| 舟山新木姜子幼苗对干旱胁迫的生理响应 |
2. 舟山市农林科学研究院,浙江 舟山 316003
2. Zhoushan Academy of Agriculture and Forestry Sciences, Zhoushan 316003, Zhejiang, China
海岛的地理隔离性质,以及外来物种的引进和不适当的管理导致海岛生态系统比内陆更加脆弱。在海岛发展过程中,由于伐林造田、外来物种的引入以及现代化城市的发展,海岛顶级植物群落面临被清除的威胁,生态环境遭到严重破坏[1-4]。近年来,植被恢复成为生态系统修复的主要工作[5-6]。海岛造林在改善海岛生态环境、促进经济发展等方面有重要的意义[7]。然而与内陆地区相比,海岛造林立地条件较为恶劣,诸如土壤贫瘠、淡水资源贫乏、季节性干旱频繁等,树苗的存活率常常低于50%,在干旱年份甚至降为0[8]。舟山列岛作为代表性的海岛,小岛众多,淡水资源普遍比较贫乏,年平均降水量也较低,季节性缺水现象十分显著,小岛造林条件较为恶劣。水分亏缺会严重影响植物的生长及代谢水平,但植物只有适应这种环境才能生存。
研究表明,一些樟科植物具有一定的抗旱能力。有学者在对水分胁迫下幼苗光合或生理特性的研究中发现,黑壳楠表现出较强的耐涝性和耐旱性,且耐涝、耐旱的能力高于香樟[9-10];香樟在轻度干旱胁迫下能通过体内抗氧化系统和渗透调节系统维持其正常生长[11];浙江楠幼苗具有较强的水分调节能力,能适应轻度干旱和水涝,抵抗重度干旱[12];楠木在生理层面对干旱和水涝都有一定的适应机制[13];普陀樟和香楠的抗旱能力高于其他海岛树种[14]。
舟山新木姜子(Neolitsea sericea)属于樟科常绿乔木,生长于海岸石缝,根系发达,抗风耐旱,耐盐碱,生长适应性强,引种范围广,是优良的海岛造林树种,同时也是国家二级珍稀保护植物,1996年被评为舟山市市树,多用于行道与花园中[15]。舟山新木姜子作为舟山海岛原著樟科植物能够适应干旱、贫瘠的土壤环境,说明此物种进化出了适应水分亏缺的特殊机制。本研究以舟山新木姜子为材料,采用土培方式模拟干旱胁迫,通过定期观察苗木在干旱胁迫下生理指标的动态变化,明确干旱强度与植物光合特性、抗氧化酶活性物质、渗透调节物质及细胞膜透性等的相互关系,以探讨舟山新木姜子的抗旱机制,为海岛地区绿化造林提供理论依据。
1 材料与方法 1.1 材料与处理试验苗种为一年生舟山新木姜子盆栽苗,2016年7月购于舟山市农林科学研究院苗木基地。试验用土为普通圃地土[10],基本理化性质如下:持水量45.83%,体积质量(容重)1.33 g/cm3,pH 5.51,有机质14.32 g/kg, 全氮0.76 g/kg。栽植用花盆的盆口直径为20 cm, 高18 cm, 每盆装入普通圃地土3 kg, 每个花盆栽植苗木1株。
试验于2016年7月在浙江海洋大学浙江省近海海洋工程技术重点实验室内进行。苗木栽植后经过30 d的缓苗期,选取无病虫害、生长基本一致的健壮幼苗120株,于8月21日开始进行干旱胁迫处理,10月5日开始复水,10月15日结束试验。结合舟山往年夏秋气候数据,采用中央空调、日光灯和加湿器等对培养环境进行人为控制,培养室环境设置为:白天光照13 h(7:00—20:00),光照强度5 000 lx, 温度27 ℃;夜晚时长11 h(20:00—7:00),温度22 ℃;湿度60%~70%。按土壤水分含量共设4个处理,分别为对照(CK)、轻度干旱胁迫(T1)、中度干旱胁迫(T2)和重度干旱胁迫(T3),其土壤含水量分别为土壤持水量的75%~80%、55%~60%、40%~45%和30%~35%。每个处理10株幼苗,3次重复,分别在处理0、15、30、45 d以及复水至适宜水分10 d后进行各项生理指标测定。
干旱胁迫处理开始前将各盆苗木浇透,当各处理组土壤水分含量分别自然降至处理范围内后开始试验。试验期间密封花盆底部以防止水分流失,并利用称量法控制各处理的土壤含水量,每隔1 d监测1次水分含量,低于水分含量下限的通过插孔注射补水至上限。定期观察和记录苗木的形态变化,并调查存活率。
1.2 测量指标与方法每株选择3片中上部叶片,标记为测定叶片,在10:00—12:30测定其光合作用参数。用Li-6400便携式光合作用测定仪(Li-COR公司,美国)测定净光合速率(net photosynthetic rate, Pn)、气孔导度(stomatal conductance, Gs)、胞间CO2浓度(intercellular CO2 concentration, Ci)、蒸腾速率(transpiration rate, Tr)等光合作用参数。根据测定的光合速率和蒸腾速率的比值(Pn/Tr),计算出水分利用效率(water use efficiency, WUE)。然后摘取叶片进行以下指标测定。
单位叶面积总叶绿素(total chlorophyll, Chl)含量测定采用丙酮-乙醇浸提法[11];超氧化物歧化酶(superoxide dismutase, SOD)活性测定采用氮蓝四唑(NBT)法[16];过氧化物酶(peroxidase, POD)活性测定采用愈创木酚法[17];丙二醛(malondialdehyde, MDA)及可溶性糖含量测定采用硫代巴比妥酸法[18];游离脯氨酸含量测定采用酸性茚三酮比色法[19];叶片细胞膜透性测定采用相对电导率(Rc)法[20]。
1.3 数据处理试验结果采用SPSS 19.0进行多变量分析,用单因素分差分析进行邓肯多重检验(P<0.05),利用Excel 2013进行数据整理与绘图。
2 结果与分析 2.1 干旱胁迫对舟山新木姜子幼苗形态特征的影响如表 1所示,在胁迫及复水期间,苗木在T1条件下表现正常。胁迫第15天时,3株苗木在T3条件下出现叶片轻微下垂和卷曲;胁迫第30天时,3株苗木在T2条件下出现叶片轻微下垂和卷曲,在T3条件下全株叶片轻微下垂,6株叶片卷曲明显,并有6株生长明显比CK矮小;胁迫第45天时,5株苗木在T2条件下出现叶片轻微下垂,3株出现叶片轻微卷曲,1株生长矮小并且叶片明显卷曲,在T3条件下全株叶片轻微下垂,并且卷曲严重,新老叶片都较短小,植株也较矮小;在复水后10 d, T1处理的苗木全部恢复,T2处理的苗木基本恢复但仍有1株生长矮小、叶片轻微卷曲,而T3处理的苗木依旧全株矮小、叶片短小,大部分叶片仍呈现出叶片轻微下垂和明显卷曲。各处理的苗木均未出现失绿、枯落或死亡现象。
| 表1 持续干旱胁迫对舟山新木姜子幼苗形态特征的影响 Table 1 Effect of continuous drought stress on morphological characteristics of Neolitsea sericea |
 |
| 点击放大 |
由图 1A可以看出,舟山新木姜子幼苗叶片单位面积的总叶绿素(Chl)含量随干旱胁迫时间的延长而逐渐降低。其中T2与T3处理组在胁迫末期(45d)的Chl含量显著低于CK(P<0.05),分别为CK的66.4%与65.8%,而T1组在胁迫期间(0~45 d)与CK的差异无统计学意义。各处理组在复水后10 d的Chl含量均有回升的趋势,其中T2处理组在复水后显著升高(P<0.05)。
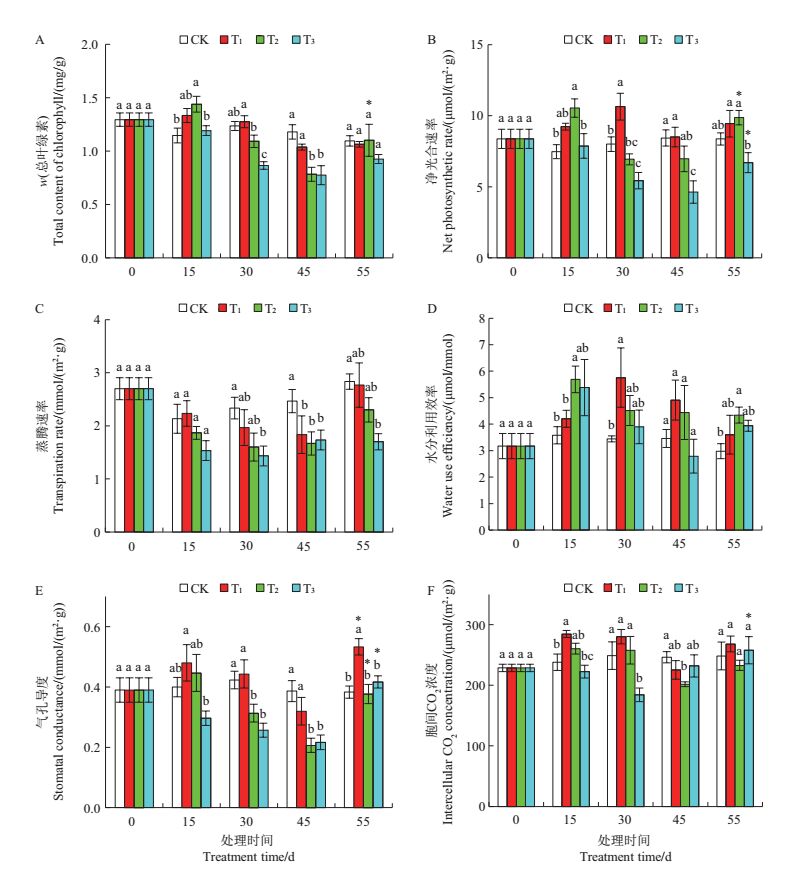 |
| CK:对照(相对含水量为75%~80%);T1:轻度干旱胁迫(相对含水量为55%~60%);T2:中度干旱胁迫(相对含水量为40%~45%);T3:重度干旱胁迫(相对含水量为30%~35%)。短栅上的不同小写字母表示在相同时间下不同胁迫处理间在P<0.05水平差异有统计学意义,n=3;*表示复水后与胁迫末期之间在P<0.05水平差异有统计学意义。 CK: Control (relative water content is 75%-80%); T1: Light drought (relative water content is 55%-60%); T2: Moderate drought (relative watercontent is 40%-45%); T3: Severe drought (relative water content is 30%-35%).Different lowercase letters above bars represent statistically significantdifferences among different stress treatments at the same time at the 0.05 probability level, n=3; single asterisk (*) indicates statistically significantdifference between the 45th day and the 55th day at the 0.05 probability level. 图1 在干旱胁迫下舟山新木姜子叶片光合指标的动态变化 Fig. 1 Dynamic changes of photosynthetic indexes in the leaves of Neolitsea sericea under drought stress |
不同强度的干旱胁迫处理对幼苗叶片净光合速率(Pn)的影响存在明显差异(图 1B)。T1处理的叶片Pn随胁迫时间的延长呈现先升高后降低的趋势,在胁迫第30天时达到最大值,且显著高于CK(P<0.05)。各处理组均在胁迫第45天时降至最低值,其中T3处理与CK相比差异有统计学意义(P<0.05)。T2及T3处理的Pn在复水后显著升高(P<0.05),其中T2组比第45天时高出29.4%。
由图 1C可以看出,各处理叶片的蒸腾速率(Tr)均随胁迫时间的延长逐渐降低,且在干旱胁迫第45天时均显著低于CK(P<0.05)。复水后各处理无显著变化,但T3组仍显著低于CK(P<0.05)。
图 1D为根据Pn/Tr计算得出的水分利用效率(WUE)的动态变化。从中可以看出,各处理随胁迫时间的延长均呈先升高后降低的趋势,其中:T1组在第30天时升至最高值且显著高于CK(P<0.05);T2、T3组在第15天时升至最高值,且T2组显著高于CK(P<0.05);各处理组的WUE均在第45天时降到最低值,但与复水后10 d相比无显著性差异,其中T2组在复水后10 d时仍保持较高的WUE,且显著高于CK(P<0.05)。
由图 1E可知,胁迫期间各处理叶片的气孔导度(Gs)随胁迫时间的变化趋势与Pn相似,均在第45天时降至最低值。复水后各处理的Gs显著回升(P<0.05),其中T1组显著高于CK与胁迫第45天时T1的Gs值(P<0.05)。
由图 1F可以看出:T1与T2处理的胞间CO2浓度(Ci)在胁迫期间呈先升高后降低的趋势,但变化并不明显,复水后也无明显变化;而T3处理的Ci呈现出相反的趋势,在第30天时显著降低至CK的74%左右(P<0.05),在第45天时又升高至与CK无显著性差异,复水后继续升高并显著高于胁迫第45天时的Ci值(P<0.05)。
2.3 干旱胁迫对舟山新木姜子幼苗叶片抗氧化酶活性的影响由图 2可以看出:T1处理的SOD与POD活性在处理15~55 d时分别维持在(264.9±4.9)U/g和(917.8±143.9)U/(g·min)的稳定水平,但T1处理的POD活性均显著高于CK(P<0.05);T2与T3处理的SOD活性在第15天时升至最高值并显著高于CK(P<0.05),分别是CK的2.653倍和2.996倍,而POD活性在第30天时升至最高值并显著高于CK(P<0.05),分别是CK的3.775倍与4.891倍,随后至45 d时,T2、T3处理的SOD与POD活性逐渐降低,但POD活性仍显著高于CK(P<0.05);复水后各处理的POD活性均显著降低,但T3处理组仍显著高于CK(P<0.05)。
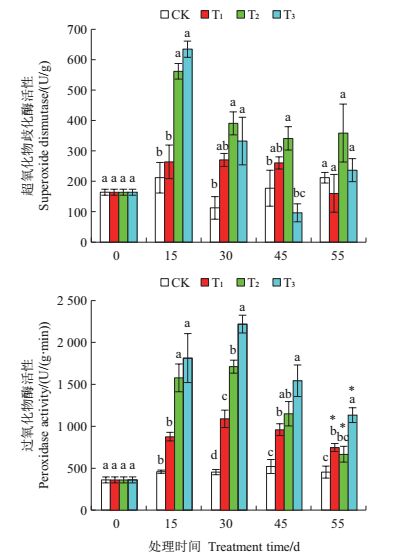 |
| 各处理及统计符号表示的含义详见图 1注。 Please see the footnote of Fig.1 for the details of each treatment and statistical sign. 图2 在干旱胁迫下舟山新木姜子幼苗叶片的超氧化物歧化酶(SOD)及过氧化物酶(POD)活性的动态变化 Fig. 2 Dynamic changes of the superoxide dismutase (SOD) and peroxidase (POD) activities in the leaves of Neolitsea sericea under drought stress |
各处理组叶片的脯氨酸含量均随胁迫时间的延长而呈升高的趋势(图 3A)。T1处理组在第30及45天时与CK存在统计学上的显著差异(P<0.05);T2、T3处理组均在第15天时显著高于CK(P<0.05),并在第45天时升至最高值,分别是CK的9.219倍与10.952倍。复水后各处理叶片的脯氨酸含量均显著降低,但T2与T3组仍显著高于CK(P<0.05)。
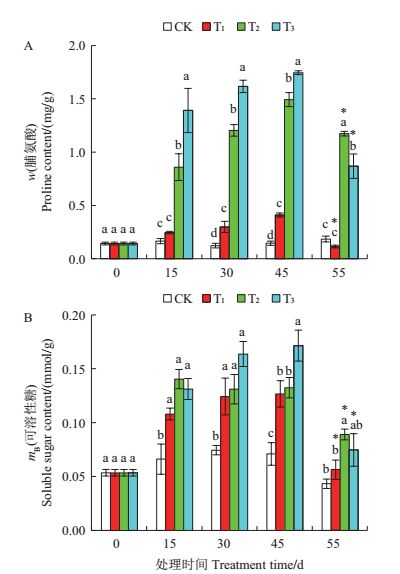 |
| 各处理及统计符号表示的含义详见图 1注。 Please see the footnote of Fig.1 for the details of each treatment and statistical sign. 图3 在干旱胁迫下舟山新木姜子幼苗叶片脯氨酸及可溶性糖含量的动态变化 Fig. 3 Dynamic changes of the proline and soluble sugar contents in the leaves of Neolitsea sericea under drought stress |
由图 3B可以看出,各处理组叶片的可溶性糖含量在干旱处理15~45 d期间均显著高于CK(P<0.05),其中T3处理组在第45天时升至最高,且显著高于其他处理组(P<0.05)。各处理在复水后10 d时的可溶性糖含量均显著低于45 d, 但T2处理组仍显著高于CK(P<0.05)。
2.5 干旱胁迫对舟山新木姜子幼苗叶片丙二醛含量及细胞膜透性的影响各处理组叶片的丙二醛(MDA)含量均在干旱胁迫第15天时显著升高(P<0.05)(图 4A)。T1、T3处理组在第15天时升至最高值,分别是CK的4倍及13.526倍,但随着胁迫时间的延长,MDA含量逐渐降低;T2处理组的MDA含量在第30天时升至最高值,是CK的8倍,随后在第45天时呈降低的趋势;复水后各处理的MDA含量均有所下降,其中T2、T3组下降显著,但仍显著高于CK(P<0.05)。
 |
| 各处理及统计符号表示的含义详见图 1注。 Please see the footnote of Fig.1 for the details of each treatment and statistical sign. 图4 在干旱胁迫下舟山新木姜子幼苗叶片的丙二醛含量及相对电导率的动态变化 Fig. 4 Dynamic changes of the malondialdehyde (MDA) content and relative conductivity (Rc) in the leaves of Neolitsea sericea under drought stress |
相对导电率(Rc)随胁迫时间的变化趋势与MDA的变化趋势类似(图 4B)。各处理组Rc均在第15天时显著升高(P<0.05),并在第30天时达到最大值,分别比CK高出了185.7%、499.2%及822.6%。复水后各处理组的Rc均显著下降,但T2与T3组仍显著高于CK(P<0.05)。
3 讨论与结论干旱胁迫会导致植物体内活性氧自由基增多,而过剩的自由基会引发膜脂过氧化,导致膜脂过氧化产物如丙二醛的积累,造成膜系统的损伤,严重时会导致植物细胞的死亡[21]。植物在逆境条件下会维持较高的抗氧化酶活性,这有利于清除活性氧,减轻对膜系统的损伤[22-23]。在本研究中,胁迫初期舟山新木姜子叶片的过氧化物酶、超氧化物歧化酶活性随丙二醛含量及相对电导率的增加而显著升高。随着过氧化物酶活性持续增加,丙二醛的清除速度已超过了积累速度,相对电导率逐渐下降,超氧化物歧化酶活性也同步回落,这时植物体已对干旱环境有了初步适应。同时,也可以推断超氧化物歧化酶在舟山新木姜子受到干旱胁迫时最先响应,而过氧化物酶对干旱胁迫的反应相对迟缓但作用时间较长。这与抗旱树种红花玉兰[24]、普陀樟[25]、杠柳[26]的研究结果一致。
在干旱胁迫下,植物能够产生或吸收更多的渗透调节物质如脯氨酸及可溶性糖等以调节体内渗透压,维持细胞膜的完整性并稳定蛋白质结构,从而保持生命活动的正常进行[27-29]。在干旱胁迫初期,舟山新木姜子叶片中可溶性糖和脯氨酸含量迅速大幅度提高,且随着干旱胁迫的持续和强度的增加而升高,有效缓解了干旱胁迫的压力,这与普陀樟[25]及杠柳[26]的研究结果一致。
干旱能使植物的光合作用受到抑制,通过气孔限制和非气孔限制来降低净光合速率。当净光合速率和胞间CO2浓度同时减小时,净光合速率下降主要由气孔导度引起,否则由非气孔限制因素引起[30]。重度干旱胁迫处理的叶片总叶绿素含量、净光合速率、气孔导度及胞间CO2浓度在胁迫试验中期均明显下降,说明此时叶片净光合速率下降受到了气孔限制因素的影响,而胁迫后期叶片总叶绿素含量、净光合速率、蒸腾速率及气孔导度持续降至最低,胞间CO2浓度却有所回升,说明此时叶片中的叶绿体受损,气孔调节功能有所丧失,净光合速率的下降则主要受非气孔限制因素的影响。对栎属植物的研究表明:一般轻度干旱胁迫不影响栎属植物幼苗的光合作用,甚至对光合作用有一定的促进作用;但随着干旱胁迫的加重,光合作用受到抑制,净光合速率迅速下降[31]。在本研究中,舟山新木姜子幼苗的光合作用有着相似的表现,即在轻度和中度干旱胁迫时能通过调节气孔导度、蒸腾速率等维持较高的净光合速率,使水分利用效率处于较高的水平,但在重度干旱胁迫下,净光合速率则随着干旱胁迫时间的延长逐渐降低,水分利用效率也有所下降。
复水10 d后,各处理组的超氧化物歧化酶活性、总叶绿素含量、净光合速率、气孔导度及胞间CO2浓度均恢复至正常水平,而在中度或重度干旱胁迫下叶片的过氧化物酶活性、可溶性糖、脯氨酸、丙二醛含量及相对电导率均有大幅降低的趋势,说明植物体尚需一定时间完全解除干旱响应;在重度干旱胁迫下的蒸腾速率仍处于较低水平,说明叶片气孔调节功能受到一定损伤。
以上分析表明,虽然舟山新木姜子在长期重度干旱胁迫下的生长受到限制,叶片的气孔调节功能丧失、细胞膜受损,导致叶片曲卷,但舟山新木姜子能通过积极调节自身抗氧化物质、渗透调节物质及提高水分利用效率等方式免受或缓解干旱带来的伤害,迅速适应轻度及中度干旱胁迫,在胁迫解除后能恢复正常生长,属于较耐旱树种,推测其可耐受的重度干旱胁迫时间为45 d。
| [1] | VITOUSEK P, LOOPE L L, ADSERSEN H, et al. Islands: Biological Diversity and Ecosystem Function. 1st ed. Berlin: Springer, 1995: 103-121. |
| [2] | FRANCISO-ORTEGA J, SANTOS-GUERRA A, KIM S C, et al. Plant genetic diversity in the Canary Islands: A conservation perspective. American Journal of Botany, 2000, 87(7): 909-919. DOI:10.2307/2656988 |
| [3] | FIEDLER P L, KAREIVA P M. Conservation Biology: For the Coming Decade. 2nd ed. New York, USA: Chapman and Hall, 1998: 317-344. |
| [4] | AVISE J C, HAMRICK J L. Conservation Genetics: Cases Histories from Nature. New York, USA: Chapman and Hall, 1996: 305-334. |
| [5] | SUDING K N. Toward an era of restoration in ecology: Successes, failures, and opportunities ahead. Annual Review of Ecology, Evolution, and Systematics, 2011, 42: 465-487. DOI:10.1146/annurev-ecolsys-102710-145115 |
| [6] | SHACKELFORD N, HOBBS R J, BURGAR J M, et al. Primed for change: Developing ecological restoration for the 21st century. Restoration Ecology, 2013, 21(3): 297-304. DOI:10.1111/rec.2013.21.issue-3 |
| [7] | HATA K, KAWAKAMI K, KACHI N. Increases in soil water content after the mortality of non-native trees in oceanic island forest ecosystems are due to reduced water loss during dry periods. Science of the Total Environment, 2016, 545/546: 372-380. DOI:10.1016/j.scitotenv.2015.12.007 |
| [8] |
袁信昌, 张晓勉, 贺位忠, 等. 3个树种在海岛困难立地造林试验. 浙江林业科技, 2015, 35(1): 68-71. YUAN X C, ZHANG X M, HE W Z, et al. Experiment on afforestation of three tree species on islands of Zhoushan. Journal of Zhejiang Forestry Science and Technology, 2015, 35(1): 68-71. (in Chinese with English abstract) |
| [9] |
丁琼, 张佑麟, 刘刚, 等. 水分胁迫对香樟和黑壳楠幼苗光合特性的影响. 西南林业大学学报, 2015, 35(4): 14-20. DING Q, ZHANG Y L, LIU G, et al. Effects of water stress on photosynthesis characteristics of Cinnamomum camphora and Lindera megaphylla seedlings. Journal of Southwest Forestry University, 2015, 35(4): 14-20. (in Chinese with English abstract) |
| [10] |
杨曼, 张佑麟, 徐振东, 等. 水分胁迫对黑壳楠和香樟幼苗生理特性的影响. 南方农业学报, 2015, 46(8): 1449-1454. YANG M, ZHANG Y L, XU Z D, et al. Effects of water stress on seedlings growth and physiological characteristics of Lindera megaphylla Hemsl. and Cinnamomum camphora (L.) Presl. Journal of Southern Agriculture, 2015, 46(8): 1449-1454. (in Chinese with English abstract) |
| [11] |
胡义, 胡庭兴, 陈洪, 等. 干旱胁迫及复水对香樟幼树生理特性及生长的影响. 西北植物学报, 2015, 35(2): 294-301. HU Y, HU T X, CHEN H, et al. Physiological properties and growth of Cinnamomum camphora saplings under drought stress and rewatering. Acta Botanica Boreali-Occidentalia Sinica, 2015, 35(2): 294-301. (in Chinese with English abstract) |
| [12] |
李冬林, 向其柏. 土壤水分状况对浙江楠幼苗的影响. 南京林业大学学报(自然科学版), 2006, 30(5): 112-114. LI D L, XIANG Q B. Effects of soil moisture status on the Phoebe chekiangensis seedlings. Journal of Nanjing Forestry University (Natural Sciences Edition), 2006, 30(5): 112-114. (in Chinese with English abstract) |
| [13] |
黄晓蓉, 李玮婷, 刘刚, 等. 水分胁迫对楠木幼苗抗逆生理特性的影响. 北方园艺, 2015(7): 68-71. HUANG X R, LI W T, LIU G, et al. Effect of water stress on growth and physiological characteristics of Phoebe zhennan S. Lee et F. N. Wei. Northern Horticulture, 2015(7): 68-71. (in Chinese with English abstract) |
| [14] |
陈闻, 赵颖, 叶正钱, 等. 干旱胁迫对5个海岛树种生长及生理特性的影响. 浙江农林大学学报, 2013, 30(4): 490-498. CHEN W, ZHAO Y, YE Z Q, et al. Growth and physiological characteristics of five island tree species with drought stress. Journal of Zhejiang A & F University, 2013, 30(4): 490-498. (in Chinese with English abstract) DOI:10.11833/j.issn.2095-0756.2013.04.005 |
| [15] | WANG Z S, AN S Q, LIU H, et al. Genetic structure of the endangered plant Neolitsea sericea (Lauraceae) from the Zhoushan archipelago using RAPD markers. Annals of Botany, 2005, 95(2): 305-313. DOI:10.1093/aob/mci027 |
| [16] | ADRIANO S, BARTOLOMEO D, CRISTOS X, et al. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Science, 2004, 166(2): 293-302. DOI:10.1016/j.plantsci.2003.09.018 |
| [17] |
高俊凤. 植物生理学实验指导. 北京: 高等教育出版社, 2006: 208-218. GAO J F. Experimental Guidance for Plant Physiology. Beijing: Higher Education Press, 2006: 208-218. (in Chinese with English abstract) |
| [18] |
刘慧娟. NaCl胁迫下喜树组培苗生长及生理生化特性研究. 杭州: 浙江农林大学, 2013: 9-11. LIU H J. Studies on the growth and physiological and biochemical characteristics of tissue culturing seedlings of Camptotheca acuminata under NaCl stress. Hangzhou: Zhejiang A & F University, 2013:9-11. (in Chinese with English abstract) http://cdmd.cnki.com.cn/Article/CDMD-10341-1014103864.htm |
| [19] | AMAKO K, CHEN G X, ASADE K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant and Cell Physiology, 1994, 35(3): 497-504. |
| [20] |
李合生. 植物生理生化实验原理和技术. 北京: 高等教育出版社, 2003: 258-260. LI H S. Experimental Principle and Technology for Plant Physiology. Beijing: Higher Education Press, 2003: 258-260. (in Chinese with English abstract) |
| [21] | SMIRNOFF N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist, 1993, 125(1): 27-58. DOI:10.1111/nph.1993.125.issue-1 |
| [22] | SELOTE D S, KHANNACHOPRA R. Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiologia Plantarum, 2006, 127(3): 494-506. DOI:10.1111/j.1399-3054.2006.00678.x |
| [23] | LI X, YAN X F, YU T. Effects of water stress on protective enzyme activities and lipid peroxidation in Phellodendron amurense seedlings. Chinese Journal of Applied Ecology, 2005, 16(12): 2353-2356. |
| [24] |
桑子阳, 马履一, 陈发菊. 干旱胁迫对红花玉兰幼苗生长和生理特性的影响. 西北植物学报, 2011, 31(1): 109-115. SANG Z Y, MA L Y, CHEN F J. Growth and physiological characteristics of Magnolia wufengensis seedlings under drought stress. Acta Botanica Boreali-Occidenalia Sinica, 2011, 31(1): 109-115. (in Chinese with English abstract) |
| [25] |
陈亚飞, 杜国坚, 岳春雷, 等. 水分胁迫对普陀樟幼苗生长及生理特性的影响. 浙江林业科技, 2009, 29(3): 24-29. CHEN Y F, DU G J, YUE C L, et al. Effect of water stress on growth and physiological properties of Cinnamomum japonicum var. chenii seedlings. Journal of Zhejiang Forestry Science and Technology, 2009, 29(3): 24-29. (in Chinese with English abstract) |
| [26] |
安玉艳, 梁宗锁, 郝文芳. 杠柳幼苗对不同强度干旱胁迫的生长与生理响应. 生态学报, 2011, 31(3): 716-725. AN Y Y, LIANG Z S, HAO W F. Growth and physiological responses of the Periploca sepium Bunge seedlings to drought stress. Acta Ecologica Sinica, 2011, 31(3): 716-725. (in Chinese with English abstract) |
| [27] | MORGAN J M. Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology, 1984, 35: 299-319. DOI:10.1146/annurev.pp.35.060184.001503 |
| [28] | KEUNEN E, PESHEV D, VANGRONSVELD J, et al. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant, Cell and Environment, 2013, 36(7): 1242-1255. DOI:10.1111/pce.2013.36.issue-7 |
| [29] | VAN DEN ENDE W, VALLURU R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging?. Journal of Experimental Botany, 2009, 60(1): 9-18. |
| [30] | FARQUHAR G D, SHARKEY T D. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology, 1982, 33: 317-345. DOI:10.1146/annurev.pp.33.060182.001533 |
| [31] | MIDGLEY G F, ARANIBAR J N, MANTLANA K B, et al. Photosynthetic and gas exchange characteristics of dominant woody plants on a moisture gradient in an African savanna. Global Change Biology, 2004, 10(3): 309-317. DOI:10.1111/gcb.2004.10.issue-3 |
 2017, Vol. 43
2017, Vol. 43


