| 左旋多巴和氯碘羟喹治疗对帕金森病猕猴血清抗氧化能力的影响 |
2. 四川农业大学实验动物工程技术中心,四川 雅安 625014
2. Engineering and Technology Center for Laboratory Animals, Sichuan Agricultural University, Ya' an 625014, Sichuan, China
帕金森病(Parkinson’ s disease,PD)是继阿尔茨海默症(Alzheimer’ s disease,AD)后的第二大神经退行性疾病,其发病率随年龄增加而上升,影响全世界大约2%的60岁以上人口[1]。PD的主要病理特征是黑质致密部多巴胺神经元变性坏死,其病理组织学标志物为出现的路易小体;PD病人的临床症状表现为运动迟缓、肌肉强直以及震颤[2]。引起PD神经变性的机制主要包括氧化应激、线粒体功能障碍、兴奋性毒性、钙的细胞毒性、营养因子缺乏、炎症过程、遗传因素、环境因素、一氧化氮毒性和细胞凋亡等[3],所有这些因素都可能相互作用,从而放大毒性,形成恶性循环,最终导致神经功能障碍、萎缩以及细胞死亡。左旋多巴(levodopa,LD)作为临床上应用最多且最有效的PD治疗药物[4],能够显著改善PD病人临床症状。但由于LD氧化代谢后会产生活性氧,可能对多巴胺神经元产生损伤,因此,目前对于LD的使用还存在争议[5]。体外研究表明,LD会对神经细胞产生毒性[6-7],但体内研究却未见异常[8-10]。氯碘羟喹(clioquinol,CQ)原本是一种抗寄生虫药,后来发现它对PD动物模型和神经细胞能够产生良好的治疗效果,并可改善临床病人的认知功能[11]。同时,CQ作为一种铁离子螯合剂,能够减少铁离子在大脑关键核团的累积[12],并在AD和PD临床试验中表现出良好的疗效[13],因此推断CQ具有神经保护功能[14]。由于无法接触到PD病人活体神经组织,不能对其大脑进行活体检查,因而无法研究PD病人中枢神经系统的病变机制;因此,分析活体状态下PD患者的脑脊液、血液、尿液以及唾液等体液样本中相关物质的改变情况就变得尤为重要。在临床研究中,由于脑脊液取样需要腰椎穿刺,使健康患者的样本获取受限,而血液样本相对容易获取,使其成为研究的首选对象。因此,本文通过测定不同药物治疗后PD猕猴血液中抗氧化水平的变化,推断其与药效的关系,从而为临床PD药物治疗效果判定提供较为科学的指导依据。
1 材料与方法 1.1 实验材料 1.1.1 实验动物健康恒河猴8只,雌雄各半,年龄3~4岁,体质量3~5 kg,由四川普莱美生物科技有限公司(国家实验用猕猴种源基地)提供[动物生产许可证:SCXK (川):2014-027]。
1.1.2 实验试剂1-甲基-4-苯基-1,2,3,6-四氢嘧啶(1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine, MPTP)、左旋多巴、卡比多巴、氯碘羟喹均购自美国Sigma公司;超氧化物歧化酶、谷胱甘肽、丙二醛、谷胱甘肽过氧化物酶和谷胱甘肽硫转移酶等测定试剂盒均购自南京建成生物工程研究所。
1.1.3 实验仪器设备全波长读数仪(Varioskan Flash,Thermo Scientific,美国),离心机(Sorvall Legend Micro 17/17R,Thermo Scientific,美国),摄像记录仪(YHS-113,北京小米科技有限责任公司),恒温水浴锅(HH-42,江苏常州国华电器有限公司),超净工作台(SW-CJ-1FD,江苏苏净集团有限公司)等。
1.2 实验方法 1.2.1 帕金森病动物模型建立造模前动物先适应环境3个月,以减少应激并降低实验误差。供试动物在昼夜交替的自然光周期下饲养,自由饮水进食。按照m (MPTP):V (生理盐水)=1 mg:1.5 mL的比例溶解,在供试动物前臂肌肉发达处按0.2 mg/(kg·d)注射,每日上午9:00—9:30完成给药;连续注射直到动物出现帕金森病主要体征状态后,根据动物承受能力酌情继续给药。实验中对动物的处置符合中华人民共和国科学技术部《关于善待实验动物的指导性意见》的规定。
1.2.2 药物治疗将成功建立的PD猕猴模型随机分为3组:3只经口喂服配比为4: 1的左旋多巴+卡比多巴15 mg/kg;3只经口喂服氯碘羟喹15 mg/kg;2只经口喂服等量生理盐水。每日上午9:00喂药,连续4周。利用自身对照法对动物给药前后相关指标进行比较研究。
1.2.3 行为评价注射MPTP和给药半小时后观察动物,进行行为学评价。为减少实验误差,降低人为干扰因素,本实验通过摄影方式记录动物行为,借助帕金森病猴临床行为评价量表[15]并结合自身的观察进行少量修订后,对PD猕猴模型以及药物治疗效果进行行为评分。评分细则如下:活动量0~3分,肌强直0~3分,运动迟缓0~3分,冻结0~2分,身体姿势0~2分,震颤0~3分,采食0~3分,手臂移动0~2分,手臂姿势0~2分。
1.2.4 生化测试连续治疗4周后,于每只猕猴前臂静脉采血,血样装存于促凝管中。促凝管静置一段时间后,以4 ℃、8000 r/min离心5 min,取上清液,分装,储存于-80 ℃冰箱中备用,待测。样品测定均严格按照试剂盒步骤进行。
1.2.5 数据处理实验数据均采用SPSS 20.0统计软件进行分析,同组治疗前后采用独立方差t检验,治疗后不同组之间的统计学差异利用单因素方差分析,并利用最小显著差数法进行多重比较。柱状图上的数据以平均值±标准差表示,P<0.05表示差异有统计学意义,P<0.01表示差异有高度统计学意义。
2 结果与分析 2.1 PD猕猴模型的建立猕猴造模前后的行为学评分结果(图 1)显示:与自身对照相比,注射MPTP的猕猴在经历7 d无症状后,其行为学评分开始逐步上升,并与MPTP在体内的累积剂量成正相关;在MPTP注射前期,猕猴未表现出明显的行为差异,仅有部分出现发声减少现象。猕猴早期行为变化包括活动量下降、手臂运动减少、肌肉僵直和冻结;随着MPTP在体内的持续累积,猕猴运动越发迟缓,最终出现姿势异常、肌肉强直和震颤等PD典型临床症状并维持稳定。这表明PD猕猴模型建立成功。
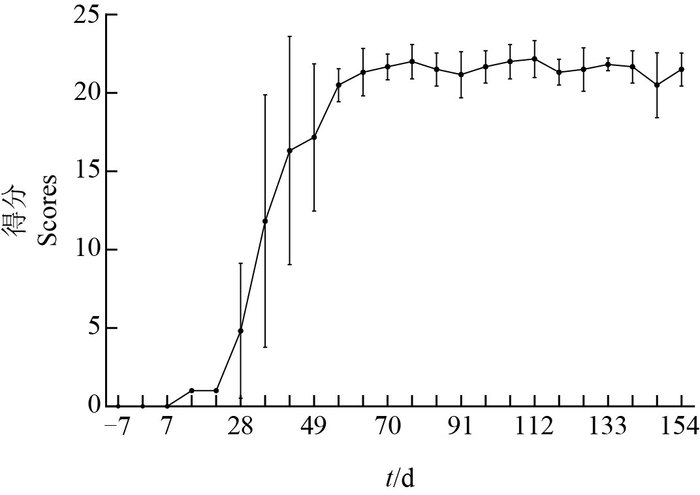 |
| 图1 慢性PD猕猴模型行为评分 Fig. 1 Behavior scores of the rhesus monkey models of chronic Parkinson' s disease |
停止注射MPTP并进行药物治疗后,猕猴行为评分均呈现下降趋势(图 2);与自身给药前后对比发现,左旋多巴(LD)组(P<0.01)和氯碘羟喹(CQ)组(P<0.05)的猕猴行为评分均显著下降,而对照组在统计学上无显著差异。
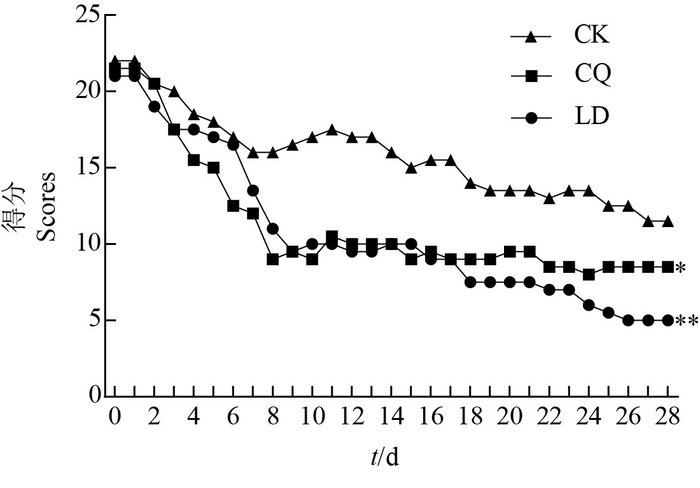 |
| CK:生理盐水;CQ:氯碘羟喹;LD:左旋多巴。*表示与对照组比较在P<0.05水平差异有统计学意义,**表示在P<0.01水平差异有高度统计学意义。 CK: Physiological saline; CQ: Clioquinol; LD: Levodopa. Compared with the CK group, the single asterisk (*) and double asterisks show statistically significant and highly significant differences at the 0.05 and 0.01 probability levels, respectively. 图2 不同药物治疗后PD猕猴行为评分 Fig. 2 Behavior scores of PD rhesus monkey after treated with different drugs |
与同组治疗前相比,LD组超氧化物歧化酶(SOD)活性增加(P<0.05);与仅注射生理盐水的对照(CK)相比,LD组和CQ组的SOD活性升高,差异有统计学意义(图 3A)。如图 3B所示:与同组治疗前相比,LD组和CK组的谷胱甘肽过氧化物酶(GPX)活性增加(P<0.05);与CK相比,LD组和CQ组的GPX活性在统计学上显著低于CK组。如图 3C所示:与同组治疗前相比,LD组和CQ组的谷胱甘肽硫转移酶(GST)活性增加(P<0.05), 且LD组的GST活性在统计学上显著高于CK组(P<0.01)和CQ组(P<0.05)。同样,与同组治疗前相比,LD组和CQ组的谷胱甘肽(GSH)含量均显著增加,差异有统计学意义(P<0.05), 且CQ组的GSH含量显著高于CK组(P<0.05)(图 3D)。与上述各指标变化相反,与同组治疗前相比,LD组的丙二醛(MDA)含量显著减少,且低于CK组和CQ组,差异均有统计学意义(P<0.05)(图 3E)。
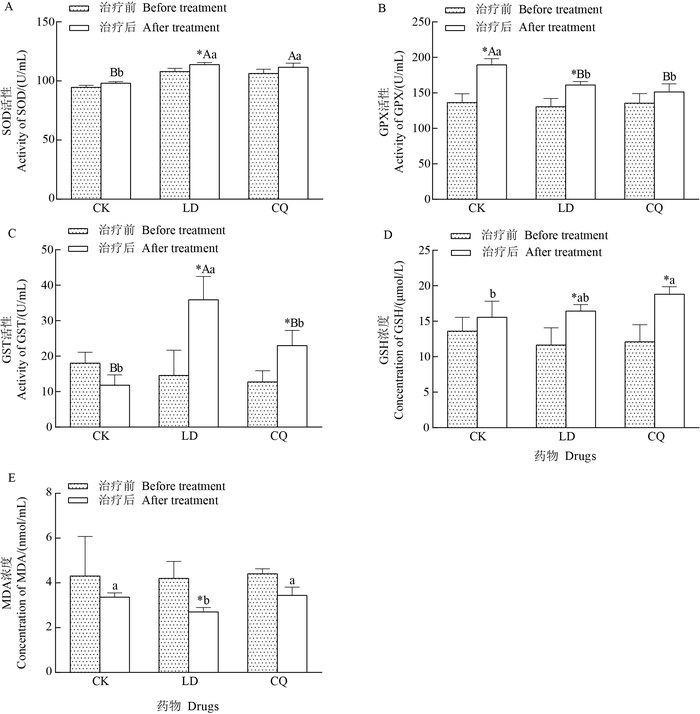 |
| CK:生理盐水;LD:左旋多巴;CQ:氯碘羟喹。SOD:超氧化物歧化酶;GPX:谷胱甘肽过氧化物酶;GST:谷胱甘肽硫转移酶;GSH:谷胱甘肽;MDA:丙二醛。*表示同组治疗前后在P<0.05水平差异有统计学意义;不同小写字母表示治疗后不同组间在P<0.05水平差异有统计学意义,不同大写字母表示在P<0.01水平差异有高度统计学意义。 CK: Physiological saline; LD: Levodopa; CQ: Clioquinol. SOD:Superoxide dismutase; GPX: Glutathione peroxidase; GST: Glutathione Stransferase; GSH: Glutathione; MDA: Malondialdehyde. Single asterisk (*) shows statistically significant difference at the 0.05 probability level before and after treatments. Different lowercase or uppercase letters above bars represent statistically significant differences among different groups after treatments at the 0.05 or 0.01 probability levels. 图3 不同药物治疗后PD猕猴血液抗氧化水平变化 Fig. 3 Change of antioxidation level in blood of PD rhesus monkey after treated with different drugs |
PD是一种由局部神经变性引起的疾病,虽然目前病因还不清楚,但研究表明,氧化应激在PD的发生发展过程中起到了关键作用[16-17]。研究表明,通过检测外周血液能够反映大脑黑质部氧化应激的水平[18]。因此,本研究通过测定不同药物治疗后PD猕猴血液中抗氧化水平的变化情况,并结合PD猕猴临床行为症状,推断其与药效的关系。
研究发现,MPTP能选择性损伤黑质部多巴胺神经元从而模拟PD症状[19-20]。同啮齿类动物相比,灵长类动物在病理上与PD病人更加相似,因此,MPTP诱导的猕猴模型是研究PD的最佳动物模型。由于急性注射MPTP不能产生PD所有临床症状[19],因此,笔者尝试通过肌肉注射MPTP,建立慢性PD猕猴模型。通过全身给药,能更好地模拟人类临床大脑双侧对称性损伤的情况。由于不同猕猴对MPTP敏感程度不一,所以MPTP对大脑黑质部神经元损伤程度不同,猕猴也表现出不同的临床行为症状,使得动物行为评分在前期波动特别大,并且只能产生轻度的PD症状;因此,本实验在猕猴前期症状出现后继续给予MPTP,以损伤更多的神经元来形成典型的PD症状。由于MPTP在多巴胺神经元中可以累积,本实验通过持续注射MPTP,猕猴大脑黑质部多巴胺神经元受到不可逆的损伤,猕猴逐渐表现出稳定的PD症状,临床行为评分趋于一致。
LD是目前治疗PD最有效的药物,作为多巴胺的天然前体,LD可以穿过血脑屏障,经神经元摄取转运并进入黑质多巴胺细胞中脱羧转变为多巴胺,从而改善PD病人的运动症状[21]。但在代谢过程中,LD会产生一定数量的活性氧,这可能会进一步加重内源性抗氧化能力下降的PD病人症状。体外研究表明,LD会对神经细胞产生损伤[6-7],但在啮齿类动物、非人类灵长类动物以及PD病人上并未发生;也有研究表明,LD能对神经细胞产生保护效果[4]。本研究发现,服用LD后,通过增强抗氧化酶的活力,加速清除机体内产生过多的自由基和过氧化物,重新维持了体内氧化剂与抗氧化剂之间的动态平衡。同时,通过补充LD还提高了脑内多巴胺的浓度,最终改善了PD猕猴的临床行为症状。CQ作为一种铁离子螯合剂,能够降低脑内铁离子浓度,增加酪氨酸羟化酶的活性[13]。由于大脑内酪氨酸羟化酶的活性受到铁离子的调控[22],因此,在CQ的作用下,通过减少脑内铁离子的累积,增强酪氨酸羟化酶的活性,促进了多巴胺的合成,从而缓解了PD的临床症状。同时,CQ通过螯合脑内的铁离子,抑制了芬顿(Fenton)反应,减少了羟自由基在脑内的累积,从而增强了机体内源性抗氧化能力。对照组猕猴行为评分也出现了下降,这可能与猕猴大脑黑质纹状体中多巴胺代偿性增加有关[19]。同时,年龄也被认为是MPTP诱导PD行为恢复的一个潜在因素,并在小鼠[23]和灵长类动物[20]上得到证实。但对照组猕猴抗氧化酶活性与脂质过氧化物无显著变化,说明猕猴行为虽有恢复但差异不显著。
4 结论本实验结果表明,PD猕猴服用LD和CQ后,通过提高血液中抗氧化酶活性,降低脂质过氧化物含量,重新维持了机体的氧化还原平衡状态,从而减轻了PD猕猴的临床症状。该结果为临床PD药物治疗提供了参考依据。
| [1] | CORNETTA T, PALMA S, APRILE I, et al. Levodopa therapy reduces DNA damage in peripheral blood cells of patients with Parkinson' s disease. Cell Biology and Toxicology, 2009, 25(4): 321-330. DOI:10.1007/s10565-008-9086-6 |
| [2] | FOLEY P, RIEDERER P. Influence of neurotoxins and oxidative stress on the onset and progression of Parkinson's disease. Journal of Neurology, 2000, 247(Suppl. 2): Ⅱ82-Ⅱ94. |
| [3] | YUAN H, ZHEGN J C, LIU P, et al. Pathogenesis of Parkinson' s disease: Oxidative stress, environmental impact factors and inflammatory processes. Neuroscience Bulletin, 2007, 23(2): 125-130. DOI:10.1007/s12264-007-0018-x |
| [4] | COLAMARTINO M, SANTORO M, DURANTI G, et al. Evaluation of levodopa and carbidopa antioxidant activity in normal human lymphocytes in vitro: Implication for oxidative stress in Parkinson' s disease. NeurotoxicityResearch, 2015, 27(2): 106-117. |
| [5] | ANDERSEN J K. Iron dysregulation and Parkinson' s disease. Journal of Alzheimer' s Disease, 2004, 6(Suppl. 6): 47-52. |
| [6] | BASMA A N, MORRIS E J, NICKLAS W J, et al. L-dopa cytotoxicity to PC12 cells in culture is via its autoxidation. Journal of Neurochemistry, 1995, 64(2): 825-832. |
| [7] | MYTILINEOU C, WALKER R H, JNOBAPTISTE R, et al. Levodopa is toxic to dopamine neurons in an in vitro but not an in vivo model of oxidative stress. Journal of Pharmacology and Experimental Therapeutics, 2003, 304(2): 792-800. DOI:10.1124/jpet.102.042267 |
| [8] | DATLA K P, BLUNT S B, DEXTER D T. Chronic L-DOPA administration is not toxic to the remaining dopaminergic nigrostriatal neurons, but instead may promote their functional recovery, in rats with partial 6-OHDA or FeCl(3) nigrostriatal lesions. Movement Disorders, 2001, 16(3): 424-434. DOI:10.1002/mds.1091 |
| [9] | LYRAS L, ZENG B Y, MCKENZIE G, et al. Chronic high dose LDOPA alone or in combination with the COMT inhibitor entacapone does not increase oxidative damage or impair the function of the nigro-striatal pathway in normal cynomologus monkeys. Journal of Neural Transmission, 2002, 109(1): 53-67. DOI:10.1007/s702-002-8236-2 |
| [10] | ZENG B Y, PEARCE R K B, MACKENZIE G M, et al. Chronic high dose L-DOPA treatment does not alter the levels of dopamine D-1, D-2 or D-3 receptor in the striatum of normal monkeys: An autoradiographic study. Journal of Neural Transmission, 2001, 108(8/9): 925-941. |
| [11] | RITCHIE C W, BUSH A I, MACKINNON A, et al. Metalprotein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Archives of Neurology, 2003, 60(12): 1685-1691. DOI:10.1001/archneur.60.12.1685 |
| [12] | LEI P, AYTON S, FINKELSTEIN D I, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nature Medicine, 2012, 18(2): 291-295. DOI:10.1038/nm.2613 |
| [13] | LEI P, AYTON S, APPUKUTTAN A T, et al. Clioquinol rescues parkinsonism and dementia phenotypes of the Tau knockout mouse. Neurobiology of Disease, 2015, 81: 168-175. DOI:10.1016/j.nbd.2015.03.015 |
| [14] | LI C, WANG J, ZHOU B. The metal chelating and chaperoning effects of clioquinol: Insights from yeast studies. Journal of Alzheimer' s Disease, 2010, 21(4): 1249-1262. DOI:10.3233/JAD-2010-100024 |
| [15] | SCHNEIDER J S, KOVELOWSKI C J. Chronic exposure to low doses of MPTP. I. Cognitive deficits in motor asymptomatic monkeys. Brain Research, 1990, 519(1/2): 122-128. |
| [16] | SHERER T B, RICHARDSON J R, TESTA C M, et al. Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson' s disease. Journal of Neurochemistry, 2007, 100(6): 1469-1479. |
| [17] | NIKAM S, NIKAM P, AHALEY S K, et al. Oxidative stress in Parkinson's disease. Indian Journal of Clinical Biochemistry, 2009, 24(1): 98-101. DOI:10.1007/s12291-009-0017-y |
| [18] | BLANDINI F, COSENTINO M, MANGIAGALLI A, et al. Modifications of apoptosis-related protein levels in lymphocytes of patients with Parkinson' s disease. The effect of dopaminergic treatment. Journal of Neural Transmission, 2004, 111(8): 1017-1030. |
| [19] | MOUNAYAR S, BOULET S, TANDé D, et al. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain, 2007, 130(11): 2898-2914. DOI:10.1093/brain/awm208 |
| [20] | OVADIA A, ZHANG Z, GASH D M. Increased susceptibility to MPTP toxicity in middle-aged rhesus monkeys. Neurobiology of Aging, 1995, 16(6): 931-937. DOI:10.1016/0197-4580(95)02012-8 |
| [21] | DEVOS D, MOREAU C, DUJARDIN K, et al. New pharmacological options for treating advanced Parkinson' s disease. Clinical Therapeutics, 2013, 35(10): 1640-1652. DOI:10.1016/j.clinthera.2013.08.011 |
| [22] | BEARD J L, KILLICK T, GONZALES E, et al. Reductions in the Labile Iron Pool activate PKC and alter monoamine metabolism. The FASEB Journal, 2006, 20(4): 193. |
| [23] | RICAURTE G A, DELANNEY L E, IRWIN I, et al. Older dopaminergic neurons do not recover from the effects of MPTP. Neuropharmacology, 1987, 26(1): 97-99. DOI:10.1016/0028-3908(87)90051-7 |
 2017, Vol. 43
2017, Vol. 43


