| 苦葛皂苷对福寿螺耗氧率及排氨率的影响 |
2. 成都市植物园,成都 610083
2. Chengdu Botanical Garden, Chengdu 610083, China
福寿螺(Pomacea canaliculata)又叫苹果螺,是两栖淡水贝类软体生物,广泛分布于中国及其他亚洲国家[1-2]。福寿螺啃食水稻、甘薯等粮食作物,影响作物产量及品质[3]。它还是广州管圆线虫(Angiostrongylus cantonensis)的中间寄主,危害人类健康[4]。因此,对福寿螺进行综合防治对农业生产及人类健康具有重要的意义。研究发现,植物中富含杀螺活性物质[5],国内外已对超过1 000多种植物进行了灭螺活性筛选,其中,油茶科植物、盾叶薯蓣、黄姜、夹竹桃、商陆、血水草、麻枫树等少数植物材料因其高活性而被人们所关注。这些植物的活性成分主要为皂苷类和生物碱类物质,其中,皂苷类物质是一类具有应用前景的杀螺活性成分[6]。JOSHI等[7]和MARTINS等[8]研究表明,从藜麦(Chenopodium quinoa)中提取的皂苷类物质能有效地防治福寿螺。现有的研究结果显示,皂苷类物质对螺体神经系统、呼吸系统、排泄系统和生殖系统等均可造成一定程度的破坏。陈盛霞等[9]发现用银杏外种皮处理钉螺,可导致钉螺体内的苹果酸脱氢酶、酸性磷酸酶等活性降低,推测其作用机制为通过干扰烟酰胺腺嘌呤二核苷酸(NADH)呼吸链来影响钉螺的呼吸代谢。呼吸与排泄是生物体新陈代谢的基本生理活动,对评估水生生物在水生态系统中的物质循环、生理状态、能量流动、代谢水平等均具有十分重要的意义[10-11]。耗氧率、排氨率及氧氮比是反映螺体呼吸代谢水平的重要指标,其变化情况反映了杀螺药物对福寿螺正常生命活动的影响程度。
苦葛(Pueraria peduncularis Grah.)是中国特有的一种豆科中药植物。四川农业大学无公害农药研究室研究发现,苦葛中的皂苷类物质对福寿螺具有极强的毒杀作用[12]。在前人研究的基础上,本试验进一步研究了苦葛皂苷不同质量浓度和培养水温对不同大小福寿螺的耗氧率、排氨率及氧氮比的影响,以期为阐明苦葛皂苷对福寿螺的毒杀机制奠定理论基础。
1 材料与方法 1.1 供试材料供试所用福寿螺(Pomacea canaliculata)采自四川农业大学温江校区农场。挑选符合要求的福寿螺,洗净后在实验室条件下(于50 L塑料箱中培养,温度、光照自然,不人工曝气,每天换水、清理死螺及粪便,并喂食白菜)培养至少2周,使其适应实验室环境。试验中用到的螺质量为(5±0.5)g、(4±0.4)g、(3±0.3)g。
苦葛皂苷由四川农业大学无公害农药研究室提供。
1.2 试验设计试验测定不同苦葛皂苷质量浓度、不同培养温度和不同螺质量处理下福寿螺耗氧率(RO)、排氨率(RN)及氧氮比(RO:N)的变化。
苦葛皂苷质量浓度处理:设20、30、40、50 mg/L4个质量浓度梯度,螺质量(5±0.5)g, 温度28 ℃。
温度处理:设26、27、28、29、30 ℃ 5个温度梯度,螺质量(5±0.5)g, 苦葛皂苷质量浓度40 mg/L。
螺质量处理:设(5±0.5)、(4±0.4)、(3±0.3)g 3个螺质量规格,温度28 ℃,苦葛皂苷质量浓度40 mg/L。
取相应大小螺3头置于500 mL碘量瓶中处理2 h, 取处理后水体测定其溶解氧含量及氨氮比值。溶解氧测定采用Winkler碘量法,氨氮比值测定采用次溴酸钠氧化法。同时将螺壳敲碎,取螺软组织于65 ℃烘箱中烘干24 h至恒量,测定其软体部干质量。各处理均设加螺不加苦葛皂苷的空白对照,每个处理3次重复。
1.3 数据计算与分析耗氧率、排氨率及氧氮比测定参考罗杰等[13]的方法。耗氧率(RO)、排氨率(RN)及氧氮比(RO:N)计算方法如下:
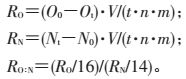
|
式中:O0、Ot分别为试验结束时清水对照、试验组水体中溶解氧质量浓度,mg/L;N0、Nt分别为试验结束时清水对照、试验组水体中氨氮质量浓度,mg/L;t为螺处理时间,h;V为试验用水体积,L;n为试验螺个数;m为福寿螺软组织干质量,g。
所有数据采用DPS 9.50进行邓肯差异分析。
2 结果与分析 2.1 不同质量浓度苦葛皂苷对福寿螺耗氧和排氨的影响由图 1A可知:苦葛皂苷处理使福寿螺的呼吸耗氧量大幅降低(P<0.05);对照组和20 mg/L苦葛皂苷处理的福寿螺单位质量耗氧率分别为0.88和0.24 mg/(g·h),两者相差3.67倍;对照组福寿螺单位质量耗氧率与苦葛皂苷各浓度处理组间差异均有统计学意义(P<0.05)。
 |
| RO:耗氧量;RN:排氨率。短栅上的不同小写字母表示在P<0.05水平差异有统计学意义。 RO: Oxygen consumption rate per unit mass; RN: Ammonia excretionrate per unit mass.Different lowercase letters above bars indicate statistically significant differences at the 0.05 probability level. 图1 不同质量浓度苦葛皂苷对福寿螺耗氧率(RO)和排氨率(RN)的影响 Fig. 1 Effects of different pedunsaponin concentrations on oxygenconsumption rate per unit mass (RO) and ammonia excretionrate per unit mass (RN) of P.canaliculata |
由图 1B可知:当苦葛皂苷质量浓度为20 mg/L时,福寿螺单位质量排氨率立即降低,与对照组差异有统计学意义(P<0.05);当其质量浓度为50 mg/L时,福寿螺单位质量排氨率相对于其他苦葛皂苷处理组略微升高,但仍比对照低48.54%;各苦葛皂苷质量浓度处理组间无交互关系(P<0.05)。
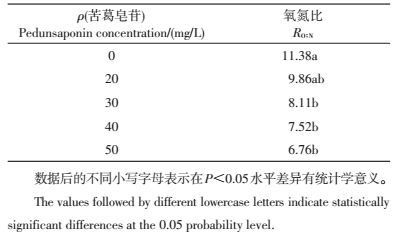
从表 1可以看出:在各质量浓度苦葛皂苷处理下福寿螺氧氮比均低于对照组;随着其质量浓度的增加,福寿螺的氧氮比呈下降趋势;苦葛皂苷质量浓度为20 mg/L的处理组与对照组差异较小,但随其质量浓度的增加,差异逐渐增大;在50 mg/L时氧氮比达到最低,比对照组低47.35%。
| 表1 在不同质量浓度苦葛皂苷处理下福寿螺的氧氮比 Table 1 Oxygen-to-nitrogen ratio (RO:N) of P.canaliculata at different pedunsaponin concentrations |
 |
| 点击放大 |
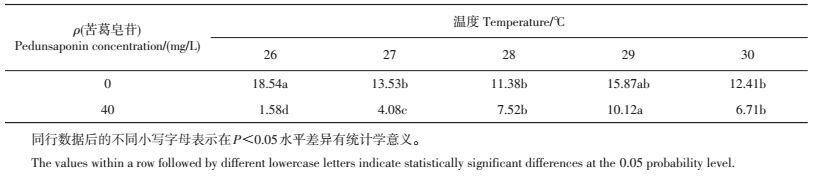
各水温下苦葛皂苷处理组福寿螺单位质量耗氧率均显著低于对照组(P<0.05)(图 2A)。随处理温度的增加,苦葛皂苷处理组与对照组福寿螺的单位质量耗氧量均呈先升高后下降的趋势,且在28 ℃时达到最高,此时,处理组与对照组单位质量耗氧率分别为0.19、0.88 mg/(g·h),即对照组福寿螺的单位质量耗氧率是苦葛皂苷处理组的4.63倍。
 |
| RO:耗氧量;RN:排氨率。短栅上的不同小写字母表示同一处理不同温度间在P<0.05水平差异有统计学意义;*表示在同一温度下处理组与对照组间在P<0.05水平差异有统计学意义。 RO: Oxygen consumption rate per unit mass; RN: Ammonia excretion rate per unit mass.Different lowercase letters above bars indicate statistically significant differences among different temperatures under the same treatment at the 0.05 probability level; single asterisk (*) indicates statistically significant difference between the treatment group and the control under the same temperature at the 0.05 probability level. 图2 不同温度下福寿螺的耗氧率(RO)和排氨率(RN) Fig. 2 Oxygen consumption rate of per unit mass (RO) and ammonia excretion rate of per unit mass (RN) of P.canaliculata under different temperatures |
从图 2B可看出:在各水温下,苦葛皂苷处理组福寿螺的单位质量排氨率均低于对照组(P<0.05);对照组福寿螺的单位质量排氨率随水温的升高呈先上升后下降再趋于平稳,而苦葛皂苷各处理组均处于较低水平;对照组排氨率在28 ℃时达到最高[0.07 mg/(g·h)],是苦葛皂苷处理组[0.02 mg/(g·h)]的3.5倍。
各温度下福寿螺苦葛皂苷处理组氧氮比均低于对照组(表 2)。在40 mg/L苦葛皂苷处理下福寿螺氧氮比呈现先升高后降低的趋势。随着温度的升高,处理组与对照组氧氮比之间的差距呈现先减小后增大的趋势,当温度为26、28、30 ℃时,对照组氧氮比分别是苦葛皂苷处理组的13.17、1.51、1.85倍。
| 表2 不同温度处理下福寿螺的氧氮比 Table 2 Oxygen-to-nitrogen ratio (RO:N) of P.canaliculata under different temperatures |
 |
| 点击放大 |
由图 3A可知:苦葛皂苷处理组中不同大小福寿螺的单位质量耗氧率均显著低于对照组(P<0.05);随着福寿螺个体的增大,苦葛皂苷处理组的单位质量耗氧率差异无统计学意义,而对照组的单位质量耗氧率逐渐增大,且5 g福寿螺与3、4 g福寿螺之间差异有统计学意义(P<0.05)。
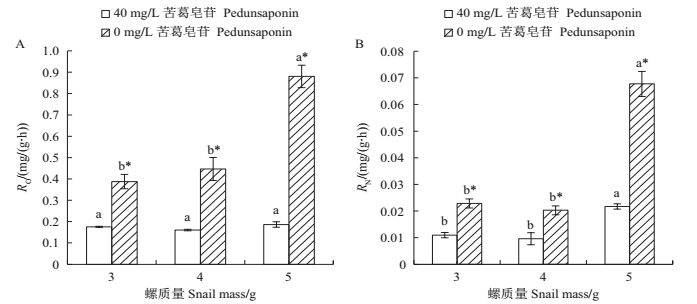 |
| RO:耗氧量;RN:排氨率。短栅上的不同小写字母表示同一处理不同螺质量间在P<0.05水平差异有统计学意义;“*”表示在同一螺质量下对照组和处理组间在P<0.05水平差异有统计学意义。 RO: Oxygen consumption rate per unit mass; RN: Ammonia excretion rate per unit mass.Different lowercase letters above bars indicate statistically significant differences among different snail masses under the same treatment at the 0.05 probability level; single asterisk (*) indicates statistically significant difference between the treatment group and the control under the same snail mass at the 0.05 probability level. 图3 不同质量福寿螺的耗氧率(RO)及排氨率(RN) Fig. 3 Oxygen consumption rate per unit mass (RO) and ammonia excretion rate per unit mass (RN) of P.canaliculata with different masses |
由图 3B可知:对照组中5 g福寿螺的单位质量排氨率显著高于3 g和4 g福寿螺,且为3 g福寿螺的2.96倍;对照组福寿螺的单位质量排氨率均显著高于苦葛皂苷处理组(P<0.05)。
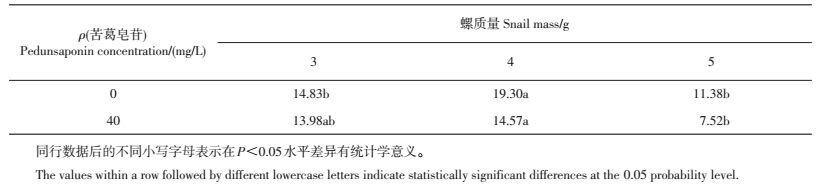
对照组及苦葛皂苷处理组的氧氮比均随福寿螺个体的增大先升高后降低;苦葛皂苷处理组的各规格福寿螺氧氮比均低于对照组,4 g螺苦葛皂苷处理组氧氮比较对照组低4.73(表 3)
| 表3 不同质量福寿螺的氧氮比 Table 3 Oxygen-to-nitrogen ratio (RO:N) of P.canaliculata with different masses |
 |
| 点击放大 |
物质代谢与能量代谢密切相关,物质代谢的异常会引发能量代谢的异常[14]。在硫化物胁迫下,日本沼虾呼吸代谢方式发生改变,由有氧呼吸转换为无氧呼吸,同时机体内磷酸精氨酸在AK催化下释放ATP为机体供能[15]。栗志民等[16]研究发现,皱肋文蛤对盐度的承受范围存在一定的阈值,当低于阈值时,耗氧率及排氨率随着盐度的升高而增加,当高于阈值时,随着盐度的增加而降低。在适宜的温度范围内,温度使贝类耗氧率及排氨率先增高后降低[16-17]。在本研究中,苦葛皂苷处理的福寿螺耗氧率及排氨率均低于对照组,表现出整体代谢水平的降低,这可能是由于苦葛皂苷对福寿螺的呼吸代谢产生影响,进而影响其能量代谢。而在不同培养温度及苦葛皂苷质量浓度条件下,苦葛皂苷处理的福寿螺耗氧率及排氨率差异均不显著,进一步说明苦葛皂苷在低质量浓度下即可对福寿螺呼吸代谢表现出极强的抑制作用,且这种抑制作用不随福寿螺生活区域水温的变化而变化。
氧氮比(RO:N)可表示生物体内蛋白质与脂肪和糖类分解代谢的比率,与新陈代谢有密切的联系[18]。当RO:N<7时,生物体代谢以蛋白质为主;当7<RO:N<24时,以蛋白质及脂肪和糖类共同代谢为主;当RO:N>24时,生物体以脂肪和糖类代谢为主[19]。试验结果表明,正常福寿螺的RO:N值范围为7~24,其代谢方式为蛋白质及脂肪和糖类共同代谢,而苦葛皂苷处理的福寿螺RO:N值比对照组低,说明福寿螺的代谢方式由以蛋白质及脂肪和糖类共同代谢朝以蛋白质代谢方向发展。吕继蓉等[20]研究发现,免疫应激会增强动物体内的蛋白质分解代谢,以维持生物体内环境稳定。由此推测,苦葛皂苷处理可有效抑制糖类和脂肪代谢能力,促使福寿螺通过增强蛋白质代谢水平进行免疫应激反应,最终导致螺体能量供应不足而中毒死亡。
目前已有研究证明,抑制糖类和脂肪代谢是一个防治有害生物的有效途径。于彩虹等[21]研究发现,皂角苷能显著抑制棉铃虫海藻糖酶活性并使相关糖类含量降低,导致棉铃虫正常的生命活动受到破坏。螺螨酯通过抑制螨体内脂肪合成,破坏脂肪代谢实现杀死害螨的目的[22]。在杀螺机制研究方面,亦有报道表明,部分灭螺活性物质对螺体的呼吸系统有较大影响,主要表现为线粒体破坏、生物氧化关键酶系活性降低[23-24]及氧化磷酸化解偶联[25]等。因此,进一步开展苦葛皂苷抑制糖类和脂肪代谢机制的研究,将有利于明确其灭螺机制,为苦葛皂苷作为灭螺剂的开发应用提供理论依据。
| [1] | YAMANISHI Y, YOSHIDA K, FUJIMORI N, et al. Predatordriven biotic resistance and propagule pressure regulate the invasive apple snail Pomacea canaliculata in Japan. Biological Invasions, 2012, 14(7): 1343-1352. DOI:10.1007/s10530-011-0158-9 |
| [2] | MOCHIDA O. Spread of freshwater Pomacea snail (Pilidae, Mollusca) from Argentina to Asia. Micronesica, 1991, 3: 51-62. |
| [3] | HALWART M. The golden apple snail Pomacea canaliculata in Asian rice farming systems: Present impact and future threat. International Journal of Pest Management, 2008, 40(2): 199-206. |
| [4] | YANG T B, WU Z D, LUN Z R. The apple snail Pomacea canaliculata, a novel vector of the rat lungworm, Angiostrongylus cantonemsis: Its introduction, spread, and control in China. Hawaii Journal of Medicine & Public Health, 2013, 72(6): 23-25. |
| [5] | AMUSON O G. Search on molluscicidal compounds for four African medicinal plants. Fitoterapia, 1995, 66(2): 113-116. |
| [6] |
王志高, 谭济才, 刘军, 等. 茶皂素、生石灰等防治稻田福寿螺的效果评估. 植物保护学报, 2011, 38(4): 363-368. WANG Z G, TAN J C, LIU J, et al. Evaluation of the controlling Pomacea canaliculata with calcium oxide, ammonium bicarbonate, Camellia oleifera powder and tea saponin. Acta Phytophylacica Sinica, 2011, 38(4): 363-368. (in Chinese with English abstract) |
| [7] | JOSHI R C, MARTIN R S, SEAZ-NAVARRETE C, et al. Efficacy of quinoa (Chenopodium quinoa) saponins against golden apple snail (Pomacea canaliculata) in the Philippines under laboratory conditions. Crop Protection, 2008, 27(3/4/5): 553-557. |
| [8] | MARTINS R S, GELMI C, DE OLIVEIRA J V, et al. Use of a saponin based molluscicide to control Pomacea canaliculata snails in Southern Brazil. Natural Product Communications, 2009, 4(10): 1327-1330. |
| [9] |
陈盛霞, 吴亮, 杨小明, 等. 银杏外种皮对钉螺的灭杀机制. 动物学报, 2007, 53(1): 190-194. CHEN S X, WU L, YANG X M, et al. Lethal effects of extracts of Ginkgo biliba sarcotesta upon the snail Oncomelania hupensis. Acta Zoologica Sinica, 2007, 53(1): 190-194. (in Chinese with English abstract) |
| [10] |
李加儿, 曹守花, 区又君, 等. 温度、盐度和pH对鲻幼鱼耗氧率、排氨率以及窒息点的影响. 中国水产科学, 2014, 21(5): 954-962. LI J E, CAO S H, OU Y J, et al. Influence of temperature, salinity, and pH on oxygen consumption rate, ammonia excretion rate, and suffocation point in juvenile Mugil cephalus. Journal of Fishery Sciences of China, 2014, 21(5): 954-962. (in Chinese with English abstract) |
| [11] |
郑侠飞, 王岩. 池塘养殖三角帆蚌的耗氧率. 浙江大学学报(农业与生命科学版), 2013, 39(4): 460-466. ZHENG X F, WANG Y. Oxygen consumption rate of freshwater pearl mussel (Hyriopsis eumingii) reared in ponds. Journal of Zhejiang University (Agriculture and Life Sciences), 2013, 39(4): 460-466. (in Chinese with English abstract) DOI:10.3785/j.issn.1008-9209.2012.09.050牋 |
| [12] |
雷博. 一种豆科植物农用活性筛选及杀福寿螺活性物质研究. 四川, 雅安: 四川农业大学, 2015: 1-55. LEI B. Screening for agriculturally useful activity of a kind of leguminous plant and study on its molluscicidal active substance against Pomacea canaliculate. Ya'an, Sichuan: Sichuan Agricultural University, 2015:1-55. (in Chinese with English abstract) http://cdmd.cnki.com.cn/Article/CDMD-10626-1016050032.htm |
| [13] |
罗杰, 刘楚吾, 李锋, 等. 盐度及规格对管角螺耗氧率和排氨率的影响. 海洋科学, 2008, 32(5): 46-50. LUO J, LIU C W, LI F, et al. Effects of salinity and specification on oxygen consumption rate and ammonia excretion rate of Hemifusus tuba (Gmelim). Marine Science, 2008, 32(5): 46-50. (in Chinese with English abstract) |
| [14] |
曹冬兴. 恶性肿瘤病人能量消耗及机体组成变化测定. 上海: 复旦大学, 2008: 1-64. CAO D X. Assessment of energy expenditure and body composition in cancer patients. Shanghai: Fudan University, 2008:1-64. (in Chinese with English abstract) http://cdmd.cnki.com.cn/article/cdmd-10246-2009017475.htm |
| [15] |
管越强, 王慧春, 李利. 硫化物胁迫对日本沼虾呼吸代谢和能量代谢酶的影响. 生态环境学报, 2009, 18(6): 2017-2021. GUAN Y Q, WANG H C, LI L. Effects of sulphide on the enzyme of respiratory metabolism and energy metabolism of Macrobrachium nipponense. Ecology and Environmental Sciences, 2009, 18(6): 2017-2021. (in Chinese with English abstract) |
| [16] |
栗志民, 刘志刚, 徐法军, 等. 体重、温度和盐度对皱肋文蛤耗氧率和排氨率的影响. 海洋科学进展, 2011, 29(4): 512-520. LI Z M, LIU Z G, XU F J, et al. Effects of body weight, temperature and salinity on oxygen consumption and ammonia excretion rate of Meretrix lyrata. Advances in Marine Science, 2011, 29(4): 512-520. (in Chinese with English abstract) |
| [17] |
范德朋, 潘鲁青, 董双林, 等. 盐度和pH对缢蛏耗氧率及排氨率的影响. 中国水产科学, 2002, 9(3): 234-238. FAN D P, PAN L Q, DONG S L, et al. Effects of salinity and pH on oxygen consumption rate and ammonia excretion rate of Sinonovacula constricta. Journal of Fishery Sciences of China, 2002, 9(3): 234-238. (in Chinese with English abstract) |
| [18] | MAYZAUD P, CONOVER R J. O:N atomic ratio as a tool to describe zooplankton metabolism. Marine Ecology: Progress Series, 1988, 45: 289-302. DOI:10.3354/meps045289 |
| [19] | PERSONLE R J, MAHE N K, LE B N, et al. Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture, 2004, 237(1/2/3/4): 269-280. |
| [20] |
吕继蓉, 陈邦云, 李小兵. 免疫应激对动物机体蛋白质代谢的影响. 饲料工业, 2005, 26(1): 42-45. Lü J R, CHEN B Y, LI X B. Effects of immunity challenge on protein metabolism in animal. Feed Industry, 2005, 26(1): 42-45. (in Chinese with English abstract) |
| [21] |
于彩虹, 梁晓贺, 卢丹, 等. 几种植物源化合物对棉铃虫海藻糖酶活性及相关碳水化合物含量的影响. 昆虫学报, 2011, 54(1): 41-49. YU C H, LIANG X H, LU D, et al. Trehalase activity and carboydrate content of the cotton bollorm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in response to several botanical compounds. Acta Entomologica Sinica, 2011, 54(1): 41-49. (in Chinese with English abstract) |
| [22] |
李翠英. 新型杀螨剂:螨危. 湖南农业, 2013(6): 25. LI C Y. Novel miticide: Envidor. Hunan Agriculture, 2013(6): 25. (in Chinese with English abstract) |
| [23] |
李洪军, 梁幼生, 戴建荣, 等. 氯硝柳胺悬浮剂对钉螺影响的酶组织化学观察. 中国血吸虫病防治杂志, 2006, 18(6): 427-432. LI H J, LIANG Y S, DAI J R, et al. Enzyme-histochemical observation on influence of suspension concentrate of niclosamide in Oncomelania hupensis snails. Chinese Journal of Schistosomiasis Control, 2006, 18(6): 427-432. (in Chinese with English abstract) |
| [24] |
谭苹, 何昌浩, 张艳, 等. 钉螺经不同药物浸泡后酶组织化学变化的观察. 中国人兽共患病杂志, 2000, 16(3): 34-37. TAN P, HE C H, ZHANG Y, et al. Observation of the changes of the enzymes for histochemistry in Oncomelania hupensis after being immersed in different chemicals. Chinese Journal of Zoonoses, 2000, 16(3): 34-37. (in Chinese with English abstract) |
| [25] | RAO I C, SINGH A, SINGH V K, et al. Effect of single and binary combinations of plant-derived molluscicides on different enzyme activities in the nervous tissue of Achatina fulica. Journal of Applied Toxicology, 2003, 23(1): 19-22. DOI:10.1002/(ISSN)1099-1263 |
 2017, Vol. 43
2017, Vol. 43


