| 镉胁迫对秋华柳植物螯合肽含量的影响 |
2. 盘龙区盘龙小学,昆明 650051
2. Panlong Primary School in Panlong District, Kunming 650051, China
近年来,土壤重金属污染被广泛关注,已成为国内外环境生态研究的热点。我国土壤污染调查公报显示,全国土壤污染以无机型为主,其超标点位占全部超标点位的82.8%,镉的点位超标率高达7.0%[1]。镉(Cd)为毒性最强的重金属元素之一,由于其水溶性强和极易被植物吸收等特点,更容易在土壤-动植物-人体间富集转移,最终危害人体健康[2-4]。植物修复可通过植物将环境中的重金属吸收富集于体内,并使其转化为毒性较小的形式,再运输至地上部分储存,从而降低环境中的重金属污染,提高其安全性[5]。现已发现了大量的超富集植物,如黑麦草(Lolium perenne)、蜈蚣草(Pteris vittata)、天蓝遏蓝菜(Thlaspi caerulescens)、孔雀草(Tagetes patula)等,但大多为草本植物,其绝对生物量小,总体富集能力相对较弱,且主根不发达,根系较浅,不能对深层受污染土壤进行修复[6]。而木本植物根系发达,吸收面积大,生物量大[7],其在重金属污染土壤上的修复应用值得重视。已有研究表明,柳属(Salix L.)和杨属(Populus L.)植物对重金属有较高的富集能力,在重金属污染土壤的植物修复中有相当可观的潜力[8]。
秋华柳(Salix variegata)为柳属多年生灌木[9],生物量大,生长快,是坡岸地带进行植被构建的优良物种[10]。相关研究表明,秋华柳地上部分积累镉和转移镉的能力强,基于生长和生物量参数的耐受指数高,且具有较强的光合耐受性,适用于镉污染区域的植物修复[11-12];但目前尚缺乏其对Cd耐受和解毒机制的进一步研究。
金属结合配体对降低重金属的毒性有重要作用。目前研究发现,多种金属结合配体对重金属胁迫下植物的生存适应性有重要意义[13]。有机酸、氨基酸等小分子有机化合物可与重金属形成稳定的螯合物,促进重金属在体内的运输,降低细胞中重金属离子的生理活性[14-15]。植物螯合肽(phytochelatins, PCs)和金属硫蛋白(metallothioneins, MTs)等巯基(—SH)多肽链配体对重金属有很强的亲和力,在维持植物细胞内稳态和重金属解毒方面具有重要意义[16],其中PCs是植物体内最重要的一种螯合剂。PCs是以谷胱甘肽(glutathione, GSH)为前体[17],在外界重金属诱导胁迫和多种酶促反应下,在细胞质内合成的一类低分子质量、富含巯基(—SH)的多肽链,其结构通式为(γ-Glu-Cys)n-Gly(n=2~11) [18]。镉(Cd)、铜(Cu)、锌(Zn)、银(Ag)、金(Au)、汞(Hg)和铅(Pb)胁迫都会引起植物体迅速合成PCs,其中,Cd诱导形成PCs的速度最快,数量最多,被认为是形成PCs的最强诱导剂[19-20]。PCs可与Cd形成无毒、低分子质量的Cd-S-PC化合物,通过液泡膜的Cd/H+逆向转运蛋白及三磷酸腺苷结合盒转运体(ATP binding cassette, ABC)进入液泡中,形成高分子质量化合物进行储存[21-22],避免游离重金属离子对植物细胞造成氧化伤害。而且,有研究表明,Cd胁迫使多种植物的GSH和PCs相关基因和蛋白表达上调[23-25]。
巯基肽螯合解毒作用的大小因物种而异。为探究Cd富集植物秋华柳的巯基肽在其耐受解毒中的作用,本文对不同程度Cd胁迫下秋华柳叶片和根系中生成的巯基肽种类和含量进行研究,以阐明秋华柳的螯合解毒机制。
1 材料与方法 1.1 试验材料2015年3月,于重庆市嘉陵江同兴街河岸段(29°41′2″ N,106°26′56″ E)剪取长15~17 cm、茎径0.7~1 cm的秋华柳枝条,带回实验室用超纯水洗净后,将其2/3处固定于1/2 Hoagland改良营养液中,置于光照培养箱中培养;每盆5株,每3天更换1次培养液。光照培养箱参数设置如下:温度25/20 ℃,相对湿度60%,光照强度10 000/0 lx,光照/黑暗时间16 h/8 h。
1.2 试验设计待秋华柳扦插苗长成完整植株后(约55 d,萌条约40 cm),于2015年8月选取生长基本一致的幼苗进行随机分组,以CdCl2·2.5H2O的形式添加,分别设置0 mg/L Cd2+(CK)、2 mg/L Cd2+(T1)、10 mg/L Cd2+(T2)、20 mg/L Cd2+(T3)、50 mg/L Cd2+(T4)共5个Cd处理水平,每个处理4次重复。
1.3 测试方法 1.3.1 植株巯基肽含量测定分别在试验0、6、12、18 d对秋华柳叶片及根系进行取样,用2 mmol/L EDTA-Na2浸泡5 min后,去除植物表面吸附的Cd2+,用高效液相色谱法(high performance liquid chromatography, HPLC)测定谷胱甘肽(GSH)和植物螯合肽2~6(PC2~6)等巯基肽含量[26]。测试仪器为安捷伦(Agilent)1200高效液相色谱系统,采用Agilent Zorbax Eclipse XDB-C18色谱柱(4.6 mm×30 mm,粒径1.8 μm)。
1.3.2 植株镉含量测定收获植物根系和叶片,用2 mmol/L EDTA-Na2浸泡5 min后,烘干至恒量。称取碾磨后的植物样0.050 0 g,消解定容,然后用电感耦合等离子体发射光谱仪(ICP-OES, Thermo Fisher iCAP 6300, 英国)测定其Cd含量。
1.4 数据分析利用SPSS 20.0软件进行数据统计分析:用重复度量(repeated measures)分析不同时间、不同Cd2+质量浓度及两者交互作用对秋华柳叶片及根系中巯基肽含量的影响;用单因素方差分析(one-way ANOVA)研究相同时间、不同Cd2+质量浓度对秋华柳叶片及根系中总巯基肽含量的影响;用邓肯多重比较(Duncan’ s multiple range test)分析不同处理间的差异;用相关分析探究秋华柳叶片和根系中各巯基肽含量与Cd积累量的相关性。采用Origin 8.5软件作图。
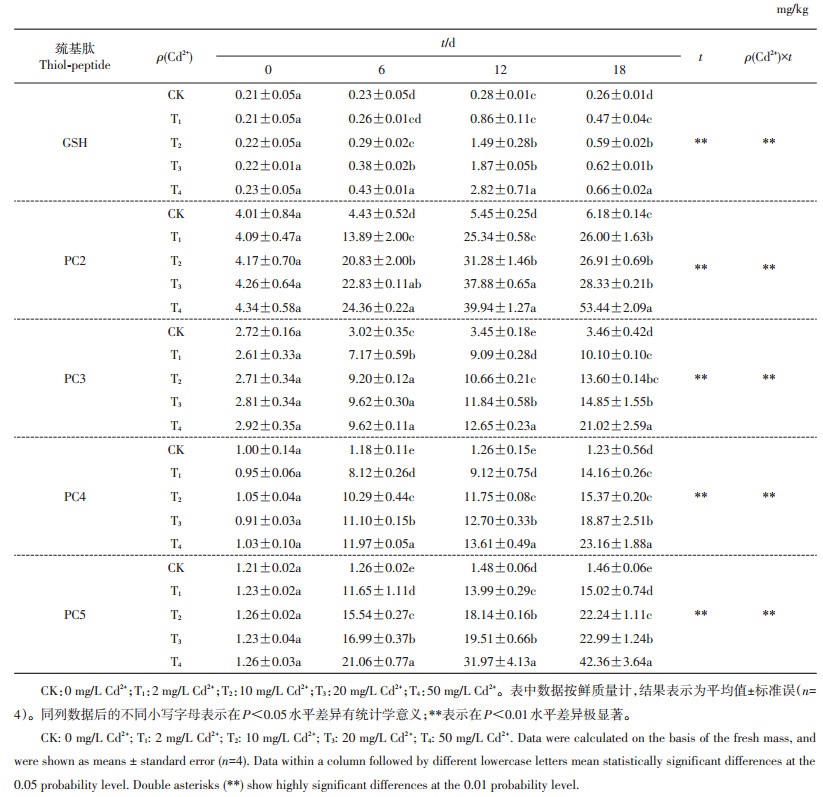
2 结果与分析 2.1 秋华柳叶片中巯基肽含量的变化特征由表 1可知,秋华柳叶片中存在GSH及PC2~ 5共5种巯基肽。0 d时,各处理组间秋华柳叶片中5种巯基肽含量在统计学上均无显著差异。6 d时,随着Cd2+处理量的增加,秋华柳叶片中5种巯基肽含量均增加,除GSH含量从T2水平开始显著高于对照外,其余4种巯基肽含量均在T1水平时即显著高于对照;其中,不同处理组间PC4和PC5的含量在统计学上均有显著差异,与对照相比,4个处理组的PC4含量依次增加了5.88、7.72、8.41和9.14倍,PC5含量依次增加了8.25、11.33、12.48和15.44倍。12 d时,T1(2 mg/L Cd2+)处理的GSH含量与CK相比无显著差异,其余各处理组的GSH含量均显著高于对照;与CK相比,不同处理组的PC2~5含量均显著增高,其中PC5含量的增幅最大,T1~T4处理分别增加了8.45、11.26、12.18和20.60倍,其次为PC4,分别增加了6.24、8.33、9.08和9.80倍;与6 d相比,所有处理组的巯基肽含量均增加,其中GSH含量的增幅最大,依次增加了2.31、4.14、3.92、5.56倍。18 d时,各处理组秋华柳叶片中5种巯基肽含量均显著高于对照,其中PC5含量的增幅最大,分别增加了9.29、14.23、14.75和28.01倍。6、12和18 d时,不同处理组秋华柳叶片中巯基肽含量均为PC2>PC5>PC4>PC3>GSH。时间对4个Cd2+处理水平下的各巯基肽含量具有极显著性效应,不同Cd2+处理量和时间的交互效应对秋华柳叶片中各巯基肽含量有极显著影响。
| 表1 不同处理时间、不同Cd2+质量浓度下秋华柳叶片中巯基肽的质量分数 Table 1 Thiol-peptide content in the leaves of Salix variegata at different Cd2+ concentrations and treatment time |
 |
| 点击放大 |
由图 1可知,秋华柳叶片中巯基肽总量随着Cd迫程度的增加和胁迫时间的延长而增加。在相同时间处理下,与对照相比,不同处理组秋华柳叶片中巯基肽的总量均显著增加,其中18 d时,T4处理组的巯基肽总量增幅最大,增加了39.33%。在相同质量浓度Cd2+处理下,T4组巯基肽总量随时间的延长增幅最大,与0 d相比,分别增加了5.90、9.34和13.40倍。
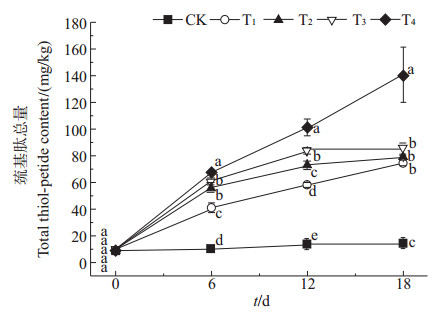 |
| CK:0 mg/L Cd2+;T1:2 mg/L Cd2+;T2:10 mg/L Cd2+;T3:20 mg/L Cd2+;T4:50 mg/L Cd2+。图上数据按鲜质量计;不同小写字母表示在P<0.05水平差异有统计学意义。 CK: 0 mg/L Cd2+; T1: 2 mg/L Cd2+; T2: 10 mg/L Cd2+; T3: 20 mg/L Cd2+; T4: 50 mg/L Cd2+. Data were calculated on the basis of the fresh mass. Different lowercase letters mean statistically significant differences at the 0.05 probability level. 图1 秋华柳叶片中巯基肽的总质量分数 Fig. 1 Total thiol-peptide content in the leaves of S. variegata |
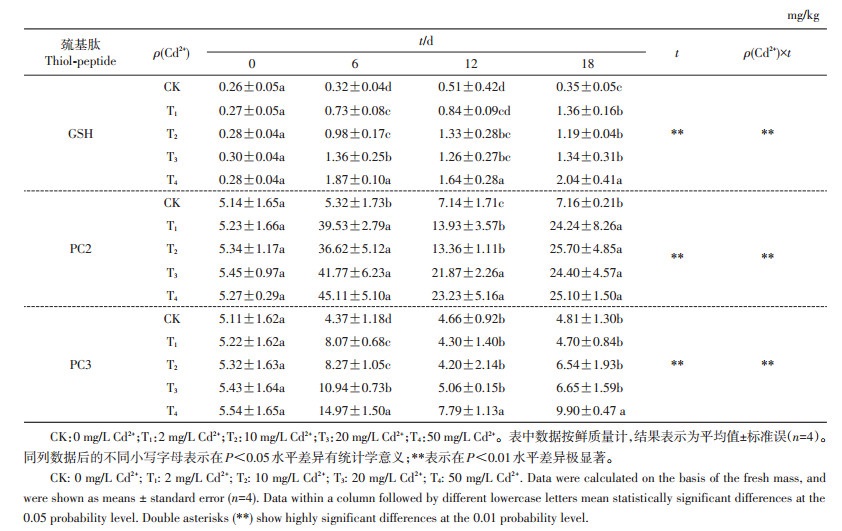
由表 2可知,在秋华柳根系中只检测到了GSH、PC2和PC3。0 d时,各处理组间秋华柳根系中3种巯基肽含量在统计学上均无显著差异。6 d时,不同处理组秋华柳根系中3种巯基肽含量均显著高于对照,其中PC2含量的增幅最大,与对照相比,4个处理组的PC2含量依次增加了6.56、5.86、6.66、7.56倍。12 d时,不同处理组秋华柳根系中3种巯基肽含量均高于对照,其中各处理组的PC2含量均显著高于对照,且增幅大于GSH和PC3;与对照相比,除T1处理的GSH含量在统计学上无显著差异外,其余3个不同质量浓度Cd2+处理的GSH含量差异均有统计学意义;PC3含量在T4水平下显著高于对照,其余各处理与对照间差异均无统计学意义。18 d时,除PC3含量在T4水平下显著高于对照外,GSH和PC2含量均在T1水平时即显著高于对照,其中PC2含量的增幅最大,与CK相比,分别增加了2.39、2.59、2.41和2.51倍。6、12和18 d时,各处理组秋华柳根系中3种巯基肽含量均为PC2>PC3>GSH。时间对4个Cd2+处理水平下的各巯基肽含量具有极显著性效应,不同Cd2+处理量和时间的交互效应对秋华柳根系中各巯基肽含量有极显著影响。
| 表2 不同处理时间、不同Cd2+质量浓度下秋华柳根系中巯基肽的质量分数 Table 2 Thiol-peptide content in the roots of Salix variegata at different Cd2+ concentrations and treatment time |
 |
| 点击放大 |
由图 2可知:在相同处理时间下,与对照(CK)相比,不同处理组秋华柳根系中的巯基肽总量均显著增加,其中6 d时,各处理组的巯基肽总量增加幅度最大,分别增加了3.82、3.58、4.40和5.18倍;在相同质量浓度的Cd2+处理下,4个处理组的秋华柳根系中的巯基肽总量随时间的延长呈先增后降再增的趋势,其中T4组在各时间处理下的巯基肽总量均显著高于CK。
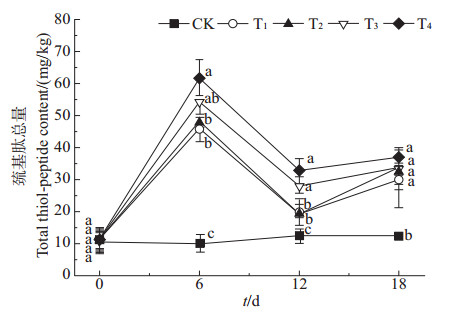 |
| CK:0 mg/L Cd2+;T1:2 mg/L Cd2+;T2:10 mg/L Cd2+;T3:20 mg/L Cd2+;T4:50 mg/L Cd2+。图上数据按鲜质量计;不同小写字母表示在P<0.05水平差异有统计学意义。 CK: 0 mg/L Cd2+; T1: 2 mg/L Cd2+; T2: 10 mg/L Cd2+; T3: 20 mg/L Cd2+; T4: 50 mg/L Cd2+. Data were calculated on the basis of the fresh mass. Different lowercase letters mean statistically significant differences at the 0.05 probability level. 图2 秋华柳根系中巯基肽的总质量分数 Fig. 2 Total thiol-peptide content in the roots of Salix variegata |
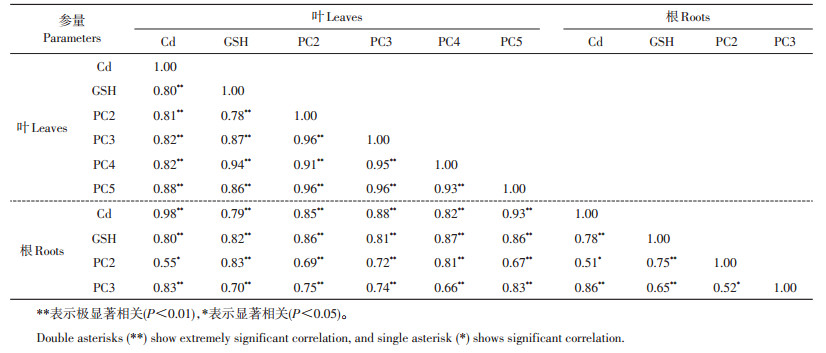
由表 3可以看出:在秋华柳叶片中,各巯基肽含量与Cd积累量以及各巯基肽含量之间均呈极显著正相关,且相关系数高达0.78以上;在秋华柳根系中,除PC2含量与Cd积累量、PC3含量之间呈显著正相关外,其余各巯基肽含量与Cd积累量以及各巯基肽含量之间均呈极显著正相关;秋华柳叶片和根系之间各巯基肽含量均呈极显著正相关,且相关系数较大。
| 表3 秋华柳叶片和根系中各巯基肽含量与镉积累量的相关性 Table 3 Correlation between thiol-peptide contents and Cd accumulation in the leaves and roots of S. variegata |
 |
| 点击放大 |
植物螯合作用是植物体内重要的耐受解毒机制,其中GSH和PCs的浓度大小直接决定其解毒作用的强弱。植物体内巯基与Cd有很强的亲合力,二者结合是Cd解毒的基本机制[27]。GSH和PCs都是植物体内普遍存在的巯基化合物,可与Cd形成无毒的络合物并存在于细胞溶胶或运输到液泡中,既解除了Cd的毒性,又阻断了Cd向其他细胞器的迁移[28-29]。在本试验中,相同时间的各Cd2+处理组及相同Cd2+处理的各时间处理组秋华柳叶片和根系中的巯基肽总量均显著高于对照组。表明在Cd胁迫下,秋华柳能有效地进行植物螯合作用,迅速合成的大量巯基化合物GSH和PCs对进入细胞内的Cd进行螯合,使其形成无毒化合物,再转运至液泡内区室化储存,降低Cd对秋华柳细胞质基质和细胞器的损害,从而提高植株对Cd的耐受和解毒能力。
GSH是由谷氨酸、半胱氨酸和甘氨酸组成的巯基三肽,它不仅是PCs合成的底物,还是细胞内一种较强的抗氧化剂,通过抗坏血酸-谷胱甘肽(ascorbic-glutathione, AsA-GSH)循环代谢参与H2O2的清除[30-31]。在本试验中,6 d和12 d时,秋华柳叶片和根系中除了T1组GSH含量与对照差异无统计学意义外,其他处理组的GSH含量均显著高于对照;18 d时,秋华柳叶片和根系中各处理组的GSH含量均显著高于对照;这与前人的研究结果[32-33]一致。这是由于随着Cd2+质量浓度的增加,产生的氧化胁迫激活了秋华柳体内的抗氧化机制,不断诱导秋华柳生成较多的GSH,而秋华柳合成PCs对GSH的消耗量低于其更新量[34],导致了秋华柳叶片和根系中的GSH含量均高于对照,且在一定质量浓度的Cd2+水平下差异显著。秋华柳叶片中的巯基肽含量与根系中的巯基肽含量呈极显著正相关,这是由于巯基肽可以在根系和叶片之间长距离运输[35-36]。许多相似的研究结果也表明,GSH在植物地上部分合成[37-39],再通过韧皮部长距离运输到根部[40-41]。但本研究发现,在不同处理时间、不同质量浓度Cd2+处理下秋华柳根系中的GSH含量明显高于叶片,这可能是由于囤积于秋华柳根系中的Cd含量远高于叶片中的Cd含量[42],根系为了清除Cd毒害产生的自由基,驱使地上部分运输更多的GSH到根部,以增强其抗氧化胁迫的能力。
在相同时间处理下,不同质量浓度Cd2+处理的秋华柳叶片和根系中的各种PCs含量均高于对照,且12 d和18 d时,秋华柳根系中的PC3含量在T4处理水平下显著高于对照,其余4个处理组叶片和根系中的PCs含量在各时间下均显著高于对照。而且,秋华柳叶片和根系中的各巯基肽含量与其对应的Cd积累量之间均呈显著正相关。表明随着Cd胁迫程度的增加,不断进入秋华柳体内的Cd诱导产生大量的巯基肽,使其螯合Cd的能力显著增强,胞质溶胶中游离Cd的浓度迅速下降,从而避免了Cd的毒害。在Cd胁迫下,叶片诱导生成4种植物螯合肽(PC2、PC3、PC4和PC5),根系生成2种植物螯合肽(PC2和PC3),这一结果与植物体内PCs的基本结构(γ-Glu-Cys)n-Gly中,n通常为2~5相一致[43]。然而,叶片中产生的PCs链长于根部,这可能与叶片和根部产生PCs的酶促反应相关。本研究中秋华柳叶片和根系的各种巯基肽含量之间均呈极显著正相关,这进一步证实了不同长度的PCs是以GSH为底物的酶促反应结果。秋华柳体内一部分GSH释放甘氨酸生成γ-谷氨酰半胱氨酸(γ-glutamylcysteine, EC)后,在PCs合成酶的催化下与另一部分GSH合成不同长度的PCs分子[44],而PCs合成酶作为PCs合成过程中重要的反馈调节酶,其活性与巯基肽和金属离子的活性密切相关[16]。因此,秋华柳体内PCs的长度可能与巯基肽和Cd的浓度相关,但其具体关系仍有待进一步研究。在以后的研究中,可考虑运用分子技术,通过上调或导入相关酶基因来提高相关酶的活性,增加巯基化合物在秋华柳体内的合成与转化,从而进一步增强秋华柳的螯合解毒能力。
综上所述,随着Cd胁迫程度的增加和处理时间的延长,秋华柳螯合Cd的能力明显增强,降低了胞质溶胶中Cd2+对细胞器的损害,从而提高了其对Cd的耐受和解毒能力。各处理组秋华柳叶片中的5种巯基肽含量均为PC2>PC5>PC4>PC3>GSH,根系中3种巯基肽含量均为PC2>PC3>GSH,且根系中的PC2含量明显高于其他巯基肽:表明在Cd胁迫下,秋华柳细胞内PC2对Cd的螯合作用明显高于其他巯基肽,尤其是在根部。
| [1] |
环境保护部, 国土资源部. 全国土壤污染状况调查公报. (2014-04-18)[2016-05-08]. http://www.mlr.gov.cn/xwdt/jrxw/201404/P020140417573876167417.pdf Ministry of Environmental Protection, Ministry of Land and Resources. Survey Bulletin of National Soil Pollution. (2014-04-18)[2016-05-08]. http://www.gtzyb.com/yaowen/2014041862262.shtml. (in Chinese) |
| [2] | JANSSEN C R, HEIJERICK D G, DE SCHAMPHELAERE K A C, et al. Environmental risk assessment of metal: Tools for incorporating bioavailability. Environmental International, 2003, 28(8): 793-800. DOI:10.1016/S0160-4120(02)00126-5 |
| [3] | DALCORSO G, FARINATI S, FURINI A. Regulatory networks of cadmium stress in plants. Plant Signaling and Behavior, 2010, 5(6): 663-667. DOI:10.4161/psb.5.6.11425 |
| [4] |
王振中, 张友梅, 邓继福, 等. 重金属在土壤生态系统中的富集及毒性效应. 应用生态学报, 2006, 17(10): 1948-1952. WANG Z Z, ZHANG Y M, DENG J F, et al. Enrichment and toxicity effect of heavy metals in soil ecosystem. Chinese Journal of Applied Ecology, 2006, 17(10): 1948-1952. (in Chinese with English abstract) DOI:10.3321/j.issn:1001-9332.2006.10.033 |
| [5] | CHERIAN S, OLIVEIRA M M. Transgenic plants in phytoremediation recent advances and new possibilities. Environmental Science and Technology, 2005, 39(24): 9377-9390. DOI:10.1021/es051134l |
| [6] | COOK R L, HESTERBERG D. Comparison of trees and grasses for rhizoremediation of petroleum hydrocarbons. International Journal of Phytoremediation, 2013, 15(9): 844-860. DOI:10.1080/15226514.2012.760518 |
| [7] | CANADELL J, JACKSON R B, EHLERINGER J B, et al. Maximum rooting depth of vegetation types at the global scale. Oecologia, 1996, 108(4): 583-595. DOI:10.1007/BF00329030 |
| [8] | SEBASTIANI L, SCEBBA F, TOGNETTI R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoides×maximowiczii) and Ⅰ-214 (P.×euramericana) exposed to industrial waste. Environmental and Experimental Botany, 2004, 52(1): 79-88. DOI:10.1016/j.envexpbot.2004.01.003 |
| [9] |
中国科学院植物研究所. 中国高等植物图鉴Ⅰ. 北京: 科学出版社, 2001: 372-379. Botany Institute of Chinese Academy of Sciences. Iconographia Cormophytorum Sinicorum. TomusⅠ. Beijing: Science Press, 2001: 372-379. (in Chinese with English abstract) |
| [10] |
李娅, 曾波, 叶小齐, 等. 水淹对三峡库区岸生植物秋华柳(Salix variegata Franch.)存活和恢复生长的影响. 生态学报, 2008, 28(5): 1923-1930. LI Y, ZENG B, YE X Q, et al. The effect of flooding on survival and recovery growth of the riparian plant Salix variegata Franch. in Three Gorges Reservoir region. Acta Ecologica Sinica, 2008, 28(5): 1923-1930. (in Chinese with English abstract) |
| [11] |
孙晓灿, 魏虹, 谢小红, 等. 水培条件下秋华柳对重金属Cd的富集特性及光合响应. 环境科学研究, 2012, 25(2): 220-225. SUN X C, WEI H, XIE X H, et al. Bioaccumulation and photosynthesis response of Salix variegata to cadmium under hydroponic culture. Research of Environmental Sciences, 2012, 25(2): 220-225. (in Chinese with English abstract) |
| [12] |
贾中民, 魏虹, 孙晓灿, 等. 秋华柳和枫杨幼苗对镉的积累和耐受性. 生态学报, 2011, 31(1): 107-114. JIA Z M, WEI H, SUN X C, et al. Accumulation and tolerance of Salix variegata and Pterocarya stenoptera seedlings to cadmium. Acta Ecologica Sinica, 2011, 31(1): 107-114. (in Chinese with English abstract) |
| [13] | CALLAHAN D L, BAKER A J M, KOLEV S D, et al. Metal ion ligands in hyperaccumulating plants. Journal of Biological Inorganic Chemistry, 2006, 11(1): 2-12. DOI:10.1007/s00775-005-0056-7 |
| [14] |
孙琴, 倪吾钟, 杨肖娥. 超积累植物体内的小分子螯合物质及其生理作用. 广东微量元素科学, 2001, 8(5): 1-8. SUN Q, NI W Z, YANG X E. Low molecular weight chelating compounds and their physiological function in hyperaccumulator. Guangdong Trace Elements Science, 2001, 8(5): 1-8. (in Chinese with English abstract) |
| [15] | RAUSER W E. Structure and function of metal chelators produced by plants: The case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochemistry and Biophysics, 1999, 31(1): 19-48. DOI:10.1007/BF02738153 |
| [16] | RAUSER W E. Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiology, 1995, 1019(4): 1141-1149. |
| [17] | YADAV S K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany, 2010, 76(2): 167-179. DOI:10.1016/j.sajb.2009.10.007 |
| [18] | COBBETT C, GOLDSBROUGH P. Phytochelatins and metallothioenins: Roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology, 2002, 53: 159-182. DOI:10.1146/annurev.arplant.53.100301.135154 |
| [19] | REDDY G N, PRASAD M N V. Heavy metal-binding proteins/peptides: Occurrence, structure, synthesis and functions. A review. Environmental and Experimental Botan, 1990, 30(3): 251-264. DOI:10.1016/0098-8472(90)90037-5 |
| [20] | COBBETT C S. Phytochelatin biosynthesis and function in heavymetal detoxification. Current Opinion in Plant Biology, 2000, 3(3): 211-216. DOI:10.1016/S1369-5266(00)00066-2 |
| [21] | SALT D E, RAUSER W E. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiology, 1995, 107(4): 1293-1301. DOI:10.1104/pp.107.4.1293 |
| [22] | SALT D E, THURMAN D A, TOMSETT A B, et al. Copper phytochelatins of Mimulus guttatus. Proceedings of the Royal Society of London, 1989, 236(1282): 79-89. DOI:10.1098/rspb.1989.0013 |
| [23] | SARRY J E, KUHN L, DUCRUIX C, et al. The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics, 2006, 6(7): 2180-2198. DOI:10.1002/(ISSN)1615-9861 |
| [24] | OGAWA I, NAKANISHI H, MORI S, et al. Time course analysis of gene regulation under cadmium stress in rice. Plant and Soil, 2009, 325(1): 97-108. |
| [25] | FAGIONI M, ZOLLA L. Does the different proteomic profile found in apical and basal leaves of spinach reveal a strategy of this plant toward cadmium pollution response?. Journal of Proteome Research, 2009, 8(5): 2519-2529. DOI:10.1021/pr8011182 |
| [26] | JU X H, TANG S, JIA Y, et al. Determination and characterization of cysteine, glutathione and phytochelatins (PC2-6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2011, 879(20): 1717-1724. DOI:10.1016/j.jchromb.2011.04.016 |
| [27] | MORELLI E, CRUZ B H, SOMOVIGO S, et al. Speciation of cadmium-γ-glutamyl peptides complexes in cells of the marine microalga Phaeodactylum tricornutum. Plant Science, 2002, 163(4): 807-813. DOI:10.1016/S0168-9452(02)00216-9 |
| [28] | LI T, DI Z, ISLAM E, et al. Rhizosphere characteristics of zinc hyperaccumulator Sedum alfredii, involved in zinc accumulation. Journal of Hazardous Materials, 2011, 185(2/3): 818-823. |
| [29] | SARWAR N, ISHAQ W, FARID G, et al. Zinc-cadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotoxicology and Environmental Safety, 2015, 122: 528-536. DOI:10.1016/j.ecoenv.2015.09.011 |
| [30] | MAY M J, VERNOUX T, LEAVER C, et al. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. Journal of Experimental Botany, 1998, 49(321): 649-667. |
| [31] | JOZEFCZAK M, KEUNEN E, SCHAT H, et al. Differential response of Arabidopsis, leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant Physiology and Biochemistry, 2014, 83: 1-9. DOI:10.1016/j.plaphy.2014.07.001 |
| [32] | MASERTI B E, FERRILLO V, AVDIS O, et al. Relationship of non-protein thiol pools and accumulated Cd or Hg in the marine macrophyte Posidonia oceanica (L.) Delile. Aquatic Toxicology, 2005, 75(3): 288-292. DOI:10.1016/j.aquatox.2005.08.008 |
| [33] | ALVAREZ-LEGORRETA T, MENDOZA-COZATL D, MORENOSANCHEZ R, et al. Thiol peptides induction in the seagrass Thalassia testudinum (Banks ex König) in response to cadmium exposure. Aquatic Toxicology, 2008, 86(1): 12-19. DOI:10.1016/j.aquatox.2007.09.001 |
| [34] |
谢彬林, 金婷婷, 刘鹏, 等. 铝胁迫下大豆根和叶中植物螯合 肽和类金属硫蛋白的变化. 中国油料作物学报, 2008, 30(2): 191-197. XIE B L, JIN T T, LIU P, et al. The change of phytochelatins and metallothioneins-like in soybean roots and leaves under Al stress. Chinese Journal of Oil Crop Sciences, 2008, 30(2): 191-197. (in Chinese with English abstract) |
| [35] | CHEN A, KOMIVES E A, SCHROEDER J I. An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiology, 2006, 141(1): 108-120. DOI:10.1104/pp.105.072637 |
| [36] | GONG J M, LEE D A, SCHROEDER J I. Long-distance root-toshoot transport of phytochelatins and cadmium in Arabidopsis. Proceedings of the National Academy of Sciences of the USA, 2003, 100(17): 10118-10123. DOI:10.1073/pnas.1734072100 |
| [37] | KOPRIVA S, RENNENGERG H. Control of sulphate assimilation and glutathione synthesis: Interaction with N and C metabolism. Journal of Experimental Botany, 2004, 55(404): 1831-1842. DOI:10.1093/jxb/erh203 |
| [38] | SOBRINO-PLATA J, ORTEGA-VILLASANTE C, FLORESCACERES M L, et al. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere, 2009, 77(7): 946-954. DOI:10.1016/j.chemosphere.2009.08.007 |
| [39] | DAGO A, ARINO C, DIAZ-CRUZ J M, et al. Analysis of phytochelatins and Hg-phytochelatin complexes in Hordeum vulgare plants stressed with Hg and Cd: HPLC study with amperometric detection. International Journal of Environmental Analytical Chemistry, 2014, 94(7): 668-678. DOI:10.1080/03067319.2013.864649 |
| [40] | LI Y, DANKHER O P, CARREIRA L, et al. The shoot-specific expression of gamma-glutamylcysteine synthetase directs the longdistance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiology, 2006, 141(1): 288-298. DOI:10.1104/pp.105.074815 |
| [41] | MENDOZA-COZATL D G, BUTKO E, SPRINGER F, et al. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiolpeptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant Journal, 2008, 54(2): 249-259. DOI:10.1111/j.1365-313X.2008.03410.x |
| [42] |
刘媛, 马文超, 张雯, 等. 镉胁迫对秋华柳根系活力及其 Ca、Mg、Mn、Zn、Fe积累的影响. 应用生态学报, 2016, 27(4): 1109-1115. LIU Y, MA W C, ZHANG W, et al. Effect of cadmium stress on root vigor and accumulation of elements of Ca, Mg, Mn, Zn, Fe in Salix variegata. Chinese Journal of Applied Ecology, 2016, 27(4): 1109-1115. (in Chinese with English abstract) |
| [43] | GONZALEZ-MENDOZA D, MORENO A Q, ZAPATA-PRREZ O. Coordinated responses of phytochelatin synthase and metallothionein genes in black mangrove, Avicennia germinans, exposed to cadmium and copper. Aquatic Toxicology, 2007, 83(4): 306-314. DOI:10.1016/j.aquatox.2007.05.005 |
| [44] |
安志装, 王校常, 严蔚东, 等. 植物螯合肽及其在重金属胁迫下 的适应机制. 植物生理学通讯, 2001, 37(5): 463-467. AN Z Z, WANG X C, YAN W D, et al. Phytochelatins and its adaptive mechanism under heavy metal stress. Plant Physiology Communications, 2001, 37(5): 463-467. (in Chinese with English abstract) |
 2017, Vol. 43
2017, Vol. 43


