

2. 湖南中医药大学 中药粉体与创新药物省部共建国家重点实验室培育基地,湖南 长沙 410208;
3. 澳门科技大学中药质量研究国家重点实验室,澳门 氹仔 999078
刘良(1957-),男,博士,教授,研究方向:中药抗炎免疫药理研究,通讯作者,E-mail: liuliang@must.edu.mo
2. Institute of Innovation and Applied Research in Chinese Medicine, Hunan University of Chinese Medicine, Changsha 410208, China;
3. State Key Lab for Quality Research in Chinese Medicine, Macau University of Science and Technology, Taipa Macau 999078, China
类风湿关节炎(rheumatoid arthritis,RA)是一种慢性炎症性自身免疫疾病,其主要病理特征为进行性关节滑膜炎症,软骨和骨破坏,最终造成关节畸形和功能丧失[1]。研究表明,RA是以大量CD4+ T细胞浸润为主的慢性滑膜炎症反应,CD4+ T细胞作为效应T细胞的重要成分,参与免疫应答过程的各个阶段,其介导的免疫反应异常被认为是RA主要发病机制之一[2]。根据其产生的细胞因子及其功能,通常将CD4+ T细胞分为辅助性T细胞1 (helper T 1,Th1)、Th2、Th17和调节性T细胞(regulatory T cell,Treg)四大亚群。Th17细胞主要分泌IL-17、IL-21等炎性细胞因子,具有促进炎性反应和促进关节软骨和骨破坏进程等多重效应。因此,本文拟收集近年来Th 17细胞参与RA发病的相关研究进展并进行综述,以期为RA病理机制研究提供参考。
1 Th17细胞概述在免疫应答的不同阶段,因抗原性质、局部微环境等因素的综合效应,CD4+ T细胞会朝不同方向分化,进而发挥其特定的生物学效应。Th17细胞是CD4+ T细胞新亚群,其主要分泌白介素IL-17A、IL-17F、IL-21等细胞因子,并表达转录因子维甲酸相关孤核受体(retinoid acid-related orphan receptorγ,RORγt)和RORα,而在自身免疫疾病中发挥重要作用。
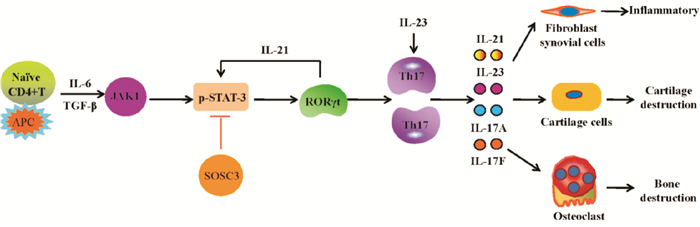
在树突细胞、单核细胞等抗原呈递细胞作用下,IL-6协同低浓度的转化生长因子-β(transforming growth factor β,TGF-β),激活JAK1,进而招募信号传导及转录激活因子-3 (signal transducer and activator of transcription-3,STAT3),并使其磷酸化,活化的JAK1/STAT3通路激活RORγt,并促进IL-21产生[3]。在IL-6和IL-21协同作用下,产生IL-23R、RORα和RORγt,使Naive CD4+ T细胞向Th17细胞分化[4],并抑制转录因子叉头蛋白3 (forkhead box protein 3,Foxp3)的负向调节作用。Th17细胞被转录因子STAT4和STAT6调控,生成IL-17,同时可产生IL-23、IL-6和肿瘤坏死因子-α (tumor necrosis factorα,TNF-α)等炎性细胞因子,IL-23可促进激活的记忆细胞产生IL-17,使Th17细胞得以存活和维持功能[5]。见Fig 1。
|
| Fig 1 Pathway of differentiation of naive CD4+ T cells into Th17 cells In the presence of antigen-presenting cells such as dendritic cells and monocytes, IL-6 is synergized with low-concentration TGF-β to activate JAK1, and then activated JAK1 recruits and phosphorates STAT3. JAK1/STAT3 signaling activates RORγt and promotes production of IL-21.Under the circumstance of abundant IL-6 and IL-21, naive CD4+ T cells differentiate into Th17 cells, and simultaneously negative regulation of Foxp3 to this differentiation is inhibited. Transcription factors STAT4 and STAT6 regulate Th17 cells to produce pro-inflammatory cytokines IL-23, IL-6, and TNF-α.IL-23 promotes activated memory CD4+ T cells to produce IL-17 which in turn maintain the survival and function of Th17 cells. |
RA早期主要表现为大量CD4+ T细胞浸润为主的慢性滑膜炎症反应。IL-17是RA早期重要细胞之一,具有强大的促炎效应,大量、广泛表达于在RA患者关节滑液、滑膜组织、外周血、外周淋巴结等处[6]。RA患者发病前外周血IL-17表达水平显著高于发病后,RA患者(病程 < 12月)外周血IL-17表达量相当高,且与C-反应蛋白(C-reactive protein,CRP)呈正相关[7]。RA患者血清Th17水平显著升高,且与疾病活动性评分28 (disease activity score 28,DAS28)呈正相关[8]。RA患者外周血Th 17、IL-6、IL-17、IL-21表达量随疾病活动程度增加而增加[9]。RA患者外周血单个核细胞Th17细胞中趋化因子受体6(chemokine receptors 6,CCR6)和RORγt表达量显著增加,且关节滑液单核细胞Th17细胞比例显著高于外周血单个核细胞[10]。树突细胞可分泌IL-23促进IL-17的增殖,而参与RA风湿结节的形成[5]。胶原性关节炎(collagen induced arthritis, CIA)大鼠关节滑膜及外周血IL-17显著升高,外周血RORγt显著增加[11]。CIA小鼠血浆IL-17、外周血和脾脏Th17细胞比例,CD4+ T细胞IL-17 mRNA表达水平均显著增加[12]。IL-17可协同IL-1β和TNF-α共同诱导T细胞和树突细胞的趋化[13]。IL-17协同TNF-α促进成纤维样滑膜细胞(fibroblast-like synoviocytes,FLSs)和上皮细胞大量分泌IL-6、IL-8、前列腺素E2(prostaglandin E,PGE2)和中性粒细胞化学引诱物[13]。上述研究表明,Th17细胞在外周血、关节滑液等部位均有表达,不仅参与了RA发病,而且与疾病活动程度及临床检测指标密切相关。
IL-21是Th17细胞分泌的促炎性细胞因子,大量表达于CD4+ T细胞及FLSs。RA患者关节滑液中CD4+ IL-21+T细胞比例显著增加,外周血CD4+ IL-21+T细胞及血清IL-21与DAS28、抗-CCP抗体、血沉和类风湿因子呈正相关[14, 15]。IL-21通过抑制Foxp3表达,上调RORC而促进Th17细胞分化与增殖[16]。研究显示,联合阻断IL-6和IL-21可有效抑制CIA小鼠脾脏CD4+ Th17细胞分化,进而减轻疾病严重程度[4]。IL-21/IL-21R可介导磷脂酰肌醇3-激酶/蛋白激酶B (phosphoinosmde-3-kinase/protein kinase B,PI3K/PKB) PI3K/Akt促进IL-23表达而促进RA-FLS异常增殖[17]。IL-21通过丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)、STAT3及PI3K/Akt通路介导FLS分泌TNF-α、IL-6等促炎性细胞因子[18]。
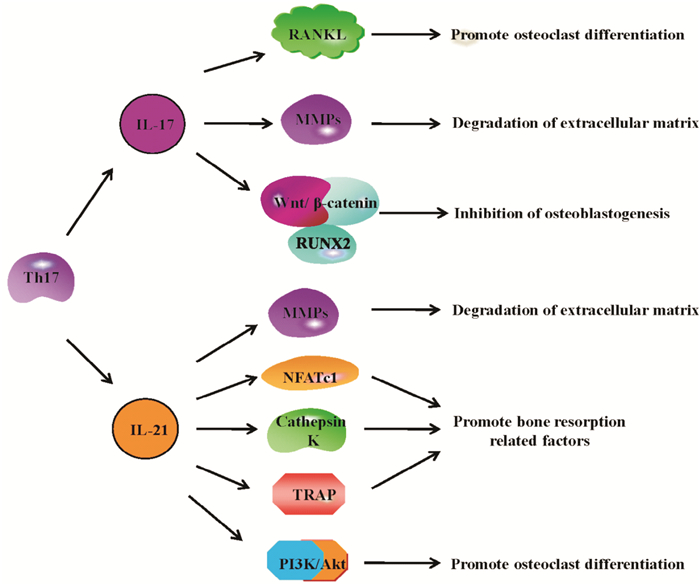
3 Th17细胞在RA骨破坏中的作用关节软骨的破坏及骨侵蚀是RA进展期重要组织学特征。研究表明,破骨细胞是介导RA骨破坏的关键细胞,核因子-κB受体活化因子配基(receptor activator of nuclear factor-κ B ligand,RANKL)是破骨细胞生成的启动信号。CD4+ T细胞介导的免疫应答在RA骨破坏中具有重要作用。IL-17A/F通过上调Runt相关转录因子-2 (runt-related transcription factor,RUNX2)蛋白,增强IL-6蛋白和基质金属蛋白酶-3 (matrix metalloproteinase-3,MMP-3)mRNA表达而抑制RA关节软骨的产生潜能[19]。骨桥蛋白可促进成骨细胞中IL-17表达而引起CIA小鼠骨质侵蚀[20]。IL-17通过活化IL-17/IL-17R/STATS-3信号通路促进RANKL表达,抑制骨保护素(osteoprotegerin,OPG)表达而介导佐剂性关节炎(adjuvant induced arthritis, AIA)大鼠FLS破骨细胞分化[21]。RA患者骨髓血浆中IL-17A/F及CD3+CD4+ IL-17+细胞百分比较骨关节炎患者显著升高,提示骨髓微环境可以促进Th17细胞和IL-17A/F过度产生而介导RA破坏[4]。IL-17促进K/BxN小鼠体内人分泌型卷曲相关蛋白1 (secreted frizzled related protein,sFRP1)分泌,减少sFRP3的分泌,进而抑Wnt/β-catenin/RUNX2通路,负调控成骨细胞生成[22]。IL-17通过诱导软骨MMP-3表达而抑制关节滑膜和软骨细胞COL2A1表达而促进骨破坏[23]。综上,IL-17主要通过以下途径参与RA骨破坏:(1)促进RANKL表达,进而促进破骨细胞分化;(2)促进MMPs表达,进而降解骨细胞外基质,引起骨破坏;(3)抑制成骨细胞生成。见Fig 2。
|
| Fig 2 Bone destruction in RA promoted by Th17 cells Th17 cells produce IL-17 and IL-21 which play an important role in bone destruction of patients with RA. IL-17 promotes bone destruction in RA through the following pathways: (1) IL-17 promotes RANKL expression and osteoclast differentiation; (2) IL-17 promotes the expression of MMPs that degrade the extracellular matrix of bone; (3) IL-17 inhibits osteoblastogenesis.IL-21 promotes bone destruction in RA through the following pathways:(1) IL-21 promotes the expression of MMPs which degrade the extracellular matrix of bone; (2) IL-21 promotes the expression of bone resorption-related factors; (3) IL-21 promotes osteoclast differentiation |
IL-21促进FLS的RANKL表达而介导破骨细胞生成[24]。IL-21促进FLS分泌介导RA骨与关节软骨破坏的关键蛋白酶MMP1、MMP2、MMP3、MMP9、MMP13而介导,其中MMP3、MMP9的活化与PI3K、STAT-3及细胞外调节蛋白激酶(extracellular regulated protein kinase 1/2,ERK1/2)有关[25]。IL-21/IL-21R相互作用可活化PI3K/Akt通路介导活化T细胞核因子1蛋白(nuclear factor of activated T-cells,NFATc1)及骨吸收相关因子组织蛋白酶k (cathepsin K)和抗酒石酸酸性磷酸酶(tartrate-resistant acid phosphatase,TRAP)表达进而介导骨破坏[17]。综上,IL-21参与RA骨破坏的途径主要有:(1)促进MMPs表达,进而降解骨细胞外基质,引起骨破坏;(2)调节骨吸收相关因子的表达;(3)促进破骨细胞分化。见Fig 2。
4 总结与展望综上所述,Th17细胞及其分泌的IL-17、IL-21等促炎性细胞因子对RA早期的滑膜炎症反应和RA晚期的骨破坏等病理环节均发挥促进作用,但对于不同病理环节的作用强度是否有差异;对于不同环节的作用机制及其效应靶点是否异同,均尚有待于进一步深入研究。因此,深入研究Th17细胞对RA不同病理环节的影响,有助于进一步揭示其针对RA不同病理环节的病理机制及其效应靶点,进而开发基于Th17细胞的RA多靶向防治药物,具有重大的研究意义和广袤的应用开发前景。
| [1] |
Liu E, Perl A. Pathogenesis and treatment of autoimmune rheumatic diseases[J]. Curr Opin Rheumatol, 2019, 31(3): 307-15. |
| [2] |
Sun J, Li L, Li L, et al. Metallothionein-1 suppresses rheumatoid arthritis pathogenesis by shifting the Th17/Tregbalance[J]. Eur J Immunol, 2018, 48(9): 1550-62. doi:10.1002/eji.201747151 |
| [3] |
Roeleveld D M, Marijnissen R J, Walgreen B, et al. Higher efficacy of anti-IL-6/IL-21 combination therapy compared to monotherapy in the induction phase of Th17-driven experimental arthritis[J]. PLos One, 2017, 12(2): e0171757. doi:10.1371/journal.pone.0171757 |
| [4] |
Halwani R, Sultana A, Vazquez-Tello A, et al. Th-17 regulatory cytokines IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17 cytokines during asthma[J]. J Asthma, 2017, 54(9): 893-904. doi:10.1080/02770903.2017.1283696 |
| [5] |
Kugyelka R, Kohl Z, Olasz K, et al. Enigma of IL-17 and Th17 cells in rheumatoid arthritis and in autoimmune animal models of arthritis[J]. Mediat Inflamm, 2016, 6145810. |
| [6] |
Zhang X, Yuan Y, Pan Z, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: A meta-analysis[J]. Clin Chim Acta, 2019, 496: 76-83. doi:10.1016/j.cca.2019.06.026 |
| [7] |
SiloŞi I, Boldeanu M V, Cojocaru M, et al. The relationship of cytokines IL-13 and IL-17 with autoantibodies profile in early rheumatoid arthritis[J]. J Immunol Res, 2016, 2016: 3109135. |
| [8] |
王玉辉. 类风湿性关节炎患者血清Th17/Treg细胞变化特征[J]. 北华大学学报(自然科学版), 2018, 19(1): 53-5. Wang Y H. Changing characteristics of serum Th17/Treg cells in patients with rheumatoid arthritis[J]. J Beihua Univ(Nat Sci), 2018, 19(1): 53-5. |
| [9] |
Sun W K, Bai Y, Yi M M, et al. Expression of T follicular helper lymphocytes with different subsets and analysis of serum IL-6, IL-17, TGF-β and MMP-3 contents in patients with rheumatoid arthritis[J]. Eur Rev Med Pharmacol Sci, 2019, 23(1): 61-9. |
| [10] |
Kaneko S, Kondo Y, Yokosawa M, et al. The RORγt-CCR6-CCL20 axis augments Th17 cells invasion into the synovia of rheumatoid arthritis patients[J]. Mod Rheumatol, 2018, 28(5): 814-25. doi:10.1080/14397595.2017.1416923 |
| [11] |
俞云, 时乐, 喻斌, 等. 栀子苷对类风湿性关节炎大鼠Th17/Treg平衡和局部炎症因子的影响[J]. 南京中医药大学学报, 2018, 34(5): 499-503. Yu Y, Shi L, Yu B, et al. The effects of geniposide on Th17/Treg balance and local inflammatory factors in rats with rheumatoid arthritis[J]. J Nanjing Univ Tradit Chin Med, 2018, 34(5): 499-503. |
| [12] |
钱飞亚, 杨培, 张明菲, 等. 滋肾通络方对胶原性关节炎小鼠Th17/Treg细胞分化及平衡的影响[J]. 中国药理学通报, 2019, 35(5): 720-6. Qian F Y, Yang P, Zhang M F, et al. Effect of ZishenTongluo formula on differentiation and balance of Th17 /Treg cells in collagen-induced arthritis[J]. Chin Pharmacol Bull, 2019, 35(5): 720-6. doi:10.3969/j.issn.1001-1978.2019.05.025 |
| [13] |
Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases[J]. Int J Mol Sci, 2019, 20(14): pii: E3394. doi:10.3390/ijms20143394 |
| [14] |
Lebre M C, Vieira P L, Tang M W, et al. Synovial IL-21/TNF-producing CD4+ T cells induce joint destruction in rheumatoid arthritis by inducing matrix metalloproteinase production by fibroblast-like synoviocytes[J]. J Leukoc Biol, 2017, 101(3): 775-83. doi:10.1189/jlb.5A0516-217RR |
| [15] |
Xing R, Sun L, Wu D, et al. Autoantibodies against interleukin-21 correlate with disease activity in patients with rheumatoid arthritis[J]. Clin Rheumatol, 2018, 37(1): 75-80. doi:10.1007/s10067-017-3862-8 |
| [16] |
Lin Z M, Yang X Q, Zhu F H, et al. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells[J]. Sci Rep, 2016, 6: 38115. doi:10.1038/srep38115 |
| [17] |
Dinesh P, Rasool M. Berberine inhibits IL-21/IL-21R mediated inflammatory proliferation of fibroblast-like synoviocytes through the attenuation of PI3K/Akt signaling pathway and ameliorates IL-21 mediated osteoclastogenesis[J]. Cytokine, 2018, 106: 54-66. doi:10.1016/j.cyto.2018.03.005 |
| [18] |
Xing R, Yang L, Jin Y, et al. Interleukin-21 induces proliferation and proinflammatory cytokine profile of fibroblast-like synoviocytes of patients with rheumatoid arthritis[J]. Scand J Immunol, 2016, 83(1): 64-71. doi:10.1111/sji.12396 |
| [19] |
Schminke B, Trautmann S, Mai B, et al. Interleukin 17 inhibits progenitor cells in rheumatoid arthritis cartilage[J]. Eur J Immunol, 2016, 46(2): 440-5. doi:10.1002/eji.201545910 |
| [20] |
Tsai C H, Liu S C, Wang Y H, et al. Osteopontin inhibition of miR-129-3p enhances IL-17 expression and monocyte migration in rheumatoid arthritis[J]. Biochim Biophys Acta Gen Subj, 2017, 1861(2): 15-22. doi:10.1016/j.bbagen.2016.11.015 |
| [21] |
Ganesan R, Rasool M. Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis[J]. Mol Immunol, 2017, 91: 134-44. doi:10.1016/j.molimm.2017.09.003 |
| [22] |
Shaw A T, Maeda Y, Gravallese E M. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis[J]. Arthritis Res Ther, 2016, 18(1): 104. doi:10.1186/s13075-016-0998-x |
| [23] |
Shui X L, Lin W, Mao C W, et al. Blockade of IL-17 alleviated inflammation in rat arthritis and MMP-13 expression[J]. Eur Rev Med Pharmacol Sci, 2017, 21(10): 2329-37. |
| [24] |
Kim K W, Kim H R, Kim B M, et al. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis[J]. Am J Pathol, 2015, 185(11): 3011-24. doi:10.1016/j.ajpath.2015.07.017 |
| [25] |
Xing R, Jin Y, Sun L, et al. Interleukin-21 induces migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis[J]. Clin Exp Immunol, 2016, 184(2): 147-58. doi:10.1111/cei.12751 |

