| 大肠杆菌表面感应机制研究进展 |
大肠杆菌是人和动物胃肠道的主要兼性厌氧菌群,包括共生性和致病性2种类型,既能在人或动物宿主体内存活,也能在环境中传播[1]。致病性大肠杆菌在环境中的传播严重威胁人和动物的健康,每年造成大量的感染和严重的经济损失[2]。粪肥和污水中的致病性大肠杆菌可经土壤传播到农作物的根系或表面,进入食品产业链,威胁人体健康[3-5]。
环境中的大肠杆菌能吸附在各种生物(植物根系或叶片)和非生物(土壤颗粒)表面,形成生物膜以存活和传播[1]。生物膜的形成过程主要包括以下5个步骤。1)初始吸附,通常是可逆过程[6-7];2)紧密吸附,即微生物通过分泌DNA、蛋白质、脂质以及多糖等胞外聚合物,改变自身的生理生化状态,与表面发生紧密结合,通常是不可逆吸附[8];3)形成微菌落:微生物不断分泌胞外聚合物(extracellular polymeric substances, EPS),并形成一层凝胶层将细胞包裹在内部区域[9];4)生物膜的成熟:微菌落内的细胞持续分泌EPS,最终形成具有三维空间结构的成熟生物膜;5)生物膜的解体:受环境因子和细胞性质等因素的影响,部分微生物从生物膜中逃离,重新分散到外部环境中[10]。在生物膜形成的多步骤过程中,微生物在固相表面的初始吸附是其形成生物膜的关键,该过程包括了微生物对固相表面的“感知”以及初始吸附后细胞的“响应”,这一过程被称为微生物的表面感应[11]。
本文重点介绍了环境微生物-大肠杆菌表面感应相关研究进展,期望能为土壤环境中病原微生物的控制提供理论依据,同时也能加深对土壤生物膜的认识,最终能揭开土壤中这些具有活性的生物膜的神秘面纱,深入理解土壤生物过程的本质,从而更好地调控生物膜参与的养分循环和污染物降解等过程,保障土壤健康。
1 表面感应概念内涵细菌生物膜形成的第一步是细胞与固相表面接触,但细菌如何知道它正在靠近或已经吸附在固相表面的这种由悬浮态向吸附态转换的过程,实际上依赖于细菌对表面的“识别”和“响应”,即“表面感应” [12]。细菌表面感应包含多种行为,在细菌与表面接近的过程中,可利用表面结构(如鞭毛和菌毛)感知固相表面,通过胞内信号系统调节细胞运动性和表面性质调控吸附。例如:副溶血性弧菌利用鞭毛感知表面,刺激scrABC系统中的质膜蛋白ScrC,诱导c-di-GMP水平上升,抑制细胞运动并促进吸附[13-14];变形杆菌的鞭毛感知表面后,鞭毛基质蛋白FliL能通过UmoA调节子调控鞭毛监管蛋白FlhD,控制细胞运动性[15];枯草芽孢杆菌利用鞭毛感知表面,触发DegS-DegU机械开关,影响枯草芽孢杆菌的吸附和生物膜的形成[16];铜绿假单胞菌利用Ⅳ型菌毛(TFP)接受表面信号,TFP相关蛋白PilY1刺激c-di-GMP水平上调,抑制鞭毛运动,促进细胞进入生物膜状态[11]。
2 大肠杆菌表面感应模式系统大肠杆菌是自然环境和哺乳动物胃肠道中普遍存在的重要微生物,是研究细菌表面感应的重要模式系统,其中,细胞的表面结构和胞内信号系统是影响大肠杆菌表面感应的主要因素。大肠杆菌能通过鞭毛、Ⅰ型菌毛和膜蛋白感知表面,通过双组分系统控制细胞运动性和表面性质(表面结构、细胞分泌、表面电荷和疏水性),调控大肠杆菌在不同表面的吸附。
2.1 表面结构大肠杆菌在表面吸附的初始阶段,细胞表面结构如鞭毛、菌毛、脂多糖、膜蛋白等是影响细菌在表面吸附的主要因素[17],环境因素如pH、离子强度和温度也可通过表面结构影响细菌在表面的吸附。
细菌鞭毛能帮助细胞克服环境中的流体作用力和细胞与表面间的静电斥力,从而诱发初始吸附[18];当细胞靠近表面,鞭毛旋转受阻,结构发生形变,鞭毛在细胞与表面间起到桥接作用[12]。由鞭毛介导的可逆吸附阶段主要受环境因素(如pH、离子强度、温度)和表面性质(褶皱、疏水性)影响[18]。PRATT等[19]利用转座子随机插入法制备大肠杆菌突变体,筛选了聚氯乙烯(polyvinyl chloride, PVC)表面生物膜形成能力有缺陷的突变体菌株,发现有超过半数的突变体菌株有鞭毛功能缺陷,这暗示了鞭毛运动性与大肠杆菌生物膜形成密切相关,这可能是由于大肠杆菌必须依赖鞭毛运动才能靠近PVC表面,也可能因为鞭毛是大肠杆菌与PVC表面直接接触的桥梁。WOOD等[20]制备大肠杆菌鞭毛突变株(fliA)和运动性缺陷突变株(motA),对比菌株形成生物膜的差异,发现运动性最好的野生型细胞成膜能力最强(≈43 μm厚,表面覆盖率21%~ 34%),含有鞭毛结构的运动性缺陷突变株motA成膜能力次之(≈13 μm厚,表面覆盖率41%~58%),无运动性也没有鞭毛结构的突变株fliA成膜能力最差(表面覆盖率小于5%)。这些结果证实了鞭毛运动性和结构对大肠杆菌生物膜形成至关重要。
菌毛是大肠杆菌与表面在不可逆吸附阶段起主要功能的细胞器,可促进细胞发生不可逆吸附[21]。大肠杆菌的Ⅰ型菌毛(主要由fim基因编码的FimA菌毛蛋白组成,末端含有特异性吸附素FimH)和P型菌毛(主要由pap基因编码的PapA菌毛蛋白组成,末端含有特异性吸附素PapG)在大肠杆菌初始吸附过程中起着重要作用。在大肠杆菌与PVC的吸附体系中,fim(Ⅰ型菌毛)基因突变株在PVC表面的成膜量较低,其主要原因是突变株在PVC表面的初始吸附量较少。Ⅰ型菌毛上的定位吸附蛋白-FimH既能诱导大肠杆菌与生物表面甘露糖的特异性结合,也能调控大肠杆菌在无机表面(如PVC)的非特异性吸附。这些结果表明,Ⅰ型菌毛是大肠杆菌在无机表面初始吸附过程中必不可少的组分[19, 22]。在大肠杆菌与聚苯乙烯的吸附体系中,编码菌毛的csgA基因突变株成膜量显著降低,ompR基因突变株显著提高csgA表达量,表明菌毛是大肠杆菌在惰性表面形成生物膜的关键结构,且受EnvZ-OmpR双组分系统调控[23]。
除了细胞膜外部结构,一些镶嵌在细胞膜上的跨膜蛋白也能调节细胞吸附,膜蛋白在表面结构与胞内信号系统间传递信号。通过双向电泳发现,吸附在涂有金膜石英晶体表面的大肠杆菌OmpA、OmpX、Slp和TolC等4种膜蛋白表达量下调,ompX突变株提高了细胞Ⅰ型菌毛和胞外多糖的表达量,降低了细胞运动性[24]。外膜脂蛋白NlpE能通过诱导Cpx系统响应表面吸附引发的细胞膜扰动,激活细菌对无机疏水性表面的响应。例如,在大肠杆菌K-12与玻璃珠混合的体系中,cpxR与nlpE突变株在玻璃表面的吸附量显著降低,约为野生型菌株吸附量的1/3;在吸附4 h的过程中,野生型大肠杆菌表达了大量的CpxR蛋白,而cpxR与nlpE突变株几乎不表达CpxR蛋白,表明NlpE蛋白能通过激活Cpx系统刺激大肠杆菌在玻璃表面的吸附[25]。
脂多糖(lipopolysaccharides, LPS)是革兰氏阴性菌细胞壁最外层的一层较厚(8~10 nm)的类脂多糖类物质,由类脂A、核心多糖和O-特异侧链组成[26]。LPS能将细胞固定在距离表面20 nm处的位置,并在细胞与表面之间形成氢键[27]。LPS也能通过调控细胞表面疏水性影响细胞对不同表面的吸附性。LPS有2种形态:A-LPS和B-LPS。A-LPS提高细胞表面疏水性,B-LPS提高细胞表面亲水性。B-LPS含量越高,细胞对亲水性玻璃表面的吸附量就越高。A-LPS和B-LPS的相对含量受环境因子控制,通过改变细胞表面性质可以选择合适的表面以供吸附,有助于细菌在环境中的存活[28]。LPS缺失,则降低了大肠杆菌在无机表面的吸附能力[29]。例如:在大肠杆菌K-12与聚苯乙烯的吸附体系中,细胞表面LPS结构基因发生突变降低了菌株的吸附量(约为野生型菌株吸附量的50%~80%);细胞在软琼脂平板上的运动性实验也表明,LPS突变株的运动性显著低于野生型菌株;利用斑点印迹法分析野生型和LPS突变株的Ⅰ型菌毛的产量,发现LPS突变株的Ⅰ型菌毛含量只有野生型细胞的20%~ 60%。这些结果表明,LPS能通过影响Ⅰ型菌毛的产量和细胞运动性影响大肠杆菌在聚苯乙烯表面的吸附[29]。
胞外聚合物(EPS)是大肠杆菌生物膜的主要组分,在大肠杆菌生物膜的形成过程中具有重要作用。大肠杆菌与表面的吸附能促进EPS的分泌,但EPS对初始吸附和生物膜形成的影响却不同。荚膜异多糖酸是大肠杆菌EPS的主要组分。DANESE等[30]检测了野生型大肠杆菌K-12和荚膜异多糖酸突变株在PVC表面的吸附量,发现二者吸附量没有显著差异;进一步利用荧光显微镜观察野生型大肠杆菌K-12和荚膜异多糖酸突变株生物膜结构,发现荚膜异多糖酸突变株的生物膜只有薄薄的一层,且表面没有复杂的三维结构。
2.2 细胞密度除了细胞表面结构,细胞密度(悬浮密度和沉积密度)也能影响细胞在表面的吸附。大肠杆菌的悬浮密度通常在1.06~1.13 g/mL之间,此时悬浮液中的细胞缓慢向固体表面沉积[31]。当大肠杆菌进入稳定期,细胞悬浮密度增加,促进细胞在表面上的快速聚集[32]。MAUTER等[33]通过改变离子强度控制大肠杆菌K-12在石英柱表面上的沉积密度,运用基因芯片和代谢组学技术测量了不同沉积密度对大肠杆菌吸附过程中基因表达的影响,结果发现:离子强度越高,大肠杆菌吸附量越高,细胞沉积密度也越高;细胞沉积密度越高,大肠杆菌中琥珀酸、脯氨酸、焦谷氨酸和色氨酸的催化基因含量越低,导致胞内的代谢物浓度越高;在高沉积密度条件下,吸附态细胞的菌毛含量比悬浮态细胞高出了约4倍;色氨酸的代谢产物能作为群体感应的信号调节大肠杆菌的运动性,表明表面细胞沉积密度能通过改变大肠杆菌的代谢强度、群体感应和表面结构影响细菌在无机表面的吸附。
2.3 胞内双组分信号系统胞内信号系统主要通过改变细胞表面结构和响应环境压力2种方式调控表面感应。调控大肠杆菌表面感应的系统主要是Cpx、Rcs和EnvZ-OmpR双组分系统。双组分系统是细菌的“神经中枢”,是最先感应到环境信号的组分,主要由位于内膜上的组氨酸激酶(histidine kinases, HK)和胞内的反应调节子(response regulator, RR)组成。当HK检测到环境信号,首先自磷酸化,然后将磷酸基传递给RR,诱导一系列下游基因表达变化[34]。
调控菌毛的表达量是胞内信号系统调控表面感应的重要途径(图 1)。Cpx系统中的CpxR蛋白是csgA的负调节因子,cpxA基因能通过CpxR的去磷酸化增加csgA转录水平,提高菌毛表达量[35];Rcs系统能调节csgDEFG的表达,RcsA和RcsB能激活csgD,抑制csgBA的表达,从而降低菌毛合成[36];EnvZOmpR系统中的OmpR蛋白能与csgD启动子结合刺激其转录,CsgD激活编码菌毛的csgBA启动子,提高菌毛表达量[37]。不同信号系统也能互相影响,OmpR只能特异性的与csgD启动子结合,而CpxR能与多个位点结合,当CpxR的识别位点与csgD-OmpR结合位点重叠时,能抑制菌毛表达[38]。
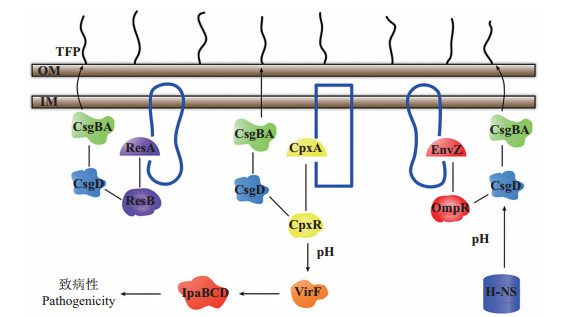 |
| 图1 大肠杆菌表面感应模型 Fig. 1 Model of Escherichia coli surface sensing |
胞内信号系统也能通过响应环境压力(渗透压、pH)调控表面感应(图 1)。当环境pH高于7.4,Cpx系统能激活virF基因,提高IpaBCD蛋白含量,促进细菌入侵宿主细胞;当pH低于6.0,virF的表达受到抑制[39]。大肠杆菌K-12在微量滴定板上的生物膜量随渗透压的升高而降低。相对于低渗透压环境,在高渗透压条件下CpxR蛋白合成量提高40%,总CpxR蛋白磷酸化水平从25%提高到超过50%,表明Cpx系统可以从转录和翻译2个水平响应渗透压变化[38]。
胞内信号系统对环境压力的响应和对表面结构的调控是一个互相联系的网络。在低渗透压条件下,OmpR和H-NS激活csgD转录,Rcs系统中的RcsB抑制csgD表达;在高渗透压条件下,CpxR磷酸化水平上升1倍,抑制csgD的表达,而H-NS仍能激活csgD[38];EnvZ-OmpR系统中的OmpA蛋白能感应渗透压变化,通过Cpx系统抑制纤维素产物,并提高大肠杆菌成膜量[40];通过抑制表面蛋白OmpX的产量可降低细胞表面疏水性和电负性,抑制EPS产量并增强细胞的群集运动,增强细胞对抗生素(丁胺卡那霉素、头孢菌素、庆大霉素、新生菌素和萘啶酸)的抗性,促进细胞在疏水性无机表面的吸附[41]。
3 展望大肠杆菌的表面感应是一个复杂的、多步骤的、互相协调的过程,通过一套复杂的系统调控生物膜的形成。目前已知大肠杆菌的菌毛和鞭毛能感知表面,但其他表面结构是否具有感知表面的能力还不得而知;而且胞内双组分系统如何改变表面结构,特别是从细胞接收信号到改变细胞行为这一过程,具体的机制仍然未知;多重调控系统共存时,系统间的互作及对表面感应的影响也不清楚,这些工作是大肠杆菌表面感应研究今后需着重解决的主要科学问题[42-43]。对于土壤系统,由于其固相组成复杂,影响大肠杆菌存活和传播的因子众多,从生物学角度去理解大肠杆菌对土壤组分的表面感应机制还是空白,这也是值得土壤学工作者今后研究的方向。
| [1] | VAN ELSAS J D, SEMENOV A V, COSTA R, et al. Survival of Escherichia coli in the environment: Fundamental and public health aspects. The ISME Journal, 2011, 5(2): 173-183. DOI:10.1038/ismej.2010.80 |
| [2] | MEAD P S, SLUTSKER L, DIETZ V, et al. Food-related illness and death in the United States. Emerging Infectious Diseases, 1999, 5(5): 607-625. DOI:10.3201/eid0505.990502 |
| [3] | SOLOMON E B, YARON S, MATTHEWS K R. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Applied and Environmental Microbiology, 2002, 68(1): 397-400. DOI:10.1128/AEM.68.1.397-400.2002 |
| [4] | ITOH Y, SUGITA-KONISHI Y, KASUGA F, et al. Enterohemorrhagic Escherichia coli O157:H7 present in radish sprouts. Applied and Environmental Microbiology, 1998, 64(4): 1532-1535. |
| [5] | NATVIG E E, INGHAM S C, INGHAM B H, et al. Salmonella enterica serovar typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Applied and Environmental Microbiology, 2002, 68(6): 2737-2744. DOI:10.1128/AEM.68.6.2737-2744.2002 |
| [6] | BULLITT E, MAKOWSKI L. Structural polymorphism of bacterial adhesion pili. Nature, 1995, 373(6510): 164-167. DOI:10.1038/373164a0 |
| [7] | THOMAS W E, NILSSON L M, FORERO M, et al. Shear-dependent " stick-and-roll"adhesion of type Ⅰ fimbriated Escherichia coli. Molecular Microbiology, 2004, 53(5): 1545-1557. DOI:10.1111/j.1365-2958.2004.04226.x |
| [8] | FLEMMING H C, WINGENDER J. The biofilm matrix. Nature Reviews Microbiology, 2010, 8(9): 623-633. |
| [9] | BORLEE B R, GOLDMAN A D, MURAKAMI K, et al. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Molecular Microbiology, 2010, 75(4): 827-842. DOI:10.1111/mmi.2010.75.issue-4 |
| [10] | COSTERTON J W, CHENG K J, GEESEY G G, et al. Bacterial biofilms in nature and disease. Annual Review of Microbiology, 1987, 41: 435-464. DOI:10.1146/annurev.mi.41.100187.002251 |
| [11] | O' TOOLE G A, WONG G C L. Sensational biofilms: Surface sensing in bacteria. Current Opinion in Microbiology, 2016, 30: 139-146. DOI:10.1016/j.mib.2016.02.004 |
| [12] | BELAS R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Cell Press, 2014, 22(9): 517-527. |
| [13] | FERREIRA R B R, ANTUNES L C M, GREENBERG E P, et al. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. Journal of Bacteriology, 2008, 190(3): 851-860. DOI:10.1128/JB.01462-07 |
| [14] | GODE- POTRATZ C J, KUSTUSCH R J, BREHENY P J, et al. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Molecular Microbiology, 2011, 79(1): 240-263. DOI:10.1111/j.1365-2958.2010.07445.x |
| [15] | CUSICK K, LEE Y Y, YOUCHAK B, et al. Perturbation of FliL interferes with Proteus mirabilis swarmer cell gene expression and differentiation. Journal of Bacteriology, 2012, 194(2): 437-447. DOI:10.1128/JB.05998-11 |
| [16] | CAIRNS L S, MARLOW V L, BISSETT E, et al. A mechanical signal transmitted by the flagellum controls signalling in Bacillus subtilis. Molecular Microbiology, 2013, 90(1): 6-21. |
| [17] | TUSON H H, WEIBET D B. Bacteria-surface interactions. Royal Society of Chemistry, 2013, 9(17): 4368-4380. |
| [18] | DONLAN R M. Biofilms: Microbial life on surfaces. Emerging Infectious Diseases, 2002, 8(9): 881-890. DOI:10.3201/eid0809.020063 |
| [19] | PRATT L A, KOLTER R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type Ⅰ pili. Molecular Microbiology, 1998, 30(2): 285-293. DOI:10.1046/j.1365-2958.1998.01061.x |
| [20] | WOOD T K, BARRIOS A F G, HERZBERG M, et al. Motility influences biofilm architecture in Escherichia coli. Applied Microbiology and Biotechnology, 2006, 72(2): 361-367. DOI:10.1007/s00253-005-0263-8 |
| [21] | CHAO Y Q, ZHANG T. Probing roles of lipopolysaccharide, type Ⅰ fimbriae, and colanic acid in the attachment of Escherichia coli strains on inert surfaces. Langmuir, 2011, 27(18): 11545-11553. DOI:10.1021/la202534p |
| [22] | HORI K, MATSUMOTO S. Bacterial adhesion: From mechanism to control. Biochemical Engineering Journal, 2010, 48(3): 424-434. DOI:10.1016/j.bej.2009.11.014 |
| [23] | VIDAL O, LONGIN R, PRIGENT-COMBARET C, et al. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: Involvement of a new ompR allele that increases curli expression. Journal of Bacteriology, 1998, 180(9): 2442-2449. |
| [24] | OTTO K, NORBECK J, LARSSON T, et al. Adhesion of type Ⅰ-fimbriated Escherichia coli to abiotic surfaces leads to altered composition of outer membrane proteins. Journal of Bacteriology, 2001, 183(8): 2445-2453. DOI:10.1128/JB.183.8.2445-2453.2001 |
| [25] | OTTO K, SILHAVY T J. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proceedings of the National Academy of Sciences of the USA, 2002, 99(4): 2287-2292. DOI:10.1073/pnas.042521699 |
| [26] | DAVEY M E, O' TOOLE G A. Microbial biofilms: From ecology to molecular genetics. Microbiology and Molecular Biology Reviews, 2000, 64(4): 847-867. DOI:10.1128/MMBR.64.4.847-867.2000 |
| [27] | SIMONI S F, HARMS H, BOSMA T N P, et al. Population heterogeneity affects transport of bacteria through sand columns at low flow rates. Environmental Science & Technology, 1998, 32(14): 2100-2105. |
| [28] | MAKIN S A, BEVERIDGE T J. The influence of A-band and Bband lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology, 1996, 142(2): 299-307. DOI:10.1099/13500872-142-2-299 |
| [29] | GENEVAUX P, BAUDA P, DUBOW M S, et al. Identification of Tn10 insertions in the rfaG, rfaP, and galU genes involved in lipopolysaccharide core biosynthesis that affect Escherichia coli adhesion. Archives of Microbiology, 1999, 172(1): 1-8. |
| [30] | DANESE P N, PRATT, L A, KOLTER R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. Journal of Bacteriology, 2000, 182(12): 3593-3596. DOI:10.1128/JB.182.12.3593-3596.2000 |
| [31] | KUBITSCHEK H E. Buoyant density variation during the cell cycle in microorganisms. CRC Critical Reviews in Microbiology, 1987, 14(1): 73-97. DOI:10.3109/10408418709104436 |
| [32] | MAKINOSHIMA H, NISHIMURA A, ISHIHAMA A. Fractionation of Escherichia coli cell populations at different stages during growth transition to stationary phase. Molecular Microbiology, 2002, 43(2): 269-279. DOI:10.1046/j.1365-2958.2002.02746.x |
| [33] | MAUTER M, FAIT A, ELIMELECH M, et al. Surface cell density effects on Escherichia coli gene expression during cell attachment. Environmental Science & Technology, 2013, 47(12): 6223-6230. |
| [34] | VOGT S L, RAIVIO T L. Just scratching the surface: An expanding view of the Cpx envelope stress response. FEMS Microbiology Letters, 2012, 326(1): 2-11. DOI:10.1111/j.1574-6968.2011.02406.x |
| [35] | DOREL C, VIDAL O, PRIGENT-COMBARET C, et al. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiology Letters, 1999, 178(1): 167-175. |
| [36] | VIANNEY A, JUBELIN G, RENAULT S, et al. Escherichia coli tol and rcs genes participate in the complex network affecting curli synthesis. Microbiology, 2005, 151(7): 2487-2497. DOI:10.1099/mic.0.27913-0 |
| [37] | PRIGENT-COMBARET C, BROMBACHER E, VIDAL O, et al. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. Journal of Bacteriology, 2001, 183(24): 7213-7223. DOI:10.1128/JB.183.24.7213-7223.2001 |
| [38] | JUBELIN G, VIANNEY A, BELOIN C, et al. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. Journal of Bacteriology, 2005, 187(6): 2038-2049. DOI:10.1128/JB.187.6.2038-2049.2005 |
| [39] | NAKAYAMA S, WATANABE H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. Journal of Bacteriology, 1995, 177(17): 5062-5069. DOI:10.1128/jb.177.17.5062-5069.1995 |
| [40] | MA Q, WOOD T K. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environmental Microbiology, 2009, 11(10): 2735-2746. DOI:10.1111/emi.2009.11.issue-10 |
| [41] | OTTO K, HERMANSSON M. Inactivation of ompX causes increased interactions of type Ⅰ fimbriated Escherichia coli with abiotic surfaces. Journal of Bacteriology, 2004, 186(1): 226-234. DOI:10.1128/JB.186.1.226-234.2004 |
| [42] | BELOIN C, ROUX A, GHIGO J M. Escherichia coli biofilms// ROMEO T. Bacterial Biofilms. Berlin Heidelberg: Springer, 2008: 249-289. |
| [43] | O'TOOLE G A, WONG G C L. Sensational biofilms: Surface sensing in bacteria. Current Opinion in Microbiology, 2016, 30:139-146. |
 2017, Vol. 43
2017, Vol. 43


