| 甘蓝型油菜黄籽突变对含油量和蛋白质含量的影响 |
2. 浙江省农业科学院病毒学与生物技术所/浙江省植物有害生物防控重点实验室,省部共建国家重点实验室培育基地, 杭州310021
2. The State Key Laboratory Breeding Base for Sustainable Control of Pest and Disease/Institute of Virology and Biotechnology, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China
甘蓝型油菜 (Brassica napus L.) 是我国主要油料作物之一, 是我国主要的食用油来源作物。传统甘蓝型油菜籽粒颜色多为黑色, 但在甘蓝型油菜近缘物种白菜型油菜 (Brassica rapa L.)、甘蓝 (Brassica orleracea L.) 及芥菜型油菜 (Brassica juncea L.) 中, 存在很多黄色籽粒的种质资源[1-2]。20世纪70年代以来, 通过种间杂交, 育种家相继培育出一些黄色籽粒的甘蓝型油菜[3-4]。相比传统的黑色籽粒的甘蓝型油菜籽, 多数研究普遍认为黄籽油菜有种皮更薄、纤维素含量更低、含油量更高、蛋白质含量更高、饼粕饲料品质好、压榨油色泽清亮等优点[5]。在我国, 刘后利等[6]最早对甘蓝型油菜黄籽与含油量的关系进行了系统的研究, 发现从同一株系分离得到的黄籽油菜单株平均含油量 (41.17±2.86)%比黑籽单株平均含油量 (40.01±2.59)%高, 特别是在黄籽出现频率比较高的株系中, 分离得到的黄籽株系平均含油量 (43.49±2.65)%比黑籽株系含油量 (40.14±2.32)%高得多。随后, 肖达人[7]对不同来源的41个甘蓝型油菜品系2年栽培样品共913个单株收获的种子进行含油量分析和颜色测定, 发现在相同的遗传背景中, 甘蓝型油菜种皮颜色与种籽含油量之间存在着显著负相关, 黄籽株系平均含油量比非黄籽 (黑籽或紫红籽) 高1.54~4.26个百分点,种皮颜色每降低一个级别时, 含油量将相应地提高0.65个百分点, 种皮切片观测表明, 种皮厚度的改变是造成种皮颜色与种子含油量相互变化的决定因素之一。后来, 王汉中等[8]研究了黄籽品系Ly84-24和黑籽品系华油13杂交及回交分离世代中种皮颜色与含油量的关系, 发现分离世代中黄籽比黑籽含油量高2.9~3.9个百分点, 种胚含油量高1.4~2.9个百分点, 皮壳含油量高3.3~4.6个百分点。简单相关分析表明, 粒色与种子含油量及胚含油量及皮壳含油量间均呈显著负相关, 与皮壳率间呈极显著正相关;偏相关分析表明, 粒色只与皮壳率和皮壳含油量偏相关显著, 与种子含油量及胚含油量间的偏相关不明显。
近年来, 随着现代分子技术的发展, 黄籽与含油量的关系也在分子水平上进行了解析。BADANI等[9]分别对3个遗传群体的种皮颜色数量遗传位点 (quantitative trait loci, QTL) 进行分子标记遗传解析, 发现种皮颜色最主效QTL和耐酸性去污剂纤维含量最主效QTL完全重叠, 都位于N18连锁群相同位置, 揭示种皮颜色主要与种皮中耐酸性去污剂纤维的合成相关。LIU等[10]采用232个株系的F9重组近交系群体, 将位于A9染色体的黄籽主效QTL也解析在耐酸性去污剂纤维QTL相同位点, 且与含油量极显著相关, 与蛋白质含量相关不显著。ZHOU等[11]采用SNP关联分析的方法发现BnaC.TT2.a基因与甘蓝型油菜中的种皮颜色和含油量有关。
在模式植物拟南芥中, 种皮色素代谢相关基因 (TT genes) 已克隆24个并阐明功能[12], 但这些基因的功能与含油量和蛋白质含量的关系都未有明确的报道。这些基因在油菜中的同源基因也有139个之多[13], 其中, BrTTG1和BjTT8分别在白菜型油菜和芥菜型油菜中证明和种皮颜色有关, 但与含油量和蛋白质含量也未有明确的关系[14-15]。在甘蓝型油菜中, 仅有一个TT基因的功能获得直接阐明, 通过RNAi技术敲除油菜中TT16的同源基因后, 油菜的生长和发育发生巨大改变, 但种皮颜色却未见明显变化, 而且含油量不仅没有预期中的提高反而降低了[16]。
在过去的研究中, 种皮颜色与含油量及蛋白质含量的关系的研究都是基于不同基因型在不同植株上的研究结果, 受环境影响较大。近年来, 我们分离获得一个胚基因型控制的黄籽油菜突变体[13], 同一个角果中既可以结出黄色种皮的油菜籽粒又可以结出褐色种皮的油菜籽粒 (图 1A), 通过对同一植株上分离的黄籽油菜和褐籽油菜 (图 1B) 进行分析可以更准确的研究黄籽突变对含油量和蛋白质含量的影响。
 |
| A:自交结实的油菜角果;B:单株自交收获的种子。 A: silique on the mutant; B: seeds harvested from single plant. 图1 基因型杂合的甘蓝型油菜黄籽突变体 Fig. 1 Bagged seeds from single plant of heterozygous yellow-seeded mutation |
油菜黄籽突变体来源于实验室田间筛选获得, 前期研究表明该黄籽受单个显性基因位点控制, 且黄籽表型受胚的基因控制, 而不是母体植株的基因控制。在黄籽基因位点为杂合的情况下, 单个植株上同时结出黄籽和褐籽, 且黄籽与褐籽比率符合3:1的分离规律 (图 1B)。本研究选取4株黄籽突变体油菜植株, 将各株套袋自交收获的种子分为黄籽和褐籽, 作为含油量和水解氨基酸成分测定的材料。
1.2 含油量测定方法油菜籽含油量测定方法参照农业部行业标准NY/T 1285—2007《油料种籽含量的测定:残余法》进行。将Ø9 cm滤纸折成油包, 编号后105 ℃烘干称量, 记为m1(精确到0.000 1);取约1 g的种子放入油包后包好, 105 ℃烘干称量, 记为m2;油包放入压面机上将种子压碎, 放入索氏抽提器抽提20 h, 取出晾干, 105 ℃烘干称量, 记为m3。每单株上黄籽褐籽各取样3次测定后取平均值。
| $ 含油量/{\text{% }}= \frac{{{m_2} -{m_3}}}{{{m_2} -{m_1}}} \times 100。 $ |
水解氨基酸的测定参照纪银福等[17]的方法进行, 从含油量测定后的烘干纸包中准确称取40~50 mg (精确到0.000 1 g) 干燥饼粕, 用10 mL浓度为6 mol/L的HCl水解, 水解后定容至50 mL, 取1 mL蒸干后溶于1 mL 0.02 mol/L盐酸溶液, 采用日立L-8900全自动氨基酸分析仪测定, 上样量20 μL。采用该方法可以测定组成蛋白质的21种氨基酸中的17种。其中谷氨酰胺被水解为谷氨酸, 天冬酰胺水解为天冬氨酸, 胱氨酸水解为半胱氨酸, 色氨酸全部水解, 不能测量。在测定的17种氨基酸中, 胱氨酸和蛋氨酸部分氧化, 不能准确测量。
1.4 定量PCR与引物设计定量PCR方法参照绝对定量PCR的方法[18], 采用油菜基因组DNA (200 ng/μL, 20 ng/μL, 2 ng/μL, 0.2 ng/μL) 为模板绘制标准曲线, 1 μg总RNA用DNA酶 (Promega) 处理后逆转录 (Invitrogen) 至20 μL体系, 完成后加ddH2O至100 μL, 取3 μL为模板在10 μL体系进行定量PCR (Promega GoTaqⓇ qPCR Master Mix), 每条引物终浓度350 μmol/L。热循环为95 ℃ 1 min预变性; 95 ℃ 10 s, 60 ℃ 30 s, 42循环; 扩增产物55~95 ℃变性温度检测。黄籽和褐籽RNA提取采用授粉后26 d种子, 在冰上用镊子和解剖刀分开种皮和种胚, 约40粒种皮和种胚液氮研磨后用1 mL Trizol提取RNA。定量PCR引物设计采用Primer3Plus网页在线软件设计, 复性温度设定60 ℃, 扩增子长度设定150~310 bp。同一基因的正反引物设定在同一个外显子上面。各基因引物设计结果见表 1。
| 表1 种皮色素代谢相关基因的定量PCR分析引物表 Table 1 Primers for qPCR analysis of seed coat pigment related genes |
 |
| 点击放大 |
透射电子显微镜观察采用成熟的收获当天的黄籽和褐籽, 用解剖刀剥下种皮, 切成1~2 mm小块, 送浙江省农业科学院电子显微镜科研平台固定, 包埋, 超薄切片, 然后上透射电子显微镜观测。
2 结果与分析 2.1 甘蓝型油菜单基因黄籽突变对含油量没有影响分别从4株基因型为杂合的油菜单株上分离出黄籽和褐籽, 含油量分析表明, 单株1上黄籽和褐籽含油量分别为 (49.58±0.26)%和 (49.90±0.28)%, 单株2上黄籽和褐籽含油量分别为 (49.65±0.27)%和 (49.36±0.25)%, 单株3上黄籽和褐籽分别为 (48.68±0.21)%和 (48.82±0.17)%, 单株4分别为 (49.41±0.19)%和 (49.46±0.13)%, 同一单株上分离出的黄籽和褐籽含油量 (图 2) 没有显著差异 (P=0.87)。
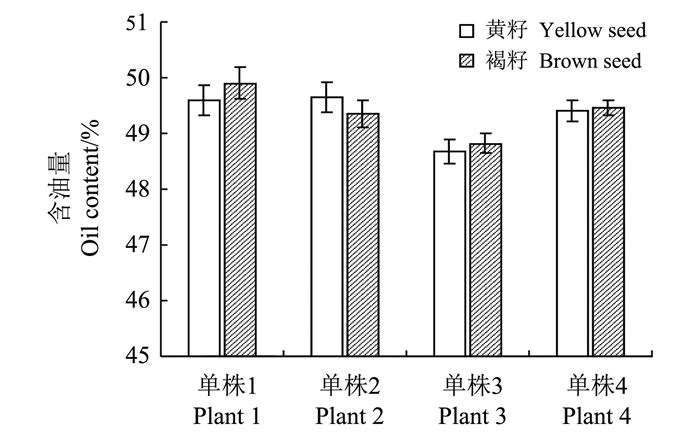 |
| 图2 来源于同一单株的黄籽油菜和黑籽油菜的含油量 Fig. 2 Oil contents of yellow seeds and brown seeds from the single plant |
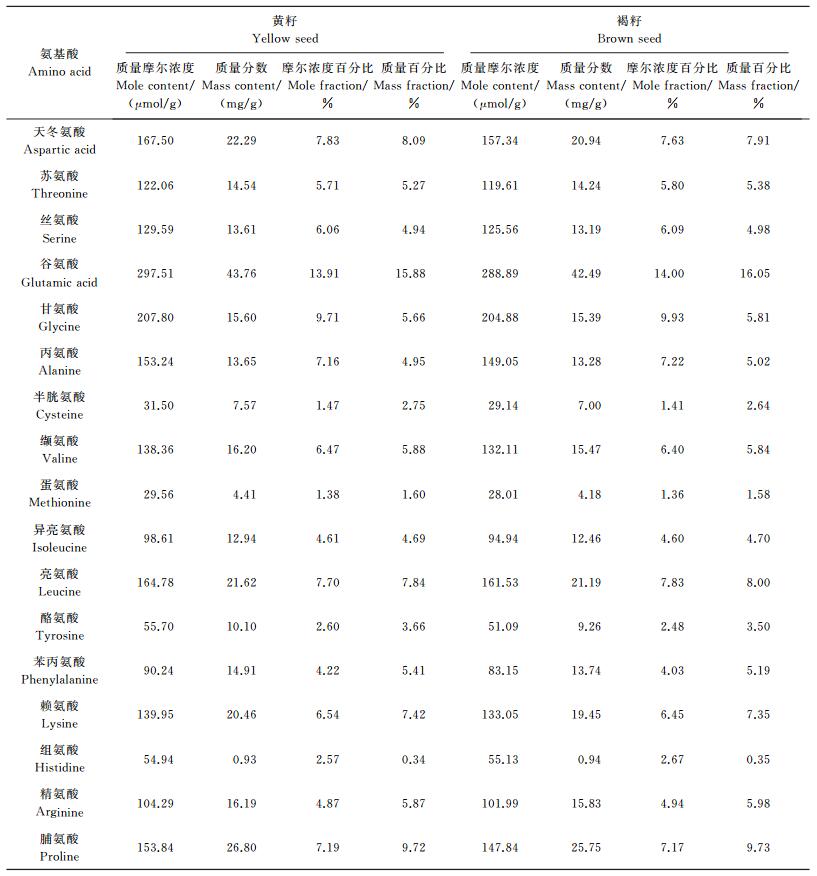
对同一单株上分离的黄籽和褐籽油菜饼粕水解后分别测定其氨基酸含量, 结果表明除蛋氨酸和组氨酸外, 其余各项氨基酸成分含量黄籽都显著高于褐籽 (P < 0.05), 不同氨基酸组分黄籽比褐籽提高相对比例在1.4%至9.0%之间, 其中酪氨酸和苯丙氨酸提升幅度最大, 分别提升9.0%和8.5%, 甘氨酸、苏氨酸、亮氨酸提升比例较低, 分别提升1.4%、2.0%、2.0%, 天冬氨酸和赖氨酸含量提升中等, 分别提升6.5%和5.2%。黄籽和褐籽饼粕中各氨基酸组分含量见表 2, 各氨基酸组分含量分布见图 3, 可测得的氨基酸总量黄籽饼粕为 (2 139.48±17.59) μmol/g, 褐籽饼粕为 (2 063.31±24.53) μmol/g, 氨基酸总量提升3.7%, 差异达到显著水平 (P=0.019 4)。
| 表2 来源于同一单株的黄籽和褐籽油菜水解氨基酸成分表 Table 2 Hydrolyzed amino acids contents in yellow and brown seed meals |
 |
| 点击放大 |
 |
| 图3 来源于同一单株的黄籽油菜和褐籽油菜饼粕中水解氨基酸成分分布 Fig. 3 Hydrolyzed amino acids contents in yellow and brown seed meal from the same plant |
采用透射电子显微镜对成熟的黄籽和褐籽种皮的超薄切片进行观察, 发现黄籽油菜内种皮细胞的细胞壁厚约0.7~4.8 μm, 越靠近外种皮细胞壁越厚;褐籽油菜内种皮细胞壁厚度约3.0~12.9 μm, 与外种皮接触的一面细胞壁特别厚, 总体上黄籽种皮内种皮细胞壁厚度显著小于褐籽种皮内种皮细胞壁厚度 (图 4)。由于细胞壁变薄, 纤维素成分降低, 蛋白质含量相对提高, 这可以解释黄籽突变后水解氨基酸含量升高的部分原因。
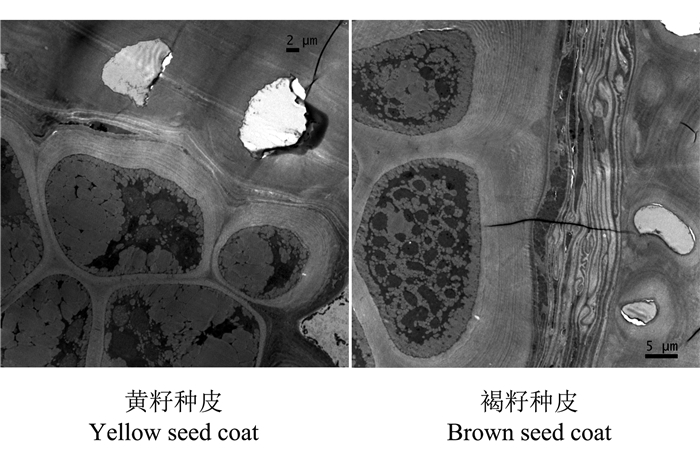 |
| 图4 来源于同一单株的黄籽和褐籽油菜种皮透射电子显微镜图片 Fig. 4 Electron microscopy of seed coat from mature yellow seed and brown seed on the single plant |
为揭示黄籽基因突变为何会造成蛋白质含量的提高而不是含油量的提高, 在转录组测序的基础上[12]挑选了部分基因进行定量PCR分析 (图 5, 表 1)。β-葡聚糖水解酶 (β-D-glucan exohydrolase, bGEH) 与细胞壁的合成有关, BnaA06g17630D是甘蓝型油菜β-葡聚糖水解酶基因家族表达量最高的一员。qPCR分析显示该基因在褐籽种皮的表达量显著高于黄籽种皮的表达量, 约是黄籽种皮的2.6倍。较高的β-葡聚糖水解酶活性暗示着褐籽种皮可能存在较高的β-葡聚糖底物, 这些底物有助于细胞壁的合成, 因此形成较厚的细胞壁。苯丙氨酸解氨酶 (phenylalanine ammonia-lyase, PAL) 是植物次生代谢调控的关键酶, 受多种逆境及环境因素影响, 是色素代谢合成的起始酶。本研究中黄籽胚的苯丙氨酸解氨酶BnaA05g28470D转录丰度显著高于其皮中该酶编码基因的转录丰度, 且褐籽中情况却相反, 皮高于胚, 指示该黄籽突变与苯丙氨酸的代谢有关, 而且前期研究中也发现黄籽胚内的游离苯丙氨酸显著多于褐籽[12], 而在本研究中也发现黄籽饼粕水解成分中苯丙氨酸提高的幅度也最大。由于酪氨酸由苯丙氨酸代谢而来, 苯丙氨酸的提升也相应提高了酪氨酸含量, 因此本研究中酪氨酸提升幅度也很大。色素代谢相关基因TT10、TT7、TT4是油菜籽粒表达最高的TT基因, 但在本研究中却发现这些基因表达不受黄籽突变的影响, 黄籽和褐籽中基因表达差异不大, 推测这些基因与胚基因型调控种皮色素代谢的关系不大。
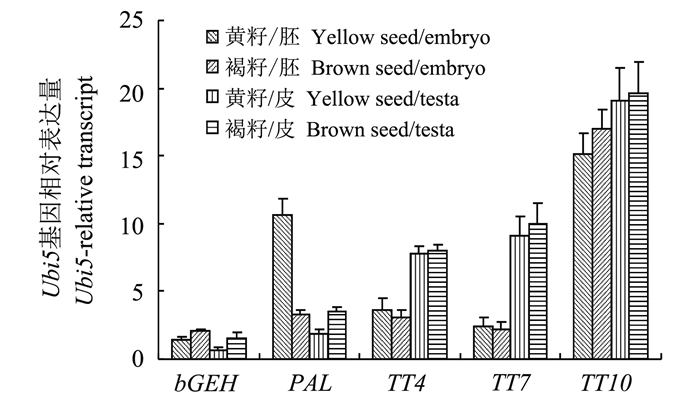 |
| 图5 种皮色素代谢相关基因的定量PCR分析 Fig. 5 Real-time qPCR analysis of seed coat pigment related genes |
本研究发现黄籽单基因突变对含油量无影响, 但对饼粕中蛋白质含量显著提高, 尤其是对组成蛋白质的苯丙氨酸成分提高最大, 同时种皮内皮层细胞壁变薄, 细胞壁变薄可导致纤维素含量降低, 因此可部分解释蛋白质含量提高的原因, 但不是唯一原因。本研究中种皮细胞壁的变薄和蛋白质含量的提高可能对种子饼粕干质量的影响大致抵消, 所以不会造成含油量的显著变化。本文及有关研究结果都显示[12], 该黄籽突变是通过胚内的苯丙氨酸代谢活动来影响种皮细胞内色素代谢过程的, 由于苯丙氨酸是色素合成的初始底物, 推测该突变基因通过将原用于种皮色素合成的苯丙氨酸改为用于胚内的蛋白质合成, 从而提高蛋白质含量, 推测这是黄籽突变引起蛋白质含量提高的另一原因。
传统的油菜种子蛋白质含量测定采用凯氏定氮法 (GB/T 5009.5—1985), 由于油菜种子中含氮化合物太多, 传统方法只能对蛋白质含量进行粗略估计。为更准确地对蛋白质含量进行测定, 本研究采用了将蛋白质水解后, 利用液相色谱对水解氨基酸进行逐个测定的方法, 相比凯氏定氮法更加准确。但是蛋白质水解法不能测定全部的氨基酸, 受方法的限制只能测定21种氨基酸中的17种, 其中谷氨酰胺被水解为谷氨酸, 天冬酰胺水解为天冬氨酸, 胱氨酸水解为半胱氨酸, 色氨酸全部水解, 不能测量, 而且在测定的17种氨基酸中, 胱氨酸和蛋氨酸部分氧化, 不能准确测量。虽然水解法存在这些缺陷, 但在本研究中完全可以用来证明黄籽突变对蛋白质含量的影响。
值得注意的是, 本研究通过对油菜饼粕氨基酸成分的分析,发现油菜饼粕中赖氨酸的含量相对其他氨基酸含量并不低, 占总蛋白质的7.4%左右, 比大豆饼粕中的赖氨酸含量 (5.4%左右) 还要高2个百分点, 相对含量比大豆高出37%, 是传统饲料玉米中赖氨酸含量 (0.96%) 的7.7倍, 绝对含量 (20 mg/g) 是玉米粉 (0.8 mg/g) 的25倍[19]。由于赖氨酸是动物有机体必需氨基酸之一, 能促进机体发育、增强免疫功能, 并有提高中枢神经组织功能的作用, 是促进机体提高蛋白质的吸收和利用, 达到营养均衡的重要氨基酸, 而且由于谷物食品中的赖氨酸含量甚低, 且在加工过程中易被破坏而缺乏, 故称为第一限制性氨基酸[20]。因此, 赖氨酸被主要用做饲料添加剂添加到谷物饲料中, 其含量是衡量饲料品质的重要指标之一。故此, 可见油菜饼粕特别是黄籽油菜饼粕是非常优良的蛋白质饲料, 适合作为赖氨酸添加剂添加到谷物饲料中。
| [1] |
SCHWETKA A. Inheritance of seed colour in turnip rape (Brassica campestris L.).
Theoretical and Applied Genetics, 1982,62 (2):161–169. DOI: 10.1007/BF00293352. |
| [2] |
徐海鹏, 刘显军, 陆赢, 等.芥菜型油菜B03染色体黄籽基因区域BAC重叠群的构建及分析.
作物研究,2016,30 (4):353–358.
XU H P, LIU X J, LIU Y, et al. Construction and analysis of BAC contigs around the yellow-seeded locus of the chromosome B03 in Brassica juncea. Crop Research, 2016,30 (4):353–358. (in Chinese with English abstract) |
| [3] |
CHEN B Y, HENEEN W K, JÖNSSON R. Resynthesis of Brassica napus L. through interspecific hybridization between Brassica alboglabra bailey and Brassica campestris L. with special emphasis on seed color.
Plant Breeding, 1988,101 (1):52–59. DOI: 10.1111/pbr.1988.101.issue-1. |
| [4] |
刘后利, 傅廷栋, 陈怀庆, 等.甘蓝型黄籽油菜的发现及其遗传行为的初步研究.
遗传学报,1979,6 (1):54.
LIU H L, FU T D, CHEN H Q, et al. Discovery of yellow seeds in Brassica napus and preliminary studies in their hereditary behavior. Acta Genetica Sinica, 1979,6 (1):54. (in Chinese with English abstract) |
| [5] |
RAHMAN M H, JOERSBO M, POULSEN M H. Development of yellow seeded Brassica napus of double low quality.
Plant Breeding, 2001,120 (6):473–478. DOI: 10.1046/j.1439-0523.2001.00639.x. |
| [6] |
刘后利, 王纫秋, 高永同.甘蓝型黄籽油菜高含油量育种初报.
华中农学院学报,1981 (1):13–20.
LIU H L, WANG R Q, GAO Y T. Preliminary report about the breeding of high oil content on yellow-seed rape of Brassica napus L. Journal of Huazhong Agricultural University, 1981 (1):13–20. (in Chinese with English abstract) |
| [7] |
肖达人.甘蓝型油菜 (Brassica napus L.) 种皮颜色与种子含油量的相关分析.
作物学报,1982,8 (4):245–254, 291.
XIAO D R. Analysis on the correlations between seed coat color and oil contents of Brassica napus L. Acta Agronomica Sinica, 1982,8 (4):245–254, 291. (in Chinese with English abstract) |
| [8] |
王汉中, 刘后利.甘蓝型油菜粒色与种子有关性状间的关系研究.
中国油料,1989 (2):32–36.
WANG H Z, LIU H L. Correlation analysis between seed color and some related traits in Brassica napus L. Chinese Journal of Oil Crop Sciences, 1989 (2):32–36. (in Chinese with English abstract) |
| [9] |
BADANI A G, SNOWDON R J, WITTKOP B, et al. Colocalization of a partially dominant gene for yellow seed colour with a major QTL influencing acid detergent fibre (ADF) content in different crosses of oilseed rape (Brassica napus).
Genome, 2006,49 :1499–509. DOI: 10.1139/g06-091. |
| [10] |
LIU L, STEIN A, WITTKOP B, et al. A knockout mutation in the lignin biosynthesis gene CCR1 explains a major QTL for acid detergent lignin content in Brassica napus seeds.
Theoretical and Applied Genetics, 2012,124 (8):1573–1586. DOI: 10.1007/s00122-012-1811-0. |
| [11] |
ZHOU L, LI Y, HUSSAIN N, LI Z, et al. Allelic variation of BnaC.TT2.a and its association with seed coat color and fatty acids in rapeseed (Brassica napus L.).
PLoS ONE, 2016,11 (1):e0146661. DOI: 10.1371/journal.pone.0146661. |
| [12] |
YU C Y. Molecular mechanism of manipulating seed coat coloration in oilseed species.
Journal of Applied Genetics, 2013,54 (2):135–145. DOI: 10.1007/s13353-012-0132-y. |
| [13] |
WANG F, HE J, SHI J, et al. Embryonal control of yellow seed coat locus ECY1 is related to alanine and phenylalanine metabolism in the seed embryo of Brassica napus.
G3-Genes Genomes Genetics, 2016,6 (4):1073–1081. |
| [14] |
ZHANG J, LU Y, YUAN Y, et al. Map-based cloning and characterization of a gene controlling hairiness and seed coat color traits in Brassica rapa.
Plant Molecular Biology, 2009,69 (5):553–563. DOI: 10.1007/s11103-008-9437-y. |
| [15] |
PADMAJA L K, AGARWAL P, GUPTA V, et al. Natural mutations in two homoeologous TT8 genes control yellow seed coat trait in allotetraploid Brassica juncea (AABB).
Theoretical and Applied Genetics, 2014,127 (2):339–347. DOI: 10.1007/s00122-013-2222-6. |
| [16] |
DENG W, CHEN G, PENG F, et al. Transparent testa16 plays multiple roles in plant development and is involved in lipid synthesis and embryo development in canola.
Plant Physiology, 2012,160 (2):978–989. DOI: 10.1104/pp.112.198713. |
| [17] |
纪银福, 石来凤.饲料中氨基酸的测定——酸水解法测试研究.
草食家畜,2007,135 (2):39–41.
JI Y F, SHI L F. Assay amino acid in feed——Acid hydrolytic method test and research. Grass-Feeding Livestock, 2007,135 (2):39–41. (in Chinese with English abstract) |
| [18] |
LIU R, LEHANE S, HE X, et al. Characterizations of odorant-binding proteins in the tsetse fly Glossina morsitans morsitans.
Cellular and Molecular Life Sciences, 2010,67 (6):919–929. DOI: 10.1007/s00018-009-0221-1. |
| [19] |
尤晓蒙. 饲料中氨基酸的HPLC检测方法研究: 保定, 河北: 河北农业大学, 2015: 33-34. YOU X M. Study on determination of amino acids in feeds by high performance liquid chromatography. Baoding, Hebe: Hebei Agricultural University, 2015:33-34. (in Chinese with English abstract) |
| [20] |
杜继忠.赖氨酸蛋白粉替代进口鱼粉对麻黄肉雏鸡的应用效果.
养禽与禽病防治,2009,11 :13–14.
DU J Z. Application effect of lysine protein powder replaced imported fishmeal on ephedra meat chicks. Poultry Husbandry and Disease Control, 2009,11 :13–14. (in Chinese with English abstract) |
 2016, Vol. 43
2016, Vol. 43


