| 油菜素内酯对盐胁迫下香樟幼苗叶片抗氧化酶活性的影响 |
香樟(Cinnamomum camphora),又名樟树,为樟科樟属常绿阔叶乔木,是常见的优良绿化树、行道树及庭荫树。樟树多喜温暖湿润气候,主根发达,深根性,萌芽力强,在南方沿海地区有一定的应用价值[1]。但香樟不耐干旱、瘠薄和盐碱土,土壤含盐量要求在0.2%以内,在酸性黄壤土中长势良好,在碱性土壤中易出现黄化病,低温、弱光以及高浓度的土壤次生盐害均会导致香樟植株生长异常,生育受到抑制乃至死亡。沿海地区土壤pH偏高,有机质含量较低,不利于香樟的生长需要,制约了其在南方沿海地区的引种推广和绿化栽培。因此,对香樟的耐盐机制进行研究具有重要意义。目前,关于樟树的耐盐性研究多集中于探讨不同盐浓度对香樟幼苗光合特性和生理特性的影响[2-3],而对于如何提高樟树耐盐性的研究仍较少,仅有研究表明施氮能缓解樟树盐胁迫带来的毒害[4]。
油菜素内酯(2, 4-epibrassinolide, EBR)是继生长素、赤霉素、细胞分裂素、乙烯、脱落酸后发现的第六大植物激素,属于类固醇类,在植物对生物和非生物的逆境响应中起主要作用[5]。大量研究表明,EBR可以提高作物对干旱[6]、低温[7]、盐害[8]、低氧[9]等逆境胁迫的抗性,可有效促进盐胁迫下植物种子萌发、幼苗生长,提高叶片光合色素含量和光合能力,增强抗氧化酶活性,降低膜脂过氧化产物丙二醛含量和质膜透性[10],且不同EBR浓度和处理方式对盐胁迫下植物的调控效果存在差异[11-12]。但是有关EBR对盐胁迫下木本植物的研究却鲜有报道,且针对EBR不同施用方式对盐胁迫下樟树幼苗影响方面的研究尚属空白。因此,本文研究了在4‰单一盐胁迫下,外源施用EBR(浸种及浸种+喷叶2种处理方式)对香樟幼苗叶片丙二醛、可溶性糖、可溶性蛋白质含量以及抗氧化酶活性的影响,分析了盐胁迫下香樟幼苗各指标的变化规律,以明确外源EBR诱导香樟抗盐性的效果,为进一步探明EBR对盐胁迫下香樟的代谢调节变化提供理论±据。
1 材料与方法 1.1 试验设计 1.1.1 浸种处理2014年11月3日在南京林业大学校园内收集香樟种子,消毒后用自来水加少许洗衣粉漂洗,除去杂物与不饱满的种子,再用H2O2处理30 min,用纱布包住瓶口,自来水流动冲洗24 h后,用滤纸吸干表面水分。将种子分为7份,每份100粒,其中2份用蒸馏水浸泡(CK1、CK2),剩余5份分别浸泡于质量浓度为0.1、0.2、0.3、0.4、0.5 mg/L的EBR溶液中,置于45 ℃恒温培养箱中培养24 h。然后,取出种子放入15~20 ℃的湿沙中层积催芽。每隔7 d往CK1处理的湿沙中添加一定量的蒸馏水,剩余处理添加一定量的4‰ NaCl以保证其相对湿度及盐度,约50 d后,待种子露白,将其播种于育苗袋中。
2015年5月2日,当苗高超过50 cm时,分别从各处理中取大小、长势一致的苗木12株,定植于25cm×25 cm的花盆中,苗木的培养基质为V(沙):V(蛭石)=1:1,约3.5 kg。在苗木生长期间(5—8月)保证水分供应,每隔25 d浇一次Hoagland营养液,每次浇0.5 L。
1.1.2 喷叶处理2015年8月1日向种子期间经不同质量浓度EBR浸泡的5组幼苗叶片表面和背面喷施质量浓度分别为0.1、0.2、0.3、0.4、0.5 mg/L的EBR溶液,CK1、CK2喷施蒸馏水,间隔期为7 d,共喷3次。9月1日除CK1处理外,其余6组进行盐处理,试验开始时持续1周不浇水,且为了保证4‰的盐分胁迫,一次性分别对6组处理加入100 mL 14%的盐溶液,以后每周浇1次水。为防止盐分流失,处理时在花盆下放置托盘,渗出的溶液再倒回盘中。2015年10月初对各指标进行测定。
1.2 测定方法丙二醛(malondialdehyde, MDA)含量和可溶性糖含量的测定采用硫代巴比妥酸法:用紫外分光光度计分别测定在450、532、600 nm波长下的吸光度,根据吸光度计算其浓度[13]。可溶性蛋白质含量及抗氧化酶活性的测定采用李合生[14]的方法。其中:可溶性蛋白质含量的测定采用考马斯亮蓝比色法;超氧化物歧化酶(superoxide dismutase, SOD)活性测定采用氮蓝四唑光还原法;过氧化物酶(peroxidase, POD)活性测定采用愈创木酚法;过氧化氢酶(catalase, CAT)活性测定采用紫外吸收法。
1.3 数据分析采用Excel 2013对数据进行处理及统计分析,用SPSS 19.0统计软件中的邓肯新复极差法对平均数进行多重比较,用Origin 8.5进行绘图。
2 结果与分析 2.1 EBR对盐胁迫下香樟幼苗可溶性蛋白质的影响由图 1可知:CK2的可溶性蛋白质含量高于CK1处理,增长了5.46%;而0.5 mg/L EBR浸种处理的可溶性蛋白质含量与CK2相比增长了31.80%,且差异有统计学意义(P<0.05);外施0.2、0.3、0.4、0.5 mg/LEBR(浸种+喷叶处理)均显著提高了香樟叶片的可溶性蛋白质含量,分别比CK2增加46.78%、60.07%、65.18%、60.16%,且在0.1~0.5 mg/L EBR质量浓度梯度中,外施0.4 mg/L EBR的效果最佳。说明在盐胁迫下采用外施EBR(浸种+喷叶处理)对提高香樟可溶性蛋白质含量的效果较好。
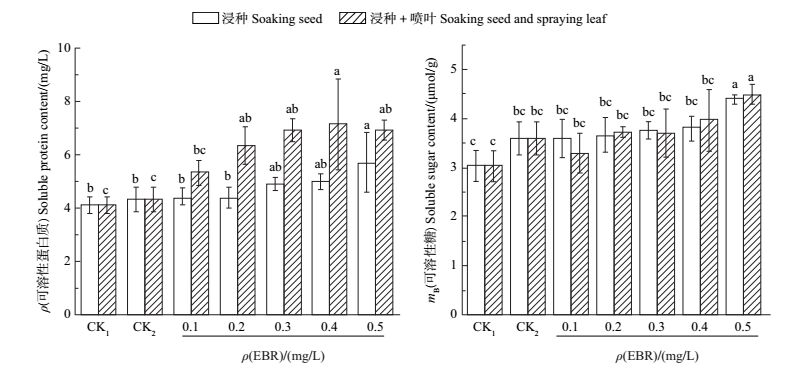 |
| CK1:对照(蒸馏水处理);CK2:对照(蒸馏水+4‰盐处理)。短栅上的不同小写字母表示不同处理间在P<0.05水平差异有统计学意义。 CK1: Control (distilled water treatment); CK2: Control (distilled water and 4‰ salt treatment). Different lowercase letters above the bars indicate statistically significant differences among different treatments at the 0.05 probability level. 图1 在4‰盐胁迫下不同质量浓度EBR对香樟幼苗叶片可溶性蛋白质和可溶性糖含量的影响 Fig. 1 Effect of different concentrations of 2, 4-epibrassionolide (EBR) on soluble protein and soluble sugar contents under 4‰ NaCl stress |
由图 1可知:CK2的可溶性糖含量高于CK1,提高了18.60%;与CK2相比,EBR质量浓度为0.5 mg/L的浸种+喷叶处理可显著提高可溶性糖含量,增加了24.80%,而其他EBR质量浓度处理间差异无统计学意义;EBR浸种处理均能提高可溶性糖含量,其中以0.5 mg/L EBR处理的效果最佳,增加了22.03%。表明在4‰盐胁迫下,外施不同质量浓度的EBR均能不同程度地提高香樟可溶性糖含量,且浸种+喷叶处理优于单一浸种处理。
2.3 EBR对盐胁迫下香樟幼苗丙二醛含量的影响细胞内丙二醛(MDA)含量的高低反映了植物遭受逆境胁迫伤害的强弱程度。由图 2可知:在4‰盐胁迫下,CK2处理的香樟幼苗叶片中MDA含量高于CK1,增加了27.10%;在浸种处理下,外源施用不同质量浓度的EBR均能不同程度地降低叶片的MDA含量,但差异无统计学意义;在浸种+喷叶处理下,与CK2相比,外施0.1、0.2、0.3 mg/L的EBR能降低MDA含量,但差异未达显著水平;而外施0.4、0.5 mg/L的EBR,MDA含量分别降低了27.67%、23.66%,且差异有统计学意义(P<0.05)。说明外施不同质量浓度的EBR能降低MDA含量,且浸种+喷叶处理的效果优于单一浸种处理;在0.1~0.5 mg/L质量浓度梯度中,EBR为0.4 mg/L时的处理效果最佳。
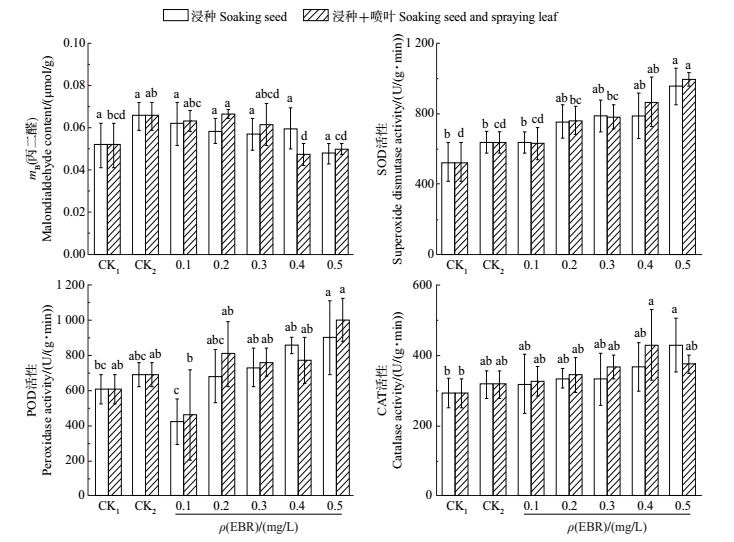 |
| CK1:对照(蒸馏水处理);CK2:对照(蒸馏水+4‰盐处理)。短栅上的不同小写字母表示不同处理间在P<0.05水平差异有统计学意义。 CK1: Control (distilled water treatment); CK2: Control (distilled water and 4‰ salt treatment). Different lowercase letters above the bars indicate statistically significant differences among different treatments at the 0.05 probability level. 图2 在4‰盐胁迫下不同质量浓度EBR对香樟幼苗叶片MDA含量及抗氧化酶活性的影响 Fig. 2 Effect of different concentrations of EBR on MDA content and antioxidant enzyme activities under 4‰ NaCl stress |
从图 2可见:与CK1相比,在CK2处理下香樟叶片的SOD、POD及CAT酶活性分别提高了21.48%、13.30%和8.65%,但差异无统计学意义,说明香樟幼苗对于低浓度盐胁迫具有一定的耐受性;采用浸种、浸种+喷叶2种处理方法外施不同质量浓度的EBR均能不同程度地提高叶片中的SOD活性,其中以0.5mg/L EBR处理的效果最佳,分别显著提高了49.49%和55.11%(P<0.05);与CK2处理相比,不同质量浓度的EBR处理均能提高香樟叶片中的CAT活性,其中以0.4 mg/L EBR的浸种+喷叶处理效果最佳,CAT活性提高了35.33%;在相同处理条件下,POD活性变化规律与SOD相似,即随着EBR质量浓度的逐渐升高,酶活性呈波动上升的趋势。综上所述,3种保护酶的活性在各处理条件下的变化趋势基本相同,它们通过协同互作共同缓解了NaCl胁迫造成的活性氧伤害,其中SOD变化幅度较大,POD次之,CAT最小,说明在抗氧化酶系统中SOD的作用相对较大。
3 讨论与结论在逆境条件下,植物细胞内的活性氧会不断积累,产生膜脂过氧化作用,造成植物体内自由基代谢平衡失调,丙二醛积累,进而损害细胞膜结构和功能的完整性[15-16]。油菜素内酯作为一种外源激素,生理活性较强,通过合理使用可提高植物的渗透调节能力和抗氧化能力,维持植株水分平衡,缓解盐胁迫的伤害,促进植株生长[17]。
丙二醛(MDA)是脂质过氧化作用的主要产物之一,其含量的高低在一定程度上反映了膜脂过氧化作用程度和对逆境反应的强弱[18]。在本研究中4‰盐胁迫使MDA含量上升,表明此时香樟幼苗叶片中活性氧的产生已超出了细胞的清除能力,导致活性氧大量积累,引起膜脂过氧化,严重破坏了膜系统。康云艳等[19]研究发现,在低氧条件下,EBR处理可以降低黄瓜的MDA含量,缓解低氧胁迫对植株的伤害。陈善娜等[20]和ÖZDEMIR等[21]的研究也表明,EBR处理可明显降低盐胁迫下水稻植株的MDA含量。本试验发现在4‰盐胁迫下,采用浸种+喷叶方式外施质量浓度为0.4 mg/L的EBR可显著降低MDA含量,与前人研究结果一致。说明外源EBR能在一定程度上抑制膜脂过氧化,维持细胞膜结构的稳定,从而保证植物体正常代谢和生长,且浸种+喷叶处理效果较好。
可溶性糖和可溶性蛋白质是植物体内重要的渗透调节物质,在植物适应逆境胁迫中发挥重要生理作用。在胁迫条件下,可溶性糖还可作为合成有机物质的碳架,为物质代谢提供能量。本试验在盐胁迫条件下,不同质量浓度的外源EBR处理能不同程度地提高香樟幼苗叶片中可溶性糖和可溶性蛋白质含量,这与前人的研究结论[22-23]一致。表明外源施用EBR可以通过促进体内渗透调节物质如可溶性糖和可溶性蛋白质的合成,来提高细胞渗透势,增强盐胁迫下幼苗的吸水能力,维持细胞正常代谢,从而缓解盐胁迫对香樟幼苗的伤害。本研究发现外施质量浓度为0.4 mg/L的EBR能显著提高叶片可溶性蛋白质含量,且以浸种+喷叶处理效果较好。
超氧化物歧化酶、过氧化物酶、过氧化氢酶是植物膜脂过氧化酶促防御系统的几个重要保护酶,它们通过相互协调、共同协作能有效清除活性氧(reactive oxygen species, ROS),是反映植物抗逆性的重要指标[24-26]。在盐胁迫下有关植物体内酶活性变化已有较多报道,但其变化规律不一致[8]。本试验结果表明,在盐胁迫下香樟幼苗叶片中的SOD、POD、CAT活性较CK1增加,说明当植株发生逆境胁迫时,叶片氧化胁迫程度加大,叶片可通过自身的调节机制提高酶活性以应对逆境胁迫[27]。外源施加EBR可进一步提高SOD、POD、CAT等保护酶的活性,这对于盐胁迫下香樟幼苗体内ROS的淬灭,减少脂质过氧化产物,维持活性氧代谢平衡,缓解盐胁迫伤害均有一定的益处。在0.1~0.5 mg/L EBR质量浓度梯度中,以0.4、0.5 mg/L的效果较为显著。这可能是由于植株的抗性与其体内的EBR水平相关,即在正常生长条件下EBR能在一定程度上提高植株的抗性,当植物遭遇逆境时,EBR的合成和累积将进一步提高抗逆性。这与李杰等[28]在低温胁迫下对辣椒的研究结果一致。
从外源EBR的2种施用方法对提高香樟叶片抗氧化酶活性的幅度来看,总体表现为经外源EBR浸种处理的效果优于未经外源EBR浸种处理(CK1),说明种子期间经EBR浸种能在一定程度上提高叶片抗氧化酶系统活性以清除ROS。同时,本研究也发现,外源EBR浸种+喷叶协同处理对提高盐胁迫下香樟的抗氧化酶活性具有显著的协同增效作用,推测植物在不同时期、不同部位对外源EBR的利用能力不同,且在种子和幼苗期同时处理更能有效地利用EBR以提高盐胁迫抗性[29]。此外,当外施EBR质量浓度为0.4、0.5 mg/L时,叶片SOD活性增幅最大,POD次之,CAT最小,表明SOD、POD对4‰盐胁迫反应更敏感。因此,EBR对盐胁迫下香樟幼苗叶片中3种保护酶的诱导效果不同,这可能是由于EBR对SOD、POD以及CAT这3种保护酶活性的调节机制不同,但其具体调节机制还需进一步论证。
综上所述,外源EBR浸种、浸种+喷叶这2种处理均能降低香樟幼苗叶片的MDA含量,诱导植株叶片中可溶性蛋白质、可溶性糖含量及抗氧化酶活性增加,从而提高植物耐盐性。就处理方式而言,浸种+喷叶协同处理的效果优于单一浸种处理,且以0.4、0.5 mg/L EBR的处理效果较好,但是对于其最佳质量浓度的确定还有待进一步扩大浓度序列步阶进行研究。虽然一定质量浓度的EBR可缓解盐胁迫对香樟幼苗叶片的伤害,但其保护作用可能不完全;同时,有关EBR浸种+喷叶处理协同诱导植物抗盐性的动态变化规律和生理机制仍有待进一步探讨。
| [1] |
韩浩章, 王晓立, 张颖, 等. 盐胁迫对秋季香樟幼苗抗氧化酶系统和光合特性的影响. 浙江农业学报, 2014, 26(5): 1235-1239. HAN H Z, WANG X L, ZHANG Y, et al. Effect of salt stress on photosynthetic characteristic and antioxidant enzyme system of Cinnamomum camphora seedlings in autumn. Acta Agriculturae Zhejiangensis, 2014, 26(5): 1235-1239. (in Chinese with English abstract) |
| [2] |
张丽华, 王晓立, 王梦秋, 等. 苏打盐碱胁迫对香樟幼苗光合特性的影响. 北方园艺, 2012(23): 91-93. ZHANG L H, WANG X L, WANG M Q, et al. Effect on camphor seedling photosynthetic characteristic of soda saline-alkali stress. Northern Horticulture, 2012(23): 91-93. (in Chinese with English abstract) |
| [3] |
梅海军, 王宁, 李自阳, 等. NaCl和Na2SO4胁迫对香樟幼苗生理特性的影响. 西北林学院学报, 2011, 26(6): 30-34. MEI H J, WANG N, LI Z Y, et al. Effects of NaCl and Na2SO4 saline stress on ecophysiological characteristics of Cinnamomum camphora seedlings. Journal of Northwest Forestry University, 2011, 26(6): 30-34. (in Chinese with English abstract) |
| [4] |
冯娟. NaCl胁迫对樟树苗木生理特性的影响及氮素的缓解效应. 福州:福建农林大学, 2013: 64-67. FENG J. Effects of NaCl stress on physiological characteristics of Cinnamomum camphora and the mitigative effect of nitrogen. Fuzhou: Fujian Agriculture and Forestry University, 2013: 64-67. (in Chinese with English abstract) |
| [5] | BAJGUZ A, HAYAT S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemisry, 2009, 47(1): 1-8. DOI:10.1016/j.plaphy.2008.10.002 |
| [6] |
邹华文. 表高油菜素内酯浸种对提高玉米幼苗抗旱性的影响. 湖北农学院学报, 2002, 22(1): 40-43. ZOU H W. Effect of epihomobrassinolide soaking seeds on the drought resistance of maize seedlings. Journal of Hubei Agricultural College, 2002, 22(1): 40-43. (in Chinese with English abstract) |
| [7] |
丁锦新, 陶晓东, 黄素青. 表油菜素内酯对黄瓜幼苗抗冷性的影响. 浙江农业科学, 1998(4): 195-197. DING J X, TAO X D, HUANG S Q. Effect of epihomobrassinolide on the cold resistance of cucumber seedling. Journal of Zhejiang Agricultural Sciences, 1998(4): 195-197. (in Chinese with English abstract) |
| [8] |
尚庆茂, 宋士清, 张志刚, 等. 外源BR诱导黄瓜(Cucumis sativus L.)幼苗的抗盐性. 中国农业科学, 2006, 39(9): 1872-1877. SHANG Q M, SONG S Q, ZHANG Z G, et al. Exogenous brassinosteroid induced the salt resistance of cucumber (Cucumis sativus L.) seedlings. Scientia Agricultura Sinica, 2006, 39(9): 1872-1877. (in Chinese with English abstract) |
| [9] | KANG Y Y, GUO S R, LI J, et al. Effect of root applied 24-epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Regulation, 2009, 57(3): 259-269. DOI:10.1007/s10725-008-9344-x |
| [10] |
吴秀, 陆晓民. 亚适宜温光盐环境下油菜素内酯对黄瓜幼苗抗氧化系统及光合作用的影响. 应用生态学报, 2015, 26(9): 2751-2757. WU X, LU X M. Effects of brassinolide on the antioxidant system and photosynthesis of cucumber seedlings under suboptimal temperature, light and salt environment. Chinese Journal of Applied Ecology, 2015, 26(9): 2751-2757. (in Chinese with English abstract) |
| [11] | SHU H M, GUO S Q, GONG Y Y, et al. Effects of brassinosteroid on salinity tolerance of cotton. Agriculture Science and Technology, 2014, 15(9): 1433-1437. |
| [12] |
刘金隆. 油菜素内酯调控三种双子叶植物耐盐性的效应及其机制. 南京:南京农业大学, 2013: 91-95. LIU J L. The effects and mechanisms of brassinolide regulating salt resistance of three dicotyledons. Nanjing: Nanjing Agricultural University, 2013: 91-95. (in Chinese with English abstract) |
| [13] |
林艳, 郭伟珍, 徐振华, 等. 大叶女贞抗寒性及冬季叶片丙二醛和可溶性糖含量的变化. 中国农学通报, 2012, 28(25): 68-72. LIN Y, GUO W Z, XU Z H, et al. Cold resistance and changes on MDA and soluble sugar of leaves of Ligustrunlucidum ait in winter. Chinese Agricultural Science Bulletin, 2012, 28(25): 68-72. (in Chinese with English abstract) DOI:10.3969/j.issn.1000-6850.2012.25.013 |
| [14] |
李合生. 植物生理生化实验原理和技术. 北京: 高等教育出版社, 2000: 164-184. LI H S. Principles and Techniques of Plant Physiological and Biochemical Experiment. Beijing: Higher Education Press, 2000: 164-184. (in Chinese with English abstract) |
| [15] | CHEN Y, XU C P, WANG N Y, et al. Effects of salicylic acid on oxidation resistance of'Nanlin 895'poplar plantlets in vitro under salt stress. Journal of Nanjing Forestry University (Natural Sciences Edition), 2012, 36(6): 17-22. |
| [16] | SHANG Q M, SONG S Q, ZHANG Z G, et al. Physiological mechanisms of salicylic acid enhancing the salt tolerance of cucumber seedling. Scientia Agricultura Sinica, 2007, 40(1): 147-152. |
| [17] | XIA X J, ZHANG Y, WU X W, et al. Brassinosteroids promote metabolism of pesticides in cucumber. Journal of Agricultural and Food Chemistry, 2009, 57(18): 8406-8413. DOI:10.1021/jf901915a |
| [18] |
裴斌, 张光灿, 张淑勇, 等. 土壤干旱胁迫对沙棘叶片光合作用和抗氧化酶活性的影响. 生态学报, 2013, 33(5): 1386-1396. PEI B, ZHANG G C, ZHANG S Y, et al. Effects of soil drought stress on photosynthetic characteristics and antioxidant enzyme activities in Hippophae rhamnoides Linn. seedlings. Acta Ecologica Sinica, 2013, 33(5): 1386-1396. (in Chinese with English abstract) |
| [19] |
康云艳, 郭世荣, 段九菊, 等. 24-表油菜素内酯对低氧胁迫下黄瓜根系抗氧化系统及无氧呼吸酶活性的影响. 植物生理与分子生物学学报, 2006, 32(5): 535-542. KANG Y Y, GUO S R, DUAN J J, et al. Effects of 24-epibrassinolide on antioxidant system and anaerobic respiratory enzyme activities in cucumber roots under hypoxia stress. Journal of Plant Physiology and Molecular Biology, 2006, 32(5): 535-542. (in Chinese with English abstract) |
| [20] |
陈善娜, 刘继梅, 游慧灵, 等. 抗寒剂和高油菜素内酯对高原水稻抗冷性的影响. 云南植物研究, 1997, 19(2): 184-190. CHEN S N, LIU J M, YOU H L, et al. The effect of cold resistant and homobrassinolide on the chilling resistance of plateau rice. Acta Botanica Yunnanica, 1997, 19(2): 184-190. (in Chinese with English abstract) |
| [21] | ÖZDEMIR F, BOR M, DEMIRAL T, et al. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regulation, 2004, 42(3): 203-211. DOI:10.1023/B:GROW.0000026509.25995.13 |
| [22] |
孙珊珊, 安勐颍, 韩烈保, 等. 外源24-表油菜素内酯对多年生黑麦草幼苗耐盐性的影响. 草地学报, 2014, 22(5): 1045-1050. SUN S S, AN M Y, HAN L B, et al. Effects of exogenously applied 24-epibrassinolide on the seedlings of perenial ryegrass under NaCl stress. Acta Agrectir Sinica, 2014, 22(5): 1045-1050. (in Chinese with English abstract) DOI:10.11733/j.issn.1007-0435.2014.05.020 |
| [23] |
张永平, 杨少军, 陈幼源. 2, 4-表油菜素内酯对高温胁迫下甜瓜幼苗抗氧化酶活性和光合作用的影响. 西北植物学报, 2011, 31(7): 1347-1354. ZHANG Y P, YANG S J, CHEN Y Y. Effects of 2, 4-epibrassinolide on antioxidant enzyme activities and photosynthesis in melon seedlings under high temperature stress. Acta Botanica BorealiOccidentalia Sinica, 2011, 31(7): 1347-1354. (in Chinese with English abstract) |
| [24] |
范志强, 姬仁磊. 外源水杨酸对低温胁迫下香樟叶片SOD活性的影响. 安徽农学通报, 2014, 20(24): 19-20. FAN Z Q, JI R L. Effect of salicylic acid on SOD activity of Cinnamomum camphora leaves under low temperature stress. Anhui Agricultural Science Bulletin, 2014, 20(24): 19-20. (in Chinese with English abstract) DOI:10.3969/j.issn.1007-7731.2014.24.009 |
| [25] | SAWADA H, SHIM I S, USUI K, et al. Adaptive mechanism of Echinochloa crusgalli Beauv. var. formosensis Ohwi under salt stress: Effect of salicylic acid on salt sensitivity. Plant Science, 2008, 174(6): 583-589. DOI:10.1016/j.plantsci.2008.03.013 |
| [26] | BAI T H, MA F W, LI C Y, et al. Effects of salicylic acid on reactive oxygen species metabolism in Malus robusta Rehd. under root-zone hypoxia stress. Acta Horticulturae Sinica, 2008, 35(2): 163-168. |
| [27] |
张润花, 郭世荣, 樊怀福, 等. 外源亚精胺对盐胁迫下黄瓜幼苗体内抗氧化酶活性的影响. 生态学杂志, 2006, 25(11): 1333-1337. ZHANG R H, GUO S R, FAN H F, et al. Effects of exogenous spermidine on anti-oxidative enzyme activities in cucumber seedlings under salt stress. Chinese Journal of Ecology, 2006, 25(11): 1333-1337. (in Chinese with English abstract) DOI:10.3321/j.issn:1000-4890.2006.11.007 |
| [28] |
李杰, 杨萍, 颉建明, 等. 2, 4-表油菜素内酯对低温胁迫下辣椒幼苗根系生长及抗氧化酶系统的影响. 核农学报, 2015, 29(5): 1001-1008. LI J, YANG P, JIE J M, et al. Effects of 2, 4-epibrassinolide on growth and antioxidant enzyme system in pepper roots under chilling stress. Journal of Nuclear Agricultural Sciences, 2015, 29(5): 1001-1008. (in Chinese with English abstract) DOI:10.11869/j.issn.100-8551.2015.05.1001 |
| [29] |
李悦, 宋士清, 王久兴. 不同BR施用方式诱导黄瓜幼苗对Ca(NO3)2胁迫抗性的研究. 西北植物学报, 2016, 36(2): 377-382. LI Y, SONG S Q, WANG J X. Inducing effects of exogenous BR application with different methods on Ca(NO3)2 stress resistance of cucumber seedlings. Acta Botanica Boreali-Occidentalia Sinica, 2016, 36(2): 377-382. (in Chinese with English abstract) |
 2017, Vol. 43
2017, Vol. 43


