOI:10.1016/j.jhazmat.2011.05.080 |
| [75] |
韩伟. 奶牛及肉牛雌激素的排放及其环境迁移特征. 北京: 北京师范大学, 2010: 37-40. HAN W. Excretion of natural estrogens in the cattle production and their environmental transportation. Beijing: Beijing Normal University, 2010: 37-40. (in Chinese with English abstract) http://d.wanfangdata.com.cn/Thesis/Y1771285 |
| [76] |
王代懿. 天然类固醇激素在土壤中的环境行为及风险控制研究. 北京: 中国矿业大学, 2015: 20-21. WANG D Y. Study on environmental behavior of natural steroid hormones in soils and its risk control. Beijing: China University of Mining & Technology, 2015: 20-21. (in Chinese with English abstract) |
| [77] | DERBY N E, HAKK H, CASEY F X M, et al. Effects of composting swine manure on nutrients and estrogens. Soil Science, 2011, 176(2): 91-98. DOI:10.1097/SS.0b013e3182088377 |
| [78] | DELAUNE P B, MOORE P A Jr. 17β-estradiol in runoff as affected by various poultry litter application strategies. Science of the Total Environment, 2013, 444(7): 26-31. |
| [79] | ZHANG F S, LI Y X, ZHANG G X, et al. The importance of nanoporosity in the stalk-derived biochar to the sorption of 17β -estradiol and retention of it in the greenhouse soil. Environmental Science and Pollution Research, 2017, 24(10): 9575-9584. DOI:10.1007/s11356-017-8630-4 |
| [80] | LABADIE P, CUNDY A B, STONE K, et al. Evidence for the migration of steroidal estrogens through river bed sediments. Environmental Science & Technology, 2007, 41(12): 4299-4304. |
| [81] | KJAER J, BACH K, BARLEBO H C, et al. Leaching of estrogenic hormones from manure-treated structured soils. Environmental Science & Technology, 2007, 41(11): 3911-3917. |
| [82] | THOMPSON M L, CASEY F X, KHAN E, et al. Occurrence and pathways of manure-borne 17β-estradiol in vadose zone water. Chemosphere, 2009, 76(4): 472-479. DOI:10.1016/j.chemosphere.2009.03.037 |
| [83] | ARNON S, DAHAN O, ELHANANY S, et al. Transport of testosterone and estrogen from dairy-farm waste lagoons to groundwater. Environmental Science & Technology, 2008, 42(15): 5521-5526. |
| [84] | FAN Z S, CASEY F X M, HAKK H, et al. Persistence and fate of 17β-estradiol and testosterone in agricultural soils. Chemosphere, 2007, 67(5): 886-895. DOI:10.1016/j.chemosphere.2006.11.040 |
| [85] |
姜鲁, 王继华, 李建忠, 等. 炔雌醇和壬基酚在土壤中的吸附-解吸特征. 环境科学, 2012, 33(11): 3885-3892. JIANG L, WANG J H, LI J Z, et al. Sorption and desorption of 17α-ethinyl estradiol and 4-n-nonylphenol in soil. Environmental Science, 2012, 33(11): 3885-3892. (in Chinese with English abstract) |
| [86] | CLARA M, STRENN B, SARACEVIC E, et al. Adsorption of bisphenol-A, 17β-estradiole and 17α-ethinylestradiole to sewage sludge. Chemosphere, 2004, 56(9): 843-851. DOI:10.1016/j.chemosphere.2004.04.048 |
| [87] |
张丰松, 李艳霞, 黄泽春, 等. 雌二醇在土壤/沉积物中的吸附特征及猪粪DOM对吸附的影响. 环境科学, 2012, 33(10): 3542-3546. ZHANG F S, LI Y X, HUANG Z C, et al. Sorption of 17β-estradiol to soils and sediment and influence of pig manure DOM. Environmental Science, 2012, 33(10): 3542-3546. (in Chinese with English abstract) |
| [88] |
王子莹, 金洁, 张哲赟, 等. 土壤和沉积物中有机质对双酚A和17α-乙炔基雌二醇的吸附行为. 环境化学, 2012, 31(5): 625-630. WANG Z Y, JIN J, ZHANG Z Y, et al. Sorption of 17α-ethinyl estradiol and bisphenol A by different soil/sediment organic matter fractions. Environmental Chemistry, 2012, 31(5): 625-630. (in Chinese with English abstract) |
| [89] | VAN EMMERIK T, ANGOVE M J, JOHNSON B B, et al. Sorption of 17 beta-estradiol onto selected soil minerals. Journal of Colloid and Interface Science, 2003, 266(1): 33-39. |
| [90] |
王联芝, 章飞芳, 薛兴亚, 等. 土壤中阴离子表面活性剂对17β-雌二醇吸附脱附的影响. 精细化工, 2008, 25(7): 691-695. WANG L Z, ZHANG F F, XUE X Y, et al. Effects of surfactant sodium dodecylbenzenesulfonate on the adsorption and desorption of 17β-estradiol on soil. Fine Chemicals, 2008, 25(7): 691-695. (in Chinese with English abstract) |
| [91] |
马晓雁, 倪梦婷, 倪永炯, 等. UV体系中3种微量类固醇雌激素的竞争降解及同趋转化. 中国环境科学, 2014, 34(4): 904-911. MA X Y, NI M T, NI Y J, et al. Competitive degradation and transformation trend of three steroid estrogens in UV system. China Environmental Science, 2014, 34(4): 904-911. (in Chinese with English abstract) |
| [92] | XUAN R, BLASSENGALE A A, WANG Q. Degradation of estrogenic hormones in a silt loam soil. Journal of Agricultural and Food Chemistry, 2008, 56(19): 9152-9158. DOI:10.1021/jf8016942 |
| [93] |
周坤, 郁晴, 孙卫玲. 复合污染体系中溶解性有机质对雌二醇降解的影响. 安全与环境学报, 2015, 15(3): 227-233. ZHOU K, YU Q, SUN W L. Effect of the dissolved organic matters on the biodegradation of 17β-estradiol in co-contaminant system. Journal of Safety and Environment, 2015, 15(3): 227-233. (in Chinese with English abstract) |
 2017, Vol. 43
2017, Vol. 43