| 土壤主要还原转化过程中微生物功能基因多样性研究进展 |
2. 浙江省农业资源与环境重点实验室,杭州 310058
2. Zhejiang Provincial Key Laboratory of Agricultural Resources and Environment, Hangzhou 310058, China
土壤是一个复杂的氧化还原体系,存在大量活跃的碳、氮、铁、硫等生源要素,以及农药、重金属等污染物质的生物地球化学循环过程,对自然界中物质代谢和能量流动具有决定性的影响。随着全社会对于土壤质量的关注度日渐提高,国家在2016年全面启动了土壤污染防治行动计划,着重强调土壤环境的改善与良性生态系统的构建。因而,开展自然复杂土壤体系中多介质界面上多要素-多过程的耦合作用与机制的研究,对探索物质循环过程、维持生态环境平衡和实现可持续发展均具有重要的现实意义。
自然环境中的氮循环、Fe/S还原、产甲烷以及有机污染物的还原脱卤等还原过程的发生本质上是微生物厌氧呼吸作用介导的电子传递过程。在该过程中,存在着不同的生源要素、蛋白组分间频繁而复杂的交互关系,如硝酸盐、三价铁、硫酸盐和卤代污染物等物质之间存在电子受体间的竞争关系,由功能基因表征的对应的蛋白酶,如亚硫酸盐还原酶、甲基辅酶M还原酶或还原脱卤酶等参与并催化着相关物质的呼吸代谢过程。
近年来,随着研究领域的深入和分子生物学技术的发展,传统的测序手段已经不能完全满足对微观尺度下土壤微生物适应特定环境的变化过程、相关元素物质的代谢机制以及污染物转化与归趋等功能性研究。因此,应用先进的分子生物学方法,如功能基因组学技术、微阵列技术、原位活性检测技术和宏基因组学技术等,利用参与相应代谢过程的功能基因的特异性来指示土壤微生物的生态功能,已成为探究微生物生态信息与特定代谢途径关系的重要纽带[1-2]。
现有研究尚未对复杂土壤体系中的物质代谢和元素耦合关系,及其可能影响的有机污染物还原一步研究氮循环、硫酸盐还原、铁还原、产甲烷以及还原脱卤等过程中的微生物生态特性,揭示相关生源要素耦合污染物还原转化的内在微生物学机制提供参考依据。
1 氮还原转化过程土壤中由微生物介导的氮素循环是生物地球化学循环的重要组成部分之一,在稳定农田生态系统、缓解全球温室效应等方面具有举足轻重的作用。梳理土壤固氮作用、反硝化作用和异化硝酸盐还原成铵作用等过程中的微生物功能基因特性,对掌握氮循环过程、提高氮素可利用性以及控制温室气体排放等意义重大[3]。
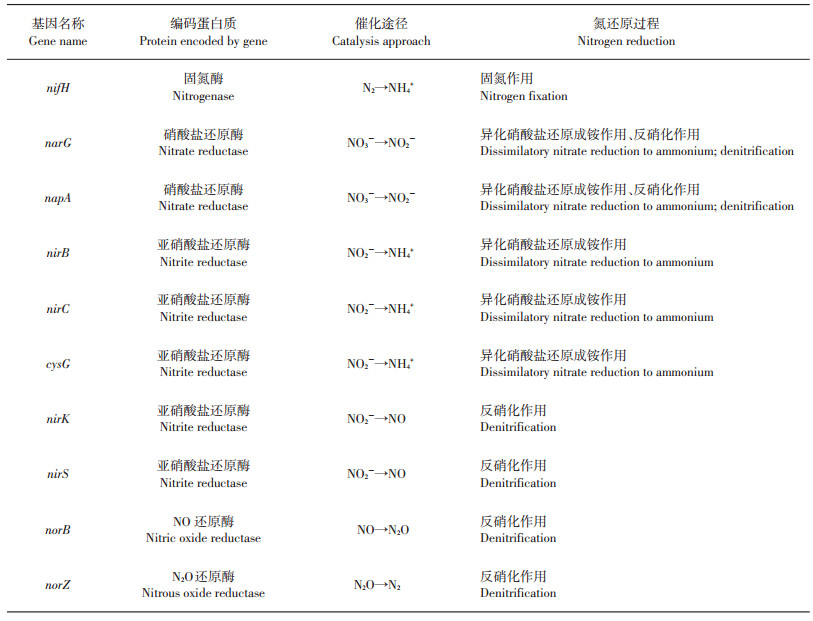
由固氮酶催化进行的生物固氮作用可以将大气中的氮气(N2)还原为生物可利用的铵态氮形式,因而是氮素进入土壤、植物等陆地生态系统中的重要途径之一。固氮酶广泛存在于固氮菌属(Azotobacter)、固氮螺菌属(Azospirillum)和中华根瘤菌属(Sinorhizobium)等微生物中,主要由2个多亚基的金属蛋白酶组成。nifD、nifK基因编码的成分Ⅰ是由2个异二聚体构成的250 kDa蛋白组分,包括N2还原的活性位点。成分Ⅱ(约70 kDa)由nifH基因编码,能够将三磷酸腺苷(adenosine triphosphate,ATP)水解与电子转移过程偶联。Fe-S中心在2个蛋白组分中共同存在,并且介导着固氮酶的生化代谢过程[4]。由于nifD、nifK基因序列相对较少,限制了对固氮酶多样性的系统研究,因此nifH基因常被用作研究固氮微生物的系统进化分析要素,为探索固氮微生物的种类和群落分布提供了重要依据[3, 5](表 1)。
| 表1 土壤中氮还原转化过程的主要功能基因[2-3, 5-13] Table 1 Main functional genes involved in soil nitrogen reduction[2-3, 5-13] |
 |
| 点击放大 |
反硝化作用是土壤中N2O最重要的产生途径,微生物可利用硝酸根作为电子受体,将其呼吸还原为气态产物,具体途径为:NO3-→NO2-→NO→N2O→N2,反应过程逐级连续,涉及的关键酶包括:硝酸盐还原酶 (nitrate reductase, Nar),亚硝酸还原酶(nitrite reductase, Nir),一氧化氮还原酶(nitric oxide reductase, Nor),氧化亚氮还原酶(nitrous oxide reductase, Nos)等,分别由各自对应的功能基因(narG/napA, nirK/nirS, norC/norB,nosZ/nosR/nosD)所编码[6-8](表 1)。硝酸盐还原酶可以催化反硝化过程的第一步,根据其附着位置不同,主要分为膜结合型硝酸盐还原酶(Nar)和周质硝酸盐还原酶(periplasmic nitrate reductase, Nap)。膜结合型硝酸盐还原酶(Nar)由α、β、γ 3个亚基构成,分别由narG、narH和narI基因编码;可溶性的周质硝酸盐还原酶(Nap)是由napA和napB基因编码的异质二聚体结构[6]。其中narG和napA基因是研究硝酸盐还原过程最重要和使用最广泛的功能基因[3]。nirK和nirS基因也是反硝化微生物群落研究中普遍使用的分子标志物,可分别编码含铜(Cu)的亚硝酸盐还原酶(NirK)和细胞色素cd1型亚硝酸盐还原酶(NirS)[3, 9]。由norB和nosZ分别编码的一氧化氮还原酶(Nor)和氧化亚氮还原酶(Nos)同样在反硝化过程中发挥着重要作用[10-11]。
异化硝酸盐还原成铵的过程主要为:在硝酸盐还原酶(Nar)的催化作用下,NO3-还原成NO2-,再由亚硝酸还原酶(Nir)催化还原为NH4+[12-13],其还原酶对应的功能基因分别为(narG/napA, nirB/nirC/cysG)(表 1)。尽管该过程与反硝化过程具有相同的硝酸盐还原酶,但在亚硝酸盐还原酶的结构和分类上(该过程中的亚硝酸盐还原酶主要可分为溶解性酶和胞外酶2类)有明显区别[14-15]。
针对氮还原转化过程中功能基因多样性的研究开展较早,同时也最为全面,在不同生态系统和农艺措施管理过程下相关微生物群落变化等研究已较为普遍。基于nirK、nirS、nosZ、nifH等功能基因靶标的分子生物学研究,对探索功能微生物的分布规律、代谢过程以及与土壤氮素循环之间的关系等方面的研究具有重要的指导意义。
2 硫酸盐还原过程硫酸盐还原过程是地球上最古老的呼吸形式之一,也是物质循环的重要组成部分。在自然条件下,硫氧化形成的硫酸盐一部分可以被植物和微生物吸收利用,另一部分则可在厌氧条件下参与由硫酸盐还原菌介导的异化硫酸盐还原过程(形成H2S)或同化还原过程(形成有机硫化物),从而成为构建硫元素循环的关键过程[16]。
异化硫酸盐还原过程是一个复杂的生物化学反应,其本质是由微生物的多种蛋白组分(如Dsr、Qmo、Hmc、Tmc、Qrc、Nuo和Rnf)催化和介导的电子传递过程。作为一种稳定的化学成分,SO42-首先要在ATP硫酸化酶和APS还原酶(APS reductase, Apr)的作用下获得电子转化为SO32-,再在异化型亚硫酸盐还原酶(dissimilatory sulfite reductase, Dsr)的催化作用下经过一系列电子转移过程,最终还原形成S2-[17](图 1)。ATP硫酸化酶是一种含有钴(Co)和锌(Zn)的金属蛋白,由sat基因编码,同时存在于硫酸盐同化和异化还原过程中,是活化硫酸盐的重要物质。aprBA基因编码的APS还原酶(Apr)是一种重要的可溶性细胞质黄素蛋白,包含2种不同形式的[4Fe-4S]簇和黄素腺嘌呤二核甘酸,广泛存在于所有的硫酸盐还原微生物以及无机营养型的硫化物氧化细菌中。异化型亚硫酸盐还原酶(Dsr)是硫酸盐还原过程中最为关键的功能蛋白之一,由α2β2结构组成,2个亚基分别被邻近的dsrA和dsrB基因所编码表征[17](图 1)。dsr基因在同一类群的硫酸盐还原菌中相当保守,但在不同类群的硫酸盐还原菌中却存在着较大的差别[18-20]。NERETIN等[21]通过纯培养实验发现,硫酸盐还原微生物中dsrAB基因的mRNA水平随着硫酸盐还原速率的增加而上升,这表明环境中的硫酸盐还原速率可以从dsr转录水平来进行评价。因此,dsr基因作为应用最为广泛的、可用于追踪参与调控硫循环功能微生物的关键分子标志物,对于研究硫酸盐还原菌的微生物群落结构和功能特性具有重要作用[22-24]。
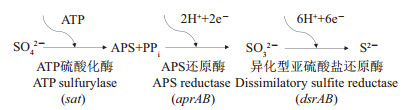 |
| 括号内为编码基因。 Encoding genes are showed in brackets. 图1 异化硫酸盐还原代谢途径[17] Fig. 1 Metabolic schemes associated with dissimilatory sulfate reduction[17] |
经过对Desulfococcus multivorans、Desulfococcus oleovorans、Desulfovibrio vulgaris、Desulfobacterium autotrophicum、Desulfobacula toluolica以及Desulfatibacillum alkenivorans等多种硫酸盐还原微生物的宏基因组测序[17, 25-28],发现其中的dsr操纵子包含多种编码基因,即dsrABEFHCMKLJOPNRS等,可表征不同功能的蛋白质组分,从而参与硫的氧化还原过程[25]。在这些基因产物中,DsrAB蛋白复合物是硫循环代谢途径中不可或缺的功能物质,不仅是催化异化型亚硫酸盐还原过程的关键酶,也是硫化物氧化过程的重要参与者[29]。而其他蛋白物质,如DsrC,可作为氧化还原中心调控硫的代谢过程,协助DsrAB发挥相关作用,并介导DsrMKJOP蛋白复合物行使与DsrAB相似的功能特性[25, 30]。除此之外,微生物中的其他多种蛋白组分也在硫酸盐还原过程中发挥着举足轻重的作用。由qmoABC基因编码的QmoABC蛋白复合物可作为电子传递通道从细胞膜向AprAB输送电子;Hmc、Tmc和Qrc蛋白参与细胞质与周质间的电子传递过程,与脱氢酶等功能物质的代谢过程息息相关;同时,由相关基因编码的Nuo、Rnf和Hdr等蛋白组分也在电子传递、能量转化、过程耦合等方面扮演着重要角色[17]。
除了异化硫酸盐还原过程,微生物还可以在同化型亚硫酸盐还原酶(sulfite reductases, SirA)的作用下吸收硫酸盐形成有机硫化物[31-32]。目前,依托基因敲除、代谢筛选、酶组学和宏基因组学等分子生物学的相关技术,针对大肠杆菌、枯草杆菌、棒状杆菌、分枝杆菌、链霉菌以及放线菌等微生物已经进行了较为详尽的代谢途径的阐述[31-33]。sirA、aprBA、fpr2-cysIXHDNYZ基因簇等功能基因编码了同化硫酸盐还原过程的大量相关的蛋白酶,如同化型亚硫酸盐还原、APS还原酶和半胱氨酸合成酶等,从而参与了无机硫化物还原中的重要步骤[32-33]。
硫酸盐还原过程常常伴随着土壤环境中多种氧化还原反应,如铁循环、污染物的还原脱卤、产甲烷及其厌氧氧化等过程,不同的蛋白质复合体(Qmo、Apr、Hdr等)及其功能基因也常常被用于共同研究硫酸盐还原菌的丰度及功能特性。
3 铁还原过程在自然环境中,铁作为最丰富、最活跃的变价金属元素,以多种化合价形式(如0、+2、+3、+6等)存在于各种氧化还原反应中,广泛参与地球生物化学循环。由微生物介导的异化铁还原过程作为最主要的铁还原途径之一,其本质是一种电子传递过程[34-37]。在厌氧条件下,异化铁还原菌能够以Fe(Ⅲ)作为外在电子受体,将其还原成Fe(Ⅱ),并通过该过程获取能量,满足自身生长发育需要[37]。
现有的研究重点针对微生物的Fe(Ⅲ)呼吸代谢途径展开,发现多种细胞色素蛋白质和相关功能酶等物质参与了电子转移和呼吸调节等过程。SAFFARINI等[38]研究发现甲基萘醌(menaquinones, MQ)是介导Fe(Ⅲ)呼吸的一个必要构件,承担着从脱氢酶中传递电子的重要角色,并在其他多种功能蛋白的作用下依次完成电子向周质细胞色素、细胞膜及Fe(Ⅲ)氧化物的传递过程。由cymA基因编码的CymA是锚定在细胞质膜上的可驱动电子从醌类(MQ)中间体到周质细胞色素的电子传递过程的四血红素周质细胞色素[39-40]。而四血红素黄素细胞色素Ifc3和小分子质量的酸性低电势的四血红素细胞色素Cct也可作为辅助途径从CymA中接递电子[41-43]。随后,由mtrA基因编码的具有非特异电子传递作用和完整细胞Fe(Ⅲ)末端还原酶本质的MtrA(周质十血红素细胞色素),可完成电子从周质向细胞外膜的传递过程[44-45],并最终在Fe(Ⅲ)末端还原酶的催化作用下,将位于细胞外膜的电子传递给Fe(Ⅲ)[46]。
在整个Fe(Ⅲ)呼吸代谢过程中,位于细胞外膜(少量在周质)的多血红素细胞色素大多具有非特异的Fe(Ⅲ)还原酶的特性,主要依赖与Fe(Ⅲ)之间存在的电势差,发挥调控与催化Fe(Ⅲ)还原、构建电子传递网络的重要作用[47-48]。从Shewanella oneidensisSR-21中获取的mtrDEF-omcA-mtrCAB基因簇[45-46],可编码3个外膜十血红素细胞色素(MtrC、MtrF和OmcA),2个周质十血红素细胞色素(MtrD和MtrA),以及2个可能的β-barre蛋白(MtrE和MtrB),参与Fe(Ⅲ)还原呼吸的多条代谢途径[49]。其中,mtrB作为最早发现的Fe(Ⅲ)还原相关的功能基因之一,其编码产物MtrB(十血红素c型细胞色素)在Fe(Ⅲ)还原蛋白的正确定位和插入外膜等方面起着重要作用[44]。此外,由ferE基因编码的铁吸收调节蛋白(ferric uptake regulator, Fur)是铁捕获系统的负调控子,在细菌Fe(Ⅲ)呼吸代谢途径中起到了关键的调控作用,ferE基因缺失将导致还原过程所需的分子质量为91 kDa的铁卟啉蛋白无法正常分泌到细胞外膜,从而抑制了Fe(Ⅲ)的还原代谢过程[48-49]。
近年来,随着相关技术手段的持续发展,针对微生物Fe(Ⅲ)还原过程的代谢机制已经得到了进一步的完善。然而从目前已分离得到的铁还原菌中尚未发现1个通用的功能基因。典型的铁还原菌[如希瓦式菌(Shewanella)、地杆菌(Geobacter)等]中虽然都以细胞色素c作为主要的电子传递蛋白,但关键功能物质的同源性并不强[50],因此,将某个功能基因作为分子标志物来表征异化型铁还原菌的微生物代谢特性和分布规律等方面的系统发育分析仍需要进行深入的研究和探索。
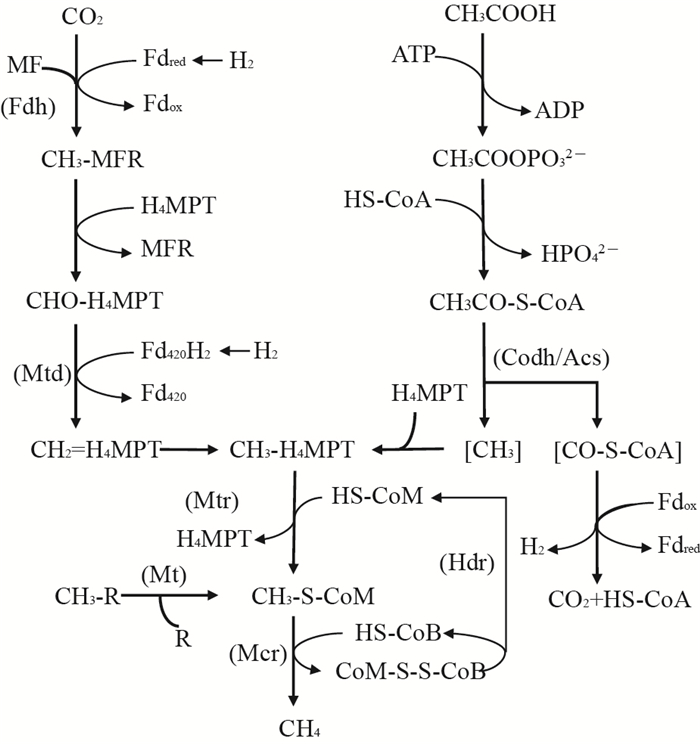
4 产甲烷过程甲烷(CH4)是重要的温室气体之一,其温室效应是二氧化碳(CO2)的20余倍,净释放量大约500 Tg/a,并且以每年约1%的速度增长[51-53]。因此,研究甲烷的产生途径、减少甲烷气体排放对于缓解全球气候变暖、维持生态环境平衡具有至关重要的作用。
目前已知的产甲烷菌主要来自广古菌门,根据碳代谢过程的不同,主要包括CO2还原、甲基裂解和乙酸发酵3种产甲烷途径,其本质是一种微生物介导、多种功能蛋白质参与的电子传递过程。其中,甲烷呋喃(methanofuran, MFR)、铁氧化还原蛋白(ferredoxin, Fd)、细胞色素(cytochrome)和辅酶F420等是电子传递的重要载体。
甲基辅酶M还原酶(methyl coenzyme M reductase, Mcr)可以在碳代谢的最后一步以辅酶B(HS-CoB)为直接的电子供体还原甲基辅酶M(CH3-S-CoM)产生甲烷,是3种代谢过程中共有的关键功能酶(图 2),由3个不同的亚基(α2β2γ2)构成的六聚体结构和含镍辅酶F430组成[54-56]。编码Mcr中α亚基的功能基因(mcrA)作为分子标志物,被广泛应用于产甲烷古菌群落结构及多样性等方面的表征研究[57]。而随着近年来技术的发展和对产甲烷微生物研究的深入,在非广古菌门的微生物中也发现了编码Mcr以及其他与产甲烷相关的功能基因[58]。EVANS等[58]通过宏基因组测序,从地下含水层中筛选得到了2株属于深古菌门的产甲烷古菌,并获得了编码甲基辅酶M还原酶的功能基因。其中:BA1菌株中还包含有mtsA、mtbA、mtaA、mttBC、mtbBC和mtrH等与产甲烷途径相关的功能基因;BA2菌株中也存在编码甲基裂解途径相关的基因,但是其能量储存和电子传递机制可能不同于BA1。该发现对于深入了解产甲烷微生物的分布与代谢机制,拓展对功能基因调控的甲烷产生机制的研究具有重要意义。同时,Mcr及其同系物也是甲烷厌氧氧化过程中的关键功能酶,在全球甲烷代谢过程中发挥着重要作用。异质二硫化物还原酶(heterodisulfide reductase, Hdr)同样是产甲烷最后关键步骤中共有的功能物质,它能以CoB-S-S-CoM作为产甲烷过程的末端电子受体,将其还原为HS-CoB和HS-CoM(图 2)。Hdr是一种膜相结合的、具有电子传递功能的蛋白复合物,由hdrABCDE基因编码产生。根据微生物细胞生长条件的不同,不同Hdr功能酶(HdrED和HdrABC)在利用传递电子、ATP合成及还原代谢过程中发挥着特有的生理功能[59-60]。
辅酶M甲基转移酶(coenzyme M methyltransferase, Mtr)是CO2还原代谢过程中的重要功能酶,可以催化甲基基团转移到辅酶M(HS-CoM)的硫醇基上形成CH3-S-CoM的过程,同时充当钠离子泵的角色,用于合成ATP[61-62](图 2)。Mtr是由8个亚基组成的嵌膜蛋白复合体,由mtrECDBAFGH基因所编码[63]。其中:含有1个钴胺素(Ⅰ)辅基的MtrA亚基能够在催化循环中不断进行甲基化和去甲基化的循环,并在该过程中产生钠离子梯度[62, 64];亚基MtrE能够将MtrA中类咕啉辅基上的甲基基团转移到辅酶M的巯基上[62-63];而MtrH亚基则能够利用CH3-H4MPT催化自由钴胺素(Ⅰ)辅基转变为甲基化的钴胺素(Ⅰ)辅基[62, 65]。
辅酶M甲基转移酶复合体(coenzyme M methyl transfer, MT)是催化和激活甲基裂解途径的酶系统,根据底物利用和代谢途径不同,主要包括MT1(MtaB、MtmB、MtbB和MttB)和MT2(MtaA、MtbA和MtsA)2个转移酶,分别由相应的转移酶基因(mta、mtm、mtb、mtt和mts等)编码表达[66]。MT1能够将底物上的甲基转移到MT1上与类咕啉蛋白结合,MT2再转移甲基与HS-CoM连接产生CH3-S-CoM(图 2)。MT2蛋白序列中都含有高度保守的结构,可以完成甲基化合物的激活和甲基的转移,并同时伴随着电子传递和能量代谢等过程[66-67]。
一氧化碳脱氢酶/乙酰辅酶A合成酶复合体(CO dehydrogenase/acetyl-CoA synthase, Codh/Acs)是解乙酸途径中的关键酶,用于剪切乙酰辅酶A上的C-C和C-S键(图 2)。它能以Fdox作为电子受体,催化产生CO2和CH3-H4SPT。同时,甲烷呋喃(MFR)、辅酶F420和F430、铁氧化还原蛋白(Fd)等也可作为氢化酶系统的电子载体,参与到产甲烷的呼吸代谢过程中[62, 68]。
甲烷产生的代谢过程体系复杂,并伴随着多种功能蛋白的偶联,因而不同的辅酶系统以及电子传递载体相互交叉,共同促成了甲烷的产生。现如今各国对于产甲烷过程相关的研究愈发深入,随着基因组学和生物信息学等新技术的发展,以功能基因作为分子靶标的研究,对人们深入理解产甲烷过程相关的代谢途径和过程调控机制具有重要意义。
5 卤代有机污染物的还原脱卤过程卤代有机污染物是全球范围内普遍使用的,具有难降解、高致癌性的芳香族或脂肪族化合物[69]。在厌氧条件下,许多微生物可以在还原脱卤酶的催化作用下以卤代有机污染物作为电子受体进行还原,自身获得能量进行生长[70]。不同的还原脱卤酶具有相同的脱卤功能,但由于卤代有机污染物种类繁多以及相关功能微生物的多样性,编码相关还原脱卤酶的具体功能基因也不尽相同。基于现代分子生物学技术,以还原脱卤功能基因作为高效的生物标志物,可以用来详细研究环境中微生物的脱卤呼吸过程[71-72]。
目前,各国学者已经通过对Sulfurospirillum、Desulfitobacterium、Dehalobacte、Dehalococcoides、Anaeromyxobacter和其他多种卤呼吸微生物进行了详尽的全基因组测序和宏基因组学研究,发现了大量具有还原脱卤功能的基因(rdh)。rdh基因簇中包含可编码具有催化活性的还原脱卤酶的rdhA基因,编码膜固蛋白的rdhB基因,以及其他与还原脱卤相关的rdhTKZECD基因等[70, 73-74]。由rdh编码的RdhA蛋白具有几个特殊的保守特征,包括2个Fe-S簇结合基序和可用于易位或跨越细胞膜的双精氨酸信号基序[74]。大多数RdhA蛋白还包含着类咕啉的辅酶因子[75]。根据可利用的污染底物不同,还原脱卤酶RdhA也包含了多种具有催化脱卤功能的不同的蛋白组分,如可利用氯乙烯的VcrA、BvcA、TceA、MbrA、PceA和PrdA[76-81],还原氯乙烷的CfrA、DcrA和DcaA[82-84],催化氯代芳烃的CbrA、CprA和CrdA[85-87],以及可利用氯苯的PcbA[88]等,不同的还原脱卤酶均由相关特定的功能基因所编码。
最早发现的rdhA基因序列是Sulfurospirillum multivorans中编码三氯乙烯还原酶的pceA基因[73],Desulfitobacterium dehalogenans中编码氯酚还原酶的cprA基因[88],及其相邻的pceB和cprB基因。随着现代分子生物学技术的发展,经过长期的研究总结发现,在Desulfitobacterium dehalogenans、D. hafniense、Desulfitobacterium chlororespirans、D. dichloroeliminans、Sulfurospirillum multivorans、Dehalobacter restrictus和Dehalococcoides mccartyi等微生物中包含着cprA、crdA、pceA、tmrA、vcrA、cbrA、mbrA和pcbA等与转录调节相关的功能基因簇,可编码特定卤代有机污染物的还原脱卤过程中的蛋白酶[69, 89-93]。
通过转录组和蛋白组学等技术方法可基于特定的功能基因分析对应的还原脱卤酶,从而研究卤代有机污染物的逐级还原过程。BISAILLON等[85]发现:在Desulfitobacterium hafniense PCP-1中的CprA3还原脱氯酶可以通过邻位脱氯,将PCP依次还原为四氯或三氯的物质;而另一种CprA5还原脱氯酶,则可以完成间位或者对位脱氯,将3,4,5-TCP还原为3,5-DCP,并继续将其脱氯转化为3-CP[94]。而编码CprA的cprA基因同样也可在Desulfitobacterium hafniense TCP-A和DCB-2菌株中发现[95]。5种在Dehalococcoides得到表征的还原脱卤酶可广泛参与到氯乙烯、氯乙烷和氯苯等的呼吸代谢过程中。其中:PceA可将PCE脱氯为TCE[74];TceA能够还原TCE为乙烯[96];VcrA可催化TCE、DCE异构体和氯乙烯(VC)转化为乙烯[97]。Dehalococcoidesides mccartyi CBDB1中的CbrA可催化完成1,2,3,4-四氯苯和1,2,3-三氯苯的还原脱氯过程[86];而BvcA也可进行TCE、DCE的一种异构体,以及VC和1,2-DCA的还原脱氯过程[83]。由此可知,在特定的微生物基因组中可能不仅包含一种还原脱卤功能基因,而且由某种基因编码的还原脱卤酶也可能会参与多种底物的代谢过程。因而,基于测序技术研究微生物的还原脱卤过程,不仅能够从基因水平丰富污染物的降解代谢体系,而且对于鉴定污染地区具有还原脱卤作用的微生物种类、促进有机污染的消减具有重要意义。
6 厌氧土壤中各氧化还原过程的耦合关系厌氧微生物催化完成了土壤环境中大量的氧化还原反应,而在此过程中产生的能量又可以驱动多种生源要素的转化。其中,C、N、Fe、S等重要生命元素的转化及污染物的还原脱卤过程之间互相耦合,彼此影响,从而共同构成了土水界面复杂的生物地球化学循环体系。
由厌氧微生物驱动的反硝化过程能够偶联多种有机和无机化合物的氧化还原反应,并显著促进CN,Fe-N和S-N等的耦合关系[98-100]。ROBERTSON等[101]通过化学计量统计和同位素标记的方法证明了自养型铁氧化及硝酸盐还原微生物的存在,该类微生物在催化完成NO3-还原过程的同时,可以耦合Fe(Ⅱ)氧化。尽管如此,相关佐证催化Fe(Ⅱ)氧化NO3-还原途径的遗传和酶学证据仍然缺乏[102]。此外,发现硝酸盐/亚硝酸盐还原菌在厌氧条件下还能够以硫化物作为电子供体,耦合N/S氧化还原反6厌氧土壤中各氧化还原过程的耦合关系应。研究表明,硝酸盐/亚硝酸盐还原耦合硫的氧化还原过程在淡水、沉积物以及厌氧土壤等各种环境中起着重要作用[103-105]。
土壤中异化型铁还原菌还原Fe(Ⅲ)的过程可以偶联多种有机物的氧化还原反应。在厌氧环境中,Fe(Ⅲ)和硫酸盐还原微生物常以互惠共生的关系存在,共同介导Fe/S的氧化还原过程,而其反应的热力学顺序取决于底物的可利用性、产物浓度和pH条件等。还原产物,如游离Fe2+和HS-等的浓度可能会因为形成FeS沉淀而降低,从而推动Fe(Ⅲ)和硫酸盐的还原[106]。此外,在碱性环境下,由硫酸盐还原产生的S0可以作为一种电子穿梭体介导微生物Fe(Ⅲ)的还原转化[107]。
除此之外,在厌氧条件下的还原脱氯过程也可以耦合其他多种元素,如NO3-、Fe(Ⅲ)、SO42-、CH4等的还原代谢反应。以严格厌氧的非专性脱氯菌Desulfitobacterium为例,它可以利用硝酸盐、亚硫酸盐、金属、腐殖酸、卤代有机化合物等多种物质作为电子受体,促进自身生长并参与其他元素的氧化还原反应[108]。其中,cprBA和pceAB基因是转录还原脱氯蛋白的重要物质,同时cprCDEKTZ,pceCT,prdAB等基因也在多株微生物中介导脱氯代谢的关键过程。有研究表明,D. hafniense TCE1可以和硫酸盐还原菌Desulfovibrio fructosivorans在低硫酸盐浓度和果糖存在的条件下形成互养共生体系,并通过种间氢传递来获取电子,从而促进PCE的还原脱氯[109-110],并推测其同样适用于与产甲烷菌的共生模式。NIGGEMYER等[111]从富铁沉积物中分离并鉴定得到的D. hafniense GBFH能够以甲酸盐作为电子供体和碳源,还原Fe(Ⅲ)、SO42-及其他多种物质。同时,D. dehalogenans、D. chlororespirans、D. hafniense PCP-1、DCB-2、TCE1和G2等多株菌在特定条件下也可以促进Fe(Ⅲ)还原[108]。此外,Anaeromyxobacter dehalogenans 2CP-C可以利用Fe(Ⅲ)作为电子受体,并通过邻位脱氯过程获得能量生长[112]。Desulfuromonas chlorethenic可以同时完成Fe(Ⅲ)还原和四氯乙烯及三氯乙烯的还原脱氯过程[113]。典型的异化铁还原菌Shewanella和Geobacter能耦合Fe(Ⅲ)还原和氯代有机化合物的还原脱氯过程[114-116]。而一些具有硫酸盐还原功能的微生物如Desulfovibrio、Desulfotomaculum、Clostridium等也可能具有还原脱氯功能,这些微生物能够通过呼吸作用代谢多氯联苯(PCBs)、二氯二苯三氯乙烷(DDT)等氯代有机污染物,并从中获取自身生长所需能量[117-119]。
与此同时,多种功能酶或细胞色素等蛋白组分在能量代谢和电子转移等过程中均发挥着重要的调控作用,如mtr基因簇的编码产物在产甲烷过程、Fe(Ⅲ)还原过程的能量合成与电子传递过程中具有关键作用;cys基因簇在介导硝酸盐还原、硫酸盐还原等循环代谢过程中扮演着关键角色;hdr基因能够耦合硫酸盐还原过程与产甲烷过程;dsr基因簇参与硫酸盐还原等过程,其产物介导的还原过程与氮的还原转化、产甲烷、有机污染物的还原脱卤以及Fe(Ⅲ)还原过程等相互耦合,在电子竞争与物质代谢方面关系密切;此外,涉及ATP合成及转化的功能基因,如sat、apr、mtr等,以及在细胞周质和细胞膜等成分间进行电子传递的功能基因,如qmo、hmc、cym等,均在微生物的多条呼吸代谢途径中起着重要作用[27-28]。因此,通过标记功能基因的方法,可以更加直观地探究和分析特定氧化还原过程中微生物的功能特性和分布特征。
7 结论与展望微生物介导着厌氧环境中的各种氧化还原反应,一方面从反应中获得能量促进自身生长,另一方面又驱动着生源要素的生物地球化学循环过程,这些过程由于微生物的参与而相互耦合,共同构成了复杂而又活跃的土壤体系。尽管我们综述了土壤各典型还原过程中的功能基因、物质转化间的耦合关系,以及与卤代有机污染物的还原脱卤过程的结合等,但不可否认土壤环境中的实际情况远远超出现有的研究对其功能和复杂程度的理解。
目前关于C、N、S、Fe等生源要素代谢过程及其对环境影响的研究依然是国际热点之一。随着分子生物学技术的发展,由微生物主导的多介质、多界面、多要素、多过程、多尺度的耦合研究是探究生物地球化学循环过程的最新的科学前沿[120]。编码环境中NO3-、Fe(Ⅲ)、SO42-和卤代有机污染物的还原和产甲烷过程中特定功能酶基因在不断地被完善,大大促进了对于元素生物地球化学循环过程的认知,推动了环境微生物生态学的纵深发展。因此,借助生物化学和生物信息学的蓬勃发展,广泛而深入地解析不同代谢过程中的功能蛋白及其编码基因,完善微生物的环境功能基因多样性,加强对元素循环机制的认识,促进污染修复和环境改善是该领域未来研究的重要内容。
| [1] | YERGEAU E, KANG S, HE Z L, et al. Functional microarray analysis of nitrogen and carbon cycling genes across an Antarctic latitudinal transect. The ISME Journal, 2007, 1(2): 163-179. DOI:10.1038/ismej.2007.24 |
| [2] |
张晶, 林先贵, 尹睿. 参与土壤氮素循环的微生物功能基因多样性研究进展. 中国生态农业学报, 2009, 17(5): 1029-1034. ZHANG J, LIN X G, YIN R. Advances in functional gene diversity of microorganism in relation to soil nitrogen cycling. Chinese Journal of Eco-Agriculture, 2009, 17(5): 1029-1034. (in Chinese with English abstract) |
| [3] | LEVY-BOOTH D J, PRESCOTT C E, GRAYSTON S J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biology & Biochemistry, 2014, 75: 11-25. |
| [4] | ZEHR J P, JENKINS B D, SHORT S M, et al. Nitrogenase gene diversity and microbial community structure: A cross-system comparison. Environmental Microbiology, 2003, 5(7): 539-554. DOI:10.1046/j.1462-2920.2003.00451.x |
| [5] |
侯海军, 秦红灵, 陈春兰, 等. 土壤氮循环微生物过程的分子生态学研究进展. 农业现代化研究, 2014, 35(5): 588-594. HOU H J, QIN H L, CHEN C L, et al. Research progress of the molecular ecology on microbiological processes in soil nitrogen cycling. Research of Agricultural Modernization, 2014, 35(5): 588-594. (in Chinese with English abstract) |
| [6] | PHILIPPOT L. Denitrifying genes in bacterial and Archaeal genomes. Biochimica et Biophysica Acta-Gene Structure and Expression, 2002, 1577(3): 355-376. DOI:10.1016/S0167-4781(02)00420-7 |
| [7] |
王莹, 胡春胜. 环境中的反硝化微生物种群结构和功能研究进展. 中国生态农业学报, 2010, 18(6): 1378-1384. WANG Y, HU C S. Research advances on community structure and function of denitrifiers. Chinese Journal of Eco-Agriculture, 2010, 18(6): 1378-1384. (in Chinese with English abstract) |
| [8] |
杨杉, 吴胜军, 蔡延江, 等. 硝态氮异化还原机制及其主导因素研究进展. 生态学报, 2016, 36(5): 1224-1232. YANG S, WU S J, CAI Y J, et al. The synergetic and competitive mechanism and the dominant factors of dissimilatory nitrate reduction processes: A review. Acta Ecologica Sinica, 2016, 36(5): 1224-1232. (in Chinese with English abstract) |
| [9] | BRAKER G, FESEFELDT A, WITZEL K P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Applied and Environmental Microbiology, 1998, 64(10): 3769-3775. |
| [10] | BRAKER G, TIEDJE J M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Applied and Environmental Microbiology, 2003, 69(6): 3476-3483. DOI:10.1128/AEM.69.6.3476-3483.2003 |
| [11] | SCALA D J, KERKHOF L J. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiology Letters, 1998, 162(1): 61-68. DOI:10.1111/fml.1998.162.issue-1 |
| [12] | STEVENS R J, LAUGHLIN R J. Measurement of nitrous oxide and di-nitrogen emissions from agricultural soils. Nutrient Cycling in Agroecosystems, 1998, 52(2/3): 131-139. DOI:10.1023/A:1009715807023 |
| [13] | STEVENS R J, LAUGHLIN R J, MALONE J P. Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biology and Biochemistry, 1998, 30(8/9): 1119-1126. |
| [14] |
殷士学. 淹水土壤中硝态氮异化还原成铵过程的研究. 南京: 南京农业大学, 2000: 55-56. YIN S X. Dissimilatory nitrate reduction to ammonium in submerged soils. Nanjing: Nanjing Agricultural University, 2000: 55-56. (in Chinese with English abstract) |
| [15] | COLE J A. Physiology, biochemistry and genetics of nitrate dissimilation to ammonia//REVSBECH N P, SORENSEN J. Denitrification in Soil and Sediment. USA: Springer, 1990:57-76. |
| [16] |
刘新展, 贺纪正, 张丽梅. 水稻土中硫酸盐还原微生物研究进展. 生态学报, 2009, 29(8): 4455-4463. LIU X Z, HE J Z, ZHANG L M. The sulfate-reducing bacteria and surfer cycle in paddy soil. Acta Ecologica Sinica, 2009, 29(8): 4455-4463. (in Chinese with English abstract) |
| [17] | DÖRRIES M, WÖHLBRAND L, KUBE M, et al. Genome and catabolic subproteomes of the marine, nutritionally versatile, sulfate-reducing bacterium Desulfococcus multivorans DSM 2059. BMC Genomics, 2016, 17: 918. DOI:10.1186/s12864-016-3236-7 |
| [18] | PÉREZ-JIMÉNEZ J R, YOUNG L Y, KERKHOF L J. Molecular characterization of sulfate-reducing bacteria in anaerobic hydrocarbon-degrading consortia and pure cultures using the dissimilatory sulfite reductase (dsrAB) genes. FEMS Microbiology Ecology, 2001, 35(2): 145-150. DOI:10.1111/fem.2001.35.issue-2 |
| [19] | WAGNER M, ROGER A J, FLAX J L, et al. Phylogeny of dissimilatory sulfite reductases supports, an early origin of sulfate respiration. Journal of Bacteriology, 1998, 180(11): 2975-2982. |
| [20] | CHIN K J, SHARMA M L, RUSSELL L A, et al. Quantifying expression of a dissimilatory (bi)sulfite reductase gene in petroleum-contaminated marine harbor sediments. Microbial Ecology, 2008, 55(3): 489-499. DOI:10.1007/s00248-007-9294-2 |
| [21] | NERETIN L N, SCHIPPERS A, PERNTHALER A, et al. Quantification of dissimilatory (bi)sulphite reductase gene expression in Desulfobacterium autotrophicum using real-time RT-PCR. Environmental Microbiology, 2003, 5(8): 660-671. DOI:10.1046/j.1462-2920.2003.00452.x |
| [22] | PETRI R, PODGORSEK L, IMHOFF J F. Phylogeny and distribution of the soxB gene among thiosulfate-oxidizing bacteria. FEMS Microbiology Letters, 2001, 197(2): 171-178. DOI:10.1111/fml.2001.197.issue-2 |
| [23] | TOUROVA T P, SLOBODOVA N V, BUMAZHKIN B K, et al. Analysis of community composition of sulfur-oxidizing bacteria in hypersaline and soda lakes using soxB as a functional molecular marker. FEMS Microbiology Ecology, 2013, 84(2): 280-289. DOI:10.1111/femsec.2013.84.issue-2 |
| [24] | ZHENG Y, BU N S, LONG X E, et al. Sulfate reducer and sulfur oxidizer respond differentially to the invasion of Spartina alterniflora, in estuarine salt marsh of China. Ecological Engineering, 2017, 99: 182-190. DOI:10.1016/j.ecoleng.2016.11.031 |
| [25] | GHOSH S, BAGCHI A. Comparative analysis of the mechanisms of sulfur anion oxidation and reduction by dsr operon to maintain environmental sulfur balance. Computational Biology and Chemistry, 2015, 59: 177-184. DOI:10.1016/j.compbiolchem.2015.07.001 |
| [26] | STRITTMATTER A W, LIESEGANG H, RALF R, et al. Genome sequence of Desulfobacterium autotrophicum HRM2, a marine sulfate reducer oxidizing organic carbon completely to carbon dioxide. Environmental Microbiology, 2009, 11(5): 1038-1055. DOI:10.1111/emi.2009.11.issue-5 |
| [27] | WÖHLBRAND L, JACOB J H, KUBE M, et al. Complete genome, catabolic sub-proteomes and key-metabolites of Desulfobacula toluolica Tol2, a marine, aromatic compound-degrading, sulfate-reducing bacterium. Environmental Microbiology, 2013, 15(5): 1334-1355. DOI:10.1111/emi.2013.15.issue-5 |
| [28] | CALLAGHAN A V, MORRIS B E L, PEREIRA I A, et al. The genome sequence of Desulfatibacillum alkenivorans AK-01: A blueprint for anaerobic alkane oxidation. Environmental Microbiology, 2012, 14(1): 101-113. DOI:10.1111/j.1462-2920.2011.02516.x |
| [29] | LOY A, DULLER S, BARANYI C, et al. Reverse dissimilatory sulfite reductase as phylogenetic marker for a subgroup of sulfur-oxidizing prokaryotes. Environmental Microbiology, 2009, 11(2): 289-299. DOI:10.1111/emi.2009.11.issue-2 |
| [30] | VENCESLAU S S, STOCKDREHER Y, DAHL C, et al. The "bacterial heterodisulfide"DsrC is a key protein in dissimilatory sulfur metabolism. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2014, 1837(7): 1148-1164. DOI:10.1016/j.bbabio.2014.03.007 |
| [31] | FISCHER M, SCHMIDT C, FALKE D, et al. Terminal reduction reactions of nitrate and sulfate assimilation in Streptomyces coelicolor, A3(2): Identification of genes encoding nitrite and sulfite reductases. Research in Microbiology, 2012, 163(5): 340-348. DOI:10.1016/j.resmic.2012.05.004 |
| [32] | PINTO R, HARRISON J S, HSU T, et al. Sulfite reduction in mycobacteria. Journal of Bacteriology, 2007, 189(18): 6714-6722. DOI:10.1128/JB.00487-07 |
| [33] | RÜCKERT C, KOCH D J, REY D A, et al. Functional genomics and expression analysis of the Corynebacterium glutamicum fpr2-cysIXHDNYZ, gene cluster involved in assimilatory sulphate reduction. BMC Genomics, 2005, 6: 121. DOI:10.1186/1471-2164-6-121 |
| [34] | FAUQUE G D, BARTON L L. Hemoproteins in dissimilatory sulfate-and sulfur-reducing prokaryotes. Advances in Microbial Physiology, 2012, 60: 1-90. DOI:10.1016/B978-0-12-398264-3.00001-2 |
| [35] | DING L J, SU J Q, XU H J, et al. Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. The ISME Journal, 2015, 9(3): 721-734. DOI:10.1038/ismej.2014.159 |
| [36] | LI H J, PENG J J, WEBER K A, et al. Phylogenetic diversity of Fe(Ⅲ)-reducing microorganisms in rice paddy soil: Enrichment cultures with different short-chain fatty acids as electron donors. Journal of Soils and Sediments, 2011, 11: 1234. DOI:10.1007/s11368-011-0371-2 |
| [37] | WEBER K A, ACHENBACH L A, COATES J D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nature Reviews Microbiology, 2006, 4(10): 752-764. DOI:10.1038/nrmicro1490 |
| [38] | SAFFARINI D A, BLUMERMAN S L, MANSOORABADI K J. Role of menaquinones in Fe(Ⅲ) reduction by membrane fractions of Shewanella putrefaciens. Journal of Bacteriology, 2002, 184(3): 846-848. DOI:10.1128/JB.184.3.846-848.2002 |
| [39] | MYERS C R, MYERS J M. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron (Ⅲ), fumarate, and nitrate by Shewanella putrefaciens MR-1. Journal of Bacteriology, 1997, 179(4): 1143-1152. DOI:10.1128/jb.179.4.1143-1152.1997 |
| [40] | MYERS J M, MYERS C R. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. Journal of Bacteriology, 2000, 182(1): 67-75. DOI:10.1128/JB.182.1.67-75.2000 |
| [41] | DOBBIN P S, BUTT J N, POWELL A K, et al. Characterization of a flavocytochrome that is induced during the anaerobic respiration of Fe3+ by Shewanella frigidimarina NCIMB400. Biochemical Journal, 1999, 342(2): 439-448. DOI:10.1042/bj3420439 |
| [42] | REYES-RAMIREZ F, DOBBIN P, SAWERS G, et al. Characterization of transcriptional regulation of Shewanella frigidimarina Fe(Ⅲ)-induced flavocytochrome c reveals a novel iron-responsive gene regulation system. Journal of Bacteriology, 2003, 185(15): 4561-4571. |
| [43] | GORDON E J, PIKE A D, HILL A E, et al. Identification and characterization of a novel cytochrome (c3) from Shewanella frigidimarina that is involved in Fe(Ⅲ) respiration. Biochemical Journal, 2000, 349(Pt1): 153-158. |
| [44] | MYERS J M, MYERS C R. MtrB is required for proper incorporation of the cytochromes omcA and omcB into the outer membrane of Shewanella putrefaciens MR-1. Applied and Environmental Microbiology, 2002, 68(11): 5585-5594. DOI:10.1128/AEM.68.11.5585-5594.2002 |
| [45] | PITTS K E, DOBBIN P S, REYESRAMIREZ F, et al. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA. Journal of Biological Chemistry, 2003, 278(30): 27758-27765. DOI:10.1074/jbc.M302582200 |
| [46] | BELIAEV A S, SAFFARINI D A, MCLAUGHLIN J L, et al. MtrC, an outer membrane decahaem c, cytochrome required for metal reduction in Shewanella putrefaciens, MR-1. Molecular Microbiology, 2001, 39(3): 722-730. DOI:10.1046/j.1365-2958.2001.02257.x |
| [47] | GASPARD S, VAZQUEZ F, HOLLIGER C. Localization and solubilization of the iron(Ⅲ) reductase of Geobacter sulfurreducens. Applied and Environmental Microbiology, 1998, 64(9): 3188-3194. |
| [48] | DICHRISTINA T J, MOORE C M, HALLER C A. Dissimilatory Fe(Ⅲ) and Mn(Ⅳ) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type Ⅱ protein secretion gene. Journal of Bacteriology, 2002, 184(1): 142-151. DOI:10.1128/JB.184.1.142-151.2002 |
| [49] |
洪义国, 许玫英, 郭俊, 等. 细菌的Fe(Ⅲ)还原. 微生物学报, 2005, 45(4): 653-656. HONG Y G, XU M Y, GUO J, et al. Bacterial Fe(Ⅲ) reduction. Acta Microbiologica Sinica, 2005, 45(4): 653-656. (in Chinese with English abstract) |
| [50] |
黎慧娟, 彭静静. 异化Fe(Ⅲ)还原微生物研究进展. 生态学报, 2012, 32(5): 1633-1642. LI H J, PENG J J. Recent advances in studies on dissimilatory Fe(Ⅲ)-reducing microorganisms. Acta Ecologica Sinica, 2012, 32(5): 1633-1642. (in Chinese with English abstract) |
| [51] | CALDWELL S L, LAIDLER J R, BREWER E A, et al. Anaerobic oxidation of methane: Mechanisms, bioenergetics, and the ecology of associated microorganisms. Environmental Science & Technology, 2008, 42(18): 6791-6799. |
| [52] | REEBURGH W S. Oceanic methane biogeochemistry. Chemical Reviews, 2007, 107(2): 486-513. DOI:10.1021/cr050362v |
| [53] | SIMPSON I J, CHEN T Y, BLAKE D R, et al. Implications of the recent fluctuations in the growth rate of tropospheric methane. Geophysical Research Letters, 2002, 29(10): 1479. |
| [54] | ERMLER U, GRABARSE W, SHIMA S, et al. Crystal structure of methyl-coenzyme M reductase: The key enzyme of biological methane formation. Science, 1997, 278(5342): 1457-1462. DOI:10.1126/science.278.5342.1457 |
| [55] | PRAKASH D, WU Y, SUH S J, et al. Elucidating the process of activation of methyl-coenzyme M reductase. Journal of Bacteriology, 2014, 196(13): 2491-2498. DOI:10.1128/JB.01658-14 |
| [56] | FRIEDRICH M W. Methyl-coenzyme M reductase genes: Unique functional markers for methanogenic and anaerobic methane-oxidizing Archaea. Methods in Enzymology, 2005, 397: 428-442. DOI:10.1016/S0076-6879(05)97026-2 |
| [57] | LUEDERS T, CHIN K J, CONRAD R, et al. Molecular analyses of methyl-coenzyme M reductase alpha-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environmental Microbiology, 2001, 3(3): 194-204. DOI:10.1046/j.1462-2920.2001.00179.x |
| [58] | EVANS P N, PARKS D H, CHADWICK G L, et al. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science, 2015, 350(6259): 434-438. DOI:10.1126/science.aac7745 |
| [59] | THAUER R K, KASTER A K, SEEDORF H, et al. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nature Reviews Microbiology, 2008, 6(8): 579-591. DOI:10.1038/nrmicro1931 |
| [60] | BUAN N R, METCALF W W. Methanogenesis by Methanosarcina acetivorans involves two structurally and functionally distinct classes of heterodisulfide reductase. Molecular Microbiology, 2010, 75(4): 843-853. DOI:10.1111/mmi.2010.75.issue-4 |
| [61] | WEISS D S, GÄRTNER P, THAUER R K. The energetics and sodium-ion dependence of N5-methyltetrahydromethanopterin: Coenzyme M methyltransferase studied with cob(Ⅰ)alamin as methyl acceptor and methylcob(Ⅲ)alamin as methyl donor. European Journal of Biochemistry, 1994, 226(3): 799-809. DOI:10.1111/ejb.1994.226.issue-3 |
| [62] |
方晓瑜, 李家宝, 芮俊鹏, 等. 产甲烷生化代谢途径研究进展. 应用与环境生物学报, 2015, 21(1): 1-9. FANG X Y, LI J B, RUI J P, et al. Research progress in biochemical pathways of methanogenesis. Chinese Journal of Applied and Environmental Biology, 2015, 21(1): 1-9. (in Chinese with English abstract) |
| [63] | GOTTSCHALK G, THAUER R K. The Na+-translocating methyltransferase complex from methanogenic archaea. Biochimica et Biophysica Acta, 2001, 1505(1): 28-36. DOI:10.1016/S0005-2728(00)00274-7 |
| [64] | GÄRTNER P, ECKER A, FISCHER R, et al. Purification and properties of N5-methyltetrahydromethanopterin: Coenzyme M methyltransferase from Methanobacterium thermoautotrophicum. European Journal of Biochemistry, 1993, 213(1): 537-545. DOI:10.1111/ejb.1993.213.issue-1 |
| [65] | HIPPLER B, THAUER R K. The energy conserving methyltetrahydromethanopterin: coenzyme M methyltransferase complex from methanogenic archaea: Function of the subunit MtrH. FEBS Letters, 1999, 449(2/3): 165-168. |
| [66] |
承磊, 郑珍珍, 王聪, 等. 产甲烷古菌研究进展. 微生物学通报, 2016, 43(5): 1143-1164. CHENG L, ZHENG Z Z, WANG C, et al. Recent advances in methanogens. Microbiology China, 2016, 43(5): 1143-1164. (in Chinese with English abstract) |
| [67] | HAGEMEIER C H, KRER M, THAUER R K, et al. Insight into the mechanism of biological methanol activation based on the crystal structure of the methanol-cobalamin methyltransferase complex. Proceedings of the National Academy of Sciences of the USA, 2006, 103(50): 18917-18922. DOI:10.1073/pnas.0603650103 |
| [68] | LIU Y, WHITMAN W B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Annals of the New York Academy of Sciences, 2008, 1125(1): 171-189. DOI:10.1196/annals.1419.019 |
| [69] | JUGDER B E, ERTAN H, LEE M, et al. Reductive dehalogenases come of age in biological destruction of organohalides. Trends in Biotechnology, 2015, 33(10): 595-610. DOI:10.1016/j.tibtech.2015.07.004 |
| [70] | HUG L A, MAPHOSA F, LEYS D, et al. Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Philosophical Transactions of the Royal Society B: Biological Sciences, 2013, 368(1616): 20120322. DOI:10.1098/rstb.2012.0322 |
| [71] | BAELUM J, NICOLAISEN M H, HOLBEN W E, et al. Direct analysis of tfdA gene expression by indigenous bacteria in phenoxy acid amended agricultural soil. The ISME Journal, 2008, 2(6): 677-687. DOI:10.1038/ismej.2008.21 |
| [72] | KOUTINAS M, KIPARISSIDES A, SILVA-ROCHA R, et al. Linking genes to microbial growth kinetics: An integrated biochemical systems engineering approach. Metabolic Engineering, 2011, 13(4): 401-413. DOI:10.1016/j.ymben.2011.02.001 |
| [73] | NEUMANN A, WOHLFARTH G G. Tetrachloroethene dehalogenase from Dehalospirillum multivorans: Cloning, sequencing of the encoding genes, and expression of the pceA gene in Escherichia coli. Journal of Bacteriology, 1998, 180(16): 4140-4145. |
| [74] | MAGNUSON J K, ROMINE M F, BURRIS D R, et al. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: Sequence of tceA and substrate range characterization. Applied and Environmental Microbiology, 2000, 66(12): 5141-5147. DOI:10.1128/AEM.66.12.5141-5147.2000 |
| [75] | KRÄUTLER B, FIEBER W, OSTERMANN S, et al. The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is norpseudo-B12, a new type of a natural corrinoid. Helvetica Chimica Acta, 2003, 86(11): 3698-3716. DOI:10.1002/(ISSN)1522-2675 |
| [76] | MAILLARD J, SCHUMACHER W, VAZQUEZ F, et al. Characterization of the corrinoid iron-sulfur protein tetrachloroethene reductive dehalogenase of Dehalobacter restrictus. Applied and Environmental Microbiology, 2003, 69(8): 4628-4638. DOI:10.1128/AEM.69.8.4628-4638.2003 |
| [77] | KRAJMALNIK- BROWN R, HOLSCHER T, THOMSON I N, et al. Genetic identification of a putative vinyl chloride reductase in Dehalococcoides sp. strain BAV1. Applied and Environmental Microbiology, 2004, 70(10): 6347-6351. DOI:10.1128/AEM.70.10.6347-6351.2004 |
| [78] | CHENG D, HE J Z. Isolation and characterization of 'Dehalococcoides' sp. strain MB, which dechlorinates tetrachloroethene to trans-1, 2-dichloroethene. Applied and Environmental Microbiology, 2009, 75(18): 5910-5918. DOI:10.1128/AEM.00767-09 |
| [79] | M LLER J A, ROSNER B M, VON ABENDROTH G, et al. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Applied and Environmental Microbiology, 2004, 70(8): 4880-4888. DOI:10.1128/AEM.70.8.4880-4888.2004 |
| [80] | TSUKAGOSHI N, EZAKI S, UENAKA T, et al. Isolation and transcriptional analysis of novel tetrachloroethene reductive dehalogenase gene from Desulfitobacterium sp. strain KBC1. Applied and Environmental Microbiology, 2006, 69(5): 543-553. |
| [81] | NAKAMURA K, MIZUMOTO M, UENO T, et al. Cloning and analysis of trichloroethene reductive dehalogenase gene and its detection by quantitative real-time PCR. Environmental Engineering Research, 2006, 43: 119-125. |
| [82] | MARZORATI M, DE FERRA F, VAN RAEMDONCK H, et al. A novel reductive dehalogenase, identified in a contaminated groundwater enrichment culture and in Desulfitobacterium dichloroeliminans strain DCA1, is linked to dehalogenation of 1, 2-dichloroethane. Applied and Environmental Microbiology, 2007, 73(9): 2990-2999. DOI:10.1128/AEM.02748-06 |
| [83] | TANG S Q, EDWARDS E A. Identification of Dehalobacter reductive dehalogenases that catalyze dechlorination of chloroform, 1, 1, 1-trichloroethane and 1, 1-dichloroethane. Philosophical Transactions of the Royal Society B: Biological Sciences, 2013, 368(1616): 20120318. DOI:10.1098/rstb.2012.0318 |
| [84] | GROSTERN A, EDWARDS E A. Characterization of a Dehalobacter coculture that dechlorinates 1, 2-dichloroethane to ethene and identification of the putative reductive dehalogenase gene. Applied and Environmental Microbiology, 2009, 75(9): 2684-2693. DOI:10.1128/AEM.02037-08 |
| [85] | BISAILLON A, BEAUDET R, LÉPINE F, et al. Identification and characterization of a novel CprA reductive dehalogenase specific to highly chlorinated phenols from Desulfitobacterium hafniense strain PCP-1. Applied and Environmental Microbiology, 2010, 76(22): 7536-7540. DOI:10.1128/AEM.01362-10 |
| [86] | ADRIAN L, RAHNENFUHRER J, GOBOM J, et al. Identification of a chlorobenzene reductive dehalogenase in Dehalococcoides sp. strain CBDB1. Applied and Environmental Microbiology, 2007, 73(23): 7717-7724. DOI:10.1128/AEM.01649-07 |
| [87] | KRASOTKINA J, WALTERS T, MARUYA K A, et al. Characterization of the B12-and iron-sulfur-containing reductive dehalogenase from Desulfitobacterium chlororespirans. Journal of Biological Chemistry, 2001, 276(44): 40991-40997. DOI:10.1074/jbc.M106217200 |
| [88] | MATTURRO B, DI L M, UBALDI C, et al. First evidence on the occurrence and dynamics of Dehalococcoides mccartyi PCB-dechlorinase genes in marine sediment during Aroclor1254 reductive dechlorination. Marine Pollution Bulletin, 2016, 112(1/ 2): 189-194. |
| [89] | VAN DE B A, SMIDT H, HAGEN W R, et al. Purification and molecular characterization of ortho-chlorophenol reductive dehalogenase, a key enzyme of halorespiration in Desulfitobacterium dehalogenans. Journal of Biological Chemistry, 1999, 274(29): 20287-20292. DOI:10.1074/jbc.274.29.20287 |
| [90] | SMIDT H, DE VOS W M. Anaerobic microbial dehalogenation. Annual Review of Microbiology, 2004, 58: 43-73. DOI:10.1146/annurev.micro.58.030603.123600 |
| [91] | SMIDT H, VANLEEST M, VANDEROOST J, et al. Transcriptional regulation of the cpr gene cluster in ortho-chlorophenol-respiring Desulfitobacterium dehalogenans. Journal of Bacteriology, 2000, 182(20): 5683-5691. DOI:10.1128/JB.182.20.5683-5691.2000 |
| [92] | FUTAGAMI T, YAMAGUCHI T, NAKAYAMA S, et al. Effects of chloromethanes on growth of and deletion of the pce gene cluster in dehalorespiring Desulfitobacterium hafniense strain Y51. Applied and Environmental Microbiology, 2006, 72(9): 5998-6003. DOI:10.1128/AEM.00979-06 |
| [93] | DESHPANDE N P, WONG Y K, MANEFIELD M, et al. Genome sequence of Dehalobacter UNSWDHB, a chloroform-dechlorinating bacterium. Genome Announcements, 2013, 1(5): e00720-13. |
| [94] | VILLEMUR R. The pentachlorophenol-dehalogenating Desulfito-bacterium hafniense strain PCP-1. Philosophical Transactions of the Royal Society B: Biological Sciences, 2013, 368(1616): 20120319. DOI:10.1098/rstb.2012.0319 |
| [95] | GAUTHIER A, BEAUDET R F, JUTEAU P, et al. Occurrence and expression of crdA and cprA5 encoding chloroaromatic reductive dehalogenases in Desulfitobacterium strains. Canadian Journal of Microbiology, 2006, 52(1): 47-55. |
| [96] | MAGNUSON J K, STERN R V, GOSSETT J M, et al. Reductive dechlorination of tetrachloroethene to ethene by a two-component enzyme pathway. Applied and Environmental Microbiology, 1998, 64(4): 1270-1275. |
| [97] | MÜLLER J A, ROSNER B M, ABENDROTH A V, et al. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Applied and Environmental Microbiology, 2004, 70(8): 4880-4888. DOI:10.1128/AEM.70.8.4880-4888.2004 |
| [98] | CANFIELD D E, JORGENSEN B B, FOSSING H, et al. Pathways of organic carbon oxidation in three continental margin sediments. Marine Geology, 1993, 113(1/2): 27-40. |
| [99] | FOSSING H, GALLARDO V A, J∅RGENSEN B B, et al. Concentration and transport of nitrate by the mat-forming sulphur bacterium Thioploca. Nature, 1995, 374(6524): 713-715. DOI:10.1038/374713a0 |
| [100] | LAUFER K, R∅Y H, J∅RGENSEN B B, et al. Evidence for the existence of autotrophic nitrate-reducing Fe(Ⅱ)-oxidizing bacteria in marine coastal sediment. Applied and Environmental Microbiology, 2016, 82(20): 6120-6131. DOI:10.1128/AEM.01570-16 |
| [101] | ROBERTSON E K, ROBERTS K L, BURDORF L D W, et al. Dissimilatory nitrate reduction to ammonium coupled to Fe(Ⅱ) oxidation in sediments of a periodically hypoxic estuary. Limnology and Oceanography, 2016, 61(1): 365-381. DOI:10.1002/lno.10220 |
| [102] | CHAKRABORTY A, RODEN E E, SCHIEBER J, et al. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(Ⅱ) oxidation in batch and continuous-flow systems. Applied and Environmental Microbiology, 2011, 77(24): 8548-8556. DOI:10.1128/AEM.06214-11 |
| [103] | BURGIN A J, HAMILTON S K. NO3--driven SO42- production in freshwater ecosystems: Implications for N and S cycling. Ecosystems, 2008, 11(6): 908-922. DOI:10.1007/s10021-008-9169-5 |
| [104] | PAYNE E K, BURGIN A J, HAMILTON S K. Sediment nitrate manipulation using porewater equilibrators reveals potential for N and S coupling in freshwaters. Aquatic Microbial Ecology, 2009, 54(3): 233-241. |
| [105] | YANG X N, HUANG S, WU Q H, et al. Nitrate reduction coupled with microbial oxidation of sulfide in river sediment. Journal of Soils and Sediments, 2012, 12(9): 1435-1444. DOI:10.1007/s11368-012-0542-9 |
| [106] | NEAL A L, TECHKARNJANARUK S, DOHNALKOVA A, et al. Iron sulfides and sulfur species produced at hematite surfaces in the presence of sulfate-reducing bacteria. Geochimica et Cosmochimica Acta, 2001, 65(2): 223-235. DOI:10.1016/S0016-7037(00)00537-8 |
| [107] | FLYNN T M, O'LOUGHLIN E J, MISHRA B, et al. Sulfur-mediated electron shuttling during bacterial iron reduction. Science, 2014, 344(6187): 1039-1042. DOI:10.1126/science.1252066 |
| [108] | VILLEMUR R, LANTHIER M, BEAUDET R, et al. The Desulfitobacterium genus. FEMS Microbiology Reviews, 2006, 30(5): 706-733. DOI:10.1111/j.1574-6976.2006.00029.x |
| [109] | DRZYZGA O, GOTTSCHAL J C. Tetrachloroethene dehalore-spiration and growth of Desulfitobacterium frappieri TCE1 in strict dependence on the activity of Desulfovibrio fructosivorans. Applied and Environmental Microbiology, 2002, 68(2): 642-649. DOI:10.1128/AEM.68.2.642-649.2002 |
| [110] | DRZYZGA O, GERRITSE J, DIJK J A, et al. Coexistence of a sulphate-reducing Desulfovibrio species and the dehalorespiring Desulfitobacterium frappieri TCE1 in defined chemostat cultures grown with various combinations of sulphate and tetrachloroethene. Environmental Microbiology, 2001, 3(2): 92-99. DOI:10.1046/j.1462-2920.2001.00157.x |
| [111] | NIGGEMYER A, SPRING S, STACKEBRANDT E, et al. Isolation and characterization of a novel As(Ⅴ)-reducing bacterium: Implications for arsenic mobilization and the genus Desulfitobacterium. Applied and Environmental Microbiology, 2001, 67(12): 5568-5580. DOI:10.1128/AEM.67.12.5568-5580.2001 |
| [112] | HE Q, SANFORD R A. Characterization of Fe(Ⅲ) reduction by chlororespiring Anaeromxyobacter dehalogenans. Applied and Environmental Microbiology, 2003, 69(5): 2712-2718. DOI:10.1128/AEM.69.5.2712-2718.2003 |
| [113] | KRUMHOLZ L R, SHARP R, FISHBAIN S S. A freshwater anaerobe coupling acetate oxidation to tetrachloroethylene dehalogenation. Applied and Environmental Microbiology, 1996, 62(11): 4108-4113. |
| [114] | CHEN M J, CAO F, LI F B, et al. Anaerobic transformation of DDT related to iron(Ⅲ) reduction and microbial community structure in paddy soils. Journal of Agricultural and Food Chemistry, 2013, 61(9): 2224-2233. DOI:10.1021/jf305029p |
| [115] | HE Y T, WILSON J T, WILKIN R T. Transformation of reactive iron minerals in a permeable reactive barrier (biowall) used to treat TCE in groundwater. Environmental Science & Technology, 2008, 42(17): 6690-6696. |
| [116] | FENG X L. Reductive transformation and mechanism of pentachlorophenol by anaerobic microbial communities enriched from paddy soil coupled with iron oxides. Hangzhou: Zhejiang University, 2014: 59-60. |
| [117] | GUERRERO-BARAJAS C, GARCÍA-PEÑA E I. Evaluation of enrichments of sulfate reducing bacteria from pristine hydrothermal vents sediments as potential inoculum for reducing trichloroethylene. World Journal of Microbiology and Biotechnology, 2010, 26(1): 21-32. DOI:10.1007/s11274-009-0136-x |
| [118] | BAO P, HU Z Y, WANG X J, et al. Dechlorination of p, p'-DDTs coupled with sulfate reduction by novel sulfate-reducing bacterium Clostridium sp. BXM. Environmental Pollution, 2012, 162: 303-310. DOI:10.1016/j.envpol.2011.11.037 |
| [119] | BARBOUR J P, SMITH J A, CHIOU C T. Sorption of aromatic organic pollutants to grasses from water. Environmental Science & Technology, 2005, 39(21): 8369-8373. |
| [120] |
宋长青, 吴金水, 陆雅海, 等. 中国土壤微生物学研究十年回顾. 地球科学进展, 2013, 28(10): 1087-1105. SONG C Q, WU J S, LU Y H, et al. Advances of soil microbiology in the last decade in China. Advances in Earth Science, 2013, 28(10): 1087-1105. (in Chinese with English abstract) DOI:10.11867/j.issn.1001-8166.2013.10.1087 |
 2017, Vol. 43
2017, Vol. 43