| 外源性亚精胺对鼠卵巢生殖激素受体基因表达的影响 |
亚精胺是广泛存在于动物、植物和微生物体内的一种多胺,可通过介导雄性动物精子发生,以及雌性动物卵泡发育、卵子发生和排卵活动,进而参与调控动物的繁殖过程[1-2]。多胺能通过与多种生殖激素建立复杂的反馈联系,进而参与调控动物繁殖[3]。多胺在维持卵巢类固醇激素水平和生成过程中具有重要作用[4]。雄性动物体内的多胺合成受到雄激素的调节[5]。FASHE等[6]研究发现,多胺合成受阻时,卵巢性激素含量显著降低。赵越超[7]研究发现,性激素可通过其受体途径影响鼠子宫内多胺代谢。大量研究证实,雄激素在治疗男性不育症上具有重要作用,亚精胺在不育男性精浆中的含量明显比正常男性低[8-10],推测亚精胺可能通过作用于雄激素及其受体来参与调控雄性动物的繁殖功能。而在雌性动物中,亚精胺是否与雄激素受体(androgen receptor, AR)存在直接或间接的作用还未有研究报道。
促卵泡激素(follicle-stimulating hormone, FSH)和促黄体激素(luteinizing hormone, LH)分别与促卵泡激素受体(follicle-stimulating hormone receptor, FSHR)和促黄体生成素受体(luteinizing hormone receptor, LHR)作用,共同参与调控动物卵泡发育和卵泡颗粒细胞增殖[11]。LH和FSH还可参与调控卵巢组织中雌激素的合成,而雌激素通过负反馈调节机制影响垂体LH和FSH的分泌[12-13]。雌激素受体(estrogen receptor, ER)包含ER1和ER2这2种亚型,双敲除ER1和ER2的雌性鼠不能排卵,说明ER对雌性动物排卵具有至关重要的调控作用[14]。研究表明,多胺可通过介导哺乳动物生殖激素合成与分泌来参与调控动物繁殖功能[4]。然而,目前有关亚精胺调控雌性动物卵巢功能的研究相对较少,而且尚未见有关亚精胺调控卵巢生殖激素受体基因表达影响的报道。因此,本试验研究了不同质量分数亚精胺对鼠卵巢组织中FSHR、LHR、ER1、ER2和AR基因表达的影响,以期为阐明多胺调控哺乳动物卵巢功能的作用机制提供理论依据。
1 材料与方法 1.1 试验动物及样品采集选取雌性健康同胞昆明鼠(SPF级试验用昆明系鼠,购于成都达硕公司),常规分笼饲养,鼠自由采食;在试验过程中对动物处置符合动物伦理学标准。6周龄时,将鼠随机分为4组,每组8只,分别腹腔注射生理盐水和0.05、0.10和0.15 mg/g(体质量)亚精胺。注射24 h后颈部脱臼处死,迅速采集鼠卵巢组织,用生理盐水洗净,滤纸吸干后,置于-80 ℃冰箱保存,备用。
1.2 引物设计和实时荧光定量聚合酶链式反应按照RNA提取试剂盒(RNAiso Plus kit,TaKaRa公司,大连)说明书提取鼠卵巢组织总RNA,然后参照反转录试剂盒说明书(TaKaRa公司,大连)将总RNA样品反转录成cDNA模板,置于-20 ℃冰箱中保存,备用。基于GenBank数据库,采用Primer Premier 5.0和Oligo 6.0软件设计特异性引物,并委托华大基因有限公司合成。引物序列信息见表 1。
| 表1 实时荧光定量聚合酶链式反应引物序列 Table 1 Primer sequences used in quantitative real-time polymerase chain reaction (PCR) |
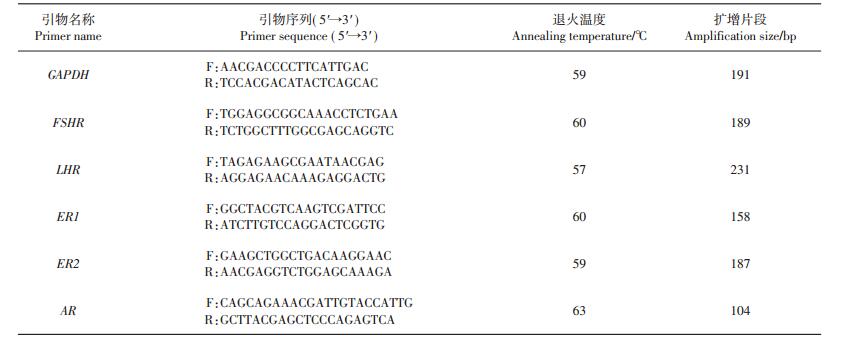 |
| 点击放大 |
实时荧光定量反应体系(10 μL):实时荧光定量聚合酶链式反应试剂盒(iQTM SYBR Green Supermix kit,Bio-Rad公司,北京)5.0 μL,上、下游引物(10 μmol/L)各0.2 μL,cDNA模板0.5 μL,用无RNA酶水补足至10 μL。反应条件:95 ℃预变性3 min;95 ℃变性10 s,57~63 ℃退火30 s,72 ℃延伸30(s采集荧光),40个循环;95 ℃保持10 s,绘制溶解曲线。用3-磷酸甘油醛脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)基因作为内参基因。每个样品设3个重复。
1.3 数据处理及统计分析采用2-ΔΔCT法处理数据[7],用GAPDH作为内参基因,以空白组基因的表达量作为对照组,计算不同质量分数亚精胺处理组卵巢组织FSHR、LHR、ER1、ER2、AR基因的相对表达量。应用SAS 9.2统计分析软件中的MEANS过程进行描述性统计分析,并进行方差分析和邓肯多重比较,结果用平均值±标准差表示,P<0.05表示差异有统计学意义。
2 结果 2.1 亚精胺对鼠LHR和FSHR基因表达的影响由图 1可知:腹腔注射0.05 mg/g亚精胺时,鼠卵巢组织中LHR相对表达量显著高于其他各组,是对照组的2.07倍(P<0.05);腹腔注射0.15 mg/g亚精胺组LHR表达量显著低于对照组,为对照组的9%(P<0.05)。腹腔注射0.15 mg/g亚精胺时,FSHR表达量显著高于对照组,是对照组的31.67倍(P<0.05);而腹腔注射0.05和0.10 mg/g亚精胺时,FSHR基因表达量与对照组相比差异无统计学意义(P>0.05)。
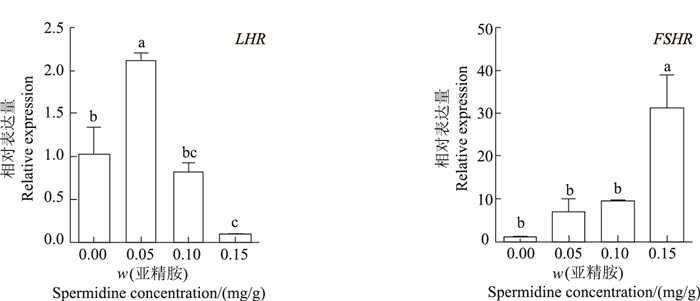 |
| 短栅上的不同小写字母表示不同质量分数亚精胺处理组之间在P<0.05水平差异有统计学意义。 Different lowercase letters above the bars represent statistically significant differences among different concentrations of spermidine at the 0.05 probability level. 图1 亚精胺对鼠卵巢LHR和FSHR表达的影响 Fig. 1 Effect of spermidine on LHR and FSHR mRNA expression in mouse ovaries |
腹腔注射0.15 mg/g亚精胺处理组中,ER1和ER2表达量均显著高于对照组,分别为对照组的1.95倍和6.43倍(P<0.05);而注射0.05和0.10 mg/g组中,ER1和ER2与对照组间差异均无统计学意义(P>0.05)(图 2)。
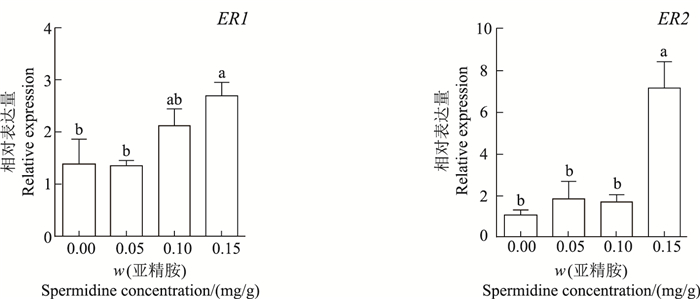 |
| 短栅上的不同小写字母表示不同质量分数亚精胺处理组之间在P<0.05水平差异有统计学意义。 Different lowercase letters above the bars represent statistically significant differences among different concentrations of spermidine at the 0.05 probability level. 图2 亚精胺对鼠卵巢ER1和ER2表达的影响 Fig. 2 Effect of spermidine on ER1 and ER2 mRNA expression in mouse ovaries |
如图 3所示:当腹腔注射0.15 mg/g亚精胺时,卵巢组织中AR相对表达量显著高于对照组和其他处理组,是对照组的4.31倍(P<0.05);而注射0.05和0.10 mg/g组中,AR表达量与对照组间差异无统计学意义(P>0.05)。
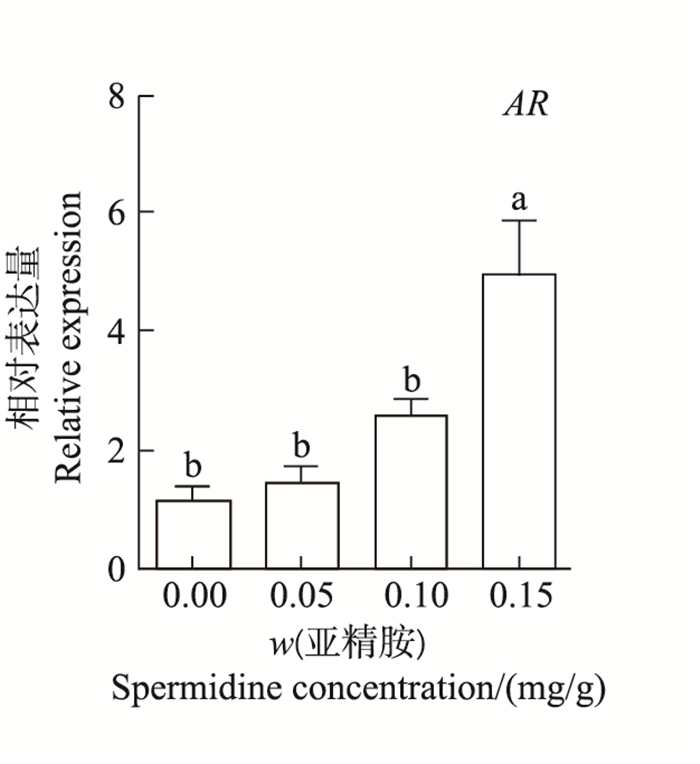 |
| 短栅上的不同小写字母表示不同质量分数亚精胺处理组之间在P<0.05水平差异有统计学意义。 Different lowercase letters above the bars represent statistically significant differences among different concentrations of spermidine at the 0.05 probability level. 图3 亚精胺对鼠卵巢AR表达的影响 Fig. 3 Effect of spermidine on AR mRNA expression in mouse ovaries |
生殖激素及其特异性受体在调控动物繁殖过程中具有关键作用,而FSH和LH在调控动物繁殖过程中具有核心作用[15-16]。在卵巢颗粒细胞中LHR表达量降低会抑制排卵功能[17];而FSHR表达量升高能增强卵巢对外源性促性腺激素的应答能力。THYSSEN等[18]研究发现,外源性腐胺和亚精胺能抑制下丘脑和垂体组织中LH和FSH的分泌;用二氟甲基鸟氨酸(一种腐胺生物合成的不可逆抑制剂)处理后FSH分泌增加:表明多胺可参与调控垂体LH和FSH分泌。本研究发现,腹腔注射亚精胺显著提高了卵巢组织中FSHR和LHR表达量:说明亚精胺也可通过介导卵巢FSHR和LHR表达来发挥其对雌性动物繁殖功能的调控作用。表皮生长因子(epidermal growth factor, EGF)作为一种卵巢旁分泌因子,通过介导卵泡发育和卵母细胞生成来调节卵巢功能。研究表明,EGF可下调哺乳动物卵巢FSHR表达[19]。BLACHOWSKI等[20]发现,多胺能参与EGF信号转导通路,提示多胺与EGF参与的生殖调控具有相关性。本研究发现,注射0.15 mg/g亚精胺显著增加了卵巢组织中FSHR表达量,推测亚精胺促进FSHR表达的作用可能与EGF有关,有待进一步研究证实。此外,本研究还发现,低质量分数亚精胺(0.05 mg/g)能促进卵巢LHR表达,但对FSHR表达影响不显著;而高质量分数亚精胺(0.15 mg/g)抑制LHR表达,但促进了FSHR表达:表明亚精胺影响FSHR和LHR表达的效应具有剂量差异。
雌激素受体基因包括ER1和ER2,在鼠卵巢组织中均有表达,但其表达量和生理作用各不相同。在未成熟和成熟的啮齿类动物和人卵巢中,ER2表达量远高于ER1[21]。研究表明,在卵巢组织中ER2的表达和分布与鼠的生殖周期存在密切联系,尤其是在发情期的鼠卵巢组织中有高度表达,其表达规律与小鼠体内雌激素水平相适应[22]。本研究发现,0.15 mg/g亚精胺处理可显著增加鼠卵巢组织中ER1和ER2表达,提示高质量分数亚精胺可通过提高ER表达进而参与调控鼠卵巢功能。
AR在调节卵巢功能和维持女性生育能力方面具有重要作用[23]。KIMURA等[24]发现,在卵泡发育早期即有AR的表达,AR缺陷型鼠易出现卵泡发育障碍和卵巢早衰。AR和雄激素水平与哺乳动物在卵巢发育过程中FSHR表达密切相关[25]。此外,雄激素还参与调控卵巢癌的发生和发展过程,AR水平及其活性增强会使卵巢癌细胞对雄激素的敏感性增大,从而促进卵巢癌变[26]。本研究结果表明,0.15 mg/g亚精胺处理组的鼠卵巢组织中AR表达显著高于对照组:表明高质量分数亚精胺可通过促进AR表达进而参与调控鼠卵巢功能。
综上所述,在本试验中,腹腔注射不同质量分数亚精胺后,鼠卵巢组织中LHR、FSHR、ER1、ER2和AR表达量相较于对照组均发生了显著变化。低质量分数的亚精胺促进LHR表达;高质量分数的亚精胺促进FSHR、ER1、ER2和AR表达,同时抑制LHR的表达,且具有剂量依赖性。说明亚精胺可通过介导鼠卵巢组织生殖激素受体基因表达来参与调控鼠卵巢功能。
| [1] |
向睿, 何珲, 康波.多胺调控动物繁殖的作用及其机制.
动物营养学报,2014,26 (11):3251–3255.
XIANG R, HE H, KANG B. Effects of polyamine regulation on animal reproduction and its mechanism. Chinese Journal of Animal Nutrition, 2014,26 (11):3251–3255. (in Chinese with English abstract) DOI: 10.3969/j.issn.1006-267x.2014.11.010. |
| [2] |
PEGG A E, CASERO R A. Current status of the polyamine research field.
Polyamines: Methods in Molecular Biology, 2011,720 :3–35. DOI: 10.1007/978-1-61779-034-8. |
| [3] |
VIJAYANATHAN V, THOMAS T J, NAIR S K, et al. Bending of the estrogen response element by polyamines and estrogen receptors alpha and beta: A fluorescence resonance energy transfer study.
The International Journal of Biochemistry and Cell Biology, 2006,38 (7):1191–1206. DOI: 10.1016/j.biocel.2005.12.015. |
| [4] |
LEFEVRE P L C, PALIN M F, MURPHY B D. Polyamines on the reproductive landscape.
Endocrine Reviews, 2011,32 (5):694–712. DOI: 10.1210/er.2011-0012. |
| [5] |
GONZALEZ-MONTELONGO M C, MARIN R, PEREZ J A, et al. Polyamines transduce the nongenomic, androgen-induced calcium sensitization in intestinal smooth muscle.
Molecular Endocrinology, 2013,27 (10):1603–1616. DOI: 10.1210/me.2013-1201. |
| [6] |
FASHE T M, KEINANEN T A, GRIGORENKO N A, et al. Cutaneous application of alpha-methylspermidine activates the growth of resting hair follicles in mice.
Amino Acids, 2010,38 (2):583–590. DOI: 10.1007/s00726-009-0421-x. |
| [7] |
赵越超. 小鼠围着床期子宫中多胺相关基因的表达、调节与功能. 哈尔滨: 东北农业大学, 2008: 61-67. ZHAO Y C. Expression, regulation and function of polyamie-related genes in mouse uterus during peri-implantation period. Harbin: Northeast Agricultural University, 2008:61-67. (in Chinese with Englishabstract) |
| [8] |
MILLER-FLEMING L, OLIN-SANDOVAL V, CAMPBELL K, et al. Remaining mysteries of molecular biology: The role of polyamines in the cell.
Journal of Molecular Biology, 2015,427 (21):3389–3406. DOI: 10.1016/j.jmb.2015.06.020. |
| [9] |
CALANDRA R S, RULLI S B, FRUNGIERI M B, et al. Polyamines in the male reproductive system.
Acta physiologica, pharmacologica et therapeutica latinoamericana: Órgano de la Asociación Latinoamericana de Ciencias Fisiológicas y[de] la Asociación Latinoamericana de Farmacología, 1996,46 (4):209–222. |
| [10] |
MELENDREZ C S, RUTTLE J L, HALLFORD D M, et al. Polyamines in ejaculated ram spermatozoa and their relationship with sperm motility.
Journal of Andrology, 1992,13 (4):293–296. |
| [11] |
KANG B, GUO J R, YANG H M, et al. Differential expression profiling of ovarian genes in prelaying and laying geese.
Poultry Science, 2009,88 (9):1975–1983. DOI: 10.3382/ps.2008-00519. |
| [12] |
NATARAJA S G, YU H N, PALMER S S. Discovery and development of small molecule allosteric modulators of glycoprotein hormone receptors.
Frontiers in Endocrinology, 2015,6 :142. |
| [13] |
MEHL N S, KHALID M, SRISUWATANASAGUL S, et al. GnRH-agonist implantation of prepubertal male cats affects their reproductive performance and testicular LH receptor and FSH receptor expression.
Theriogenology, 2016,85 (5):841–848. DOI: 10.1016/j.theriogenology.2015.10.031. |
| [14] |
LAU K M, TO K F. Importance of estrogenic signaling and its mediated receptors in prostate cancer.
International Journal of Molecular Sciences, 2016,17 (9):1434. DOI: 10.3390/ijms17091434. |
| [15] |
AYOUB M A, YVINEC R, JEGOT G, et al. Profiling of FSHR negative allosteric modulators on LH/CGR reveals biased antagonism with implications in steroidogenesis.
Molecular and Cellular Endocrinology, 2016,436 :10–22. DOI: 10.1016/j.mce.2016.07.013. |
| [16] |
LÓPEZ-DOVAL S, SALGADO R, LAFUENTE A. The expression of several reproductive hormone receptors can be modified by perfluorooctane sulfonate (PFOS) in adult male rats.
Chemosphere, 2016,155 :488–497. DOI: 10.1016/j.chemosphere.2016.04.081. |
| [17] |
SAMARDZIJA D, POGRMIC-MAJKIC K, FA S, et al. Atrazine blocks ovulation via suppression of Lhr and Cyp19a1 mRNA and estradiol secretion in immature gonadotropin?treated rats.
Reproductive Toxicology, 2016,61 :10–18. DOI: 10.1016/j.reprotox.2016.02.009. |
| [18] |
THYSSEN S M, HOCKL P F, CHAMSON A, et al. Effects of polyamines on the release of gonadotropin-releasing hormone and gonadotropins in developing female rats.
Experimental Biology and Medicine, 2002,227 (4):276–281. |
| [19] |
LIU K C, GE W. Differential regulation of gonadotropin receptors (fshr and lhcgr) by epidermal growth factor (EGF) in the zebrafish ovary.
General and Comparative Endocrinology, 2013,181 :288–294. DOI: 10.1016/j.ygcen.2012.07.032. |
| [20] |
BLACHOWSKI S, MOTYL T, GRZELKOWSKA K, et al. Involvement of polyamines in epidermal growth factor (EGF), transforming growth factor (TGF)-alpha and-beta 1 action on culture of L6 and fetal bovine myoblasts.
International Journal of Biochemistry, 1994,26 (7):891–897. DOI: 10.1016/0020-711X(94)90082-5. |
| [21] |
SAUNDERS P T K, MILLAR M R, WILLIAMS K, et al. Differential expression of estrogen receptor-alpha and-beta and androgen receptor in the ovaries of marmosets and humans.
Biology of Reproduction, 2000,63 (4):1098–1105. DOI: 10.1095/biolreprod63.4.1098. |
| [22] |
郑可佳, 杨彩荣, 张岩, 等.ERβ及p-ERβ在小鼠发情周期卵巢内的表达.
东北农业大学学报,2009,40 (7):85–89.
ZHENG K J, YANG C R, ZHANG Y, et al. Study on the expression of ERβ and p-ERβ on mouse ovary during the oestrous cycle. Journal of Northeast Agricultural University, 2009,40 (7):85–89. (in Chinese with English abstract) |
| [23] |
WALTERS K A. Role of androgens in normal and pathological ovarian function.
Reproduction, 2015,149 (4):193–218. DOI: 10.1530/REP-14-0517. |
| [24] |
KIMURA S, MATSUMOTO T, MATSUYAMA R, et al. Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure.
Trends in Endocrinology and Metabolism, 2007,18 (5):183–189. DOI: 10.1016/j.tem.2007.04.002. |
| [25] |
DU X, LI Q Q, PAN Z X, et al. Androgen receptor and miRNA-126* axis controls follicle-stimulating hormone receptor expression in porcine ovarian granulosa cells.
Reproduction, 2016,152 (2):161–169. DOI: 10.1530/REP-15-0517. |
| [26] |
ZHU T Y, YUAN J, XIE Y D, et al. Association of androgen receptor CAG repeat polymorphism and risk of epithelial ovarian cancer.
Gene, 2016,575 (2):743–746. DOI: 10.1016/j.gene.2015.09.054. |
 2017, Vol. 43
2017, Vol. 43


