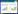文章信息
- 陈国华, 张浩宇, 韩升波, 何建鑫
- CHEN Guohua, ZHANG Haoyu, HAN Shengbo, HE Jianxin
- 基于血清CA50、TSGF、TPA的中晚期乳腺癌放化疗敏感性列线图预测模型的建立与评价
- Establishment and evaluation of the model for predicting the sensitivity to radiochemotherapy in patients with middle- and advanced-stage breast cancer based on serum CA50, TSGF, and TPA
- 中国医科大学学报, 2024, 53(3): 240-245
- Journal of China Medical University, 2024, 53(3): 240-245
-
文章历史
- 收稿日期:2023-06-13
- 网络出版时间:2024-03-04 16:54:50
乳腺癌是全球范围常见的女性肿瘤,最新数据显示,2020年新发乳腺癌226万例,这也是全球女性癌症死亡的主要原因[1]。乳腺癌早期无特异症状,临床症状明显时患者多处于中晚期,此时已错过最佳治疗时期,只能采用综合治疗。放化疗是治疗中晚期乳腺癌的首选方法,可提高患者生存期,降低组织转移和复发风险,但部分患者对放化疗不敏感,导致治疗效果不佳[2]。近年来研究[3-4]发现,血清糖类抗原50(carbohydrate antigen 50,CA50)、肿瘤特异性生长因子(tumor specific growth factor,TSGF)、组织多肽抗原(tissue polypeptide antigen,TPA)等肿瘤标志物可用于乳腺癌等肿瘤的早期诊断、预后评估、疾病转归等,但关于在中晚期乳腺癌患者中CA50、TSGF、TPA联合应用的临床研究少见。因此,本研究分析了中晚期乳腺癌放化疗不敏感和敏感患者中血清CA50、TSGF、TPA的表达情况,构建列线图预测模型,并对该模型进行验证与评价。
1 材料与方法 1.1 研究对象选取2020年10月至2022年10月我院收治的82例中晚期乳腺癌患者,年龄42~72岁,平均(56.65±5.21)岁;体质量指数(body mass index,BMI)18.7~25.4 kg/m2,平均(21.43±1.34)kg/m2;月经状态:绝经期52例,非绝经期30例;病理类型:导管内原位癌61例,浸润性导管癌4例,浸润性小叶癌17例。
纳入标准:符合《中国抗癌协会乳腺癌诊治指南与规范(2017年版)》[5]诊断标准,经病理检查确诊;为中晚期乳腺癌;预计生存期≥3个月;入组前3个月内未接受过放化疗等治疗。排除标准:妊娠期或哺乳期女性;患有其他恶性肿瘤;患有急慢性感染性疾病,如艾滋病、麻疹、病毒性肝炎等;患有自身免疫性疾病、内分泌疾病、血液系统疾病;严重心肝肾等器官功能障碍;意识或语言障碍;凝血功能障碍。本研究获得我院伦理委员会批准(批号:20200510005)。
1.2 治疗方法化疗方法:135 mg/m2紫杉醇(美国Bristol-Myers Squibb公司,国药准字HJ20171227,规格5 mL:30 mg)静脉滴注1次。放疗方法:胸壁和锁骨区域用6和9 MV电子线照射,照射16次后,在胸壁处加盖0.5 cm组织等效膜,2 Gy/次,1次/d,5次/周,总量50 Gy。21 d为1个疗程,持续2个疗程。
根据实体瘤疗效评价标准[6],将82例患者分为敏感组(n = 57)和不敏感组(n = 25)。
1.3 观察指标 1.3.1 一般资料包括年龄、肿瘤直径、BMI、月经状态(绝经、未绝经)、脉管侵犯(有、无)、TNM分期(Ⅲa期、Ⅲb期、Ⅳ期)、淋巴结转移(有、无)、淋巴结转移数量、孕激素受体(阳性、阴性)、雌激素受体(阳性、阴性)、病理类型(导管内原位癌、浸润性导管癌、浸润性小叶癌)、分化程度(中分化、低分化)、肿瘤位置(外侧象限、中央或内侧象限)。
1.3.2 治疗前后血清CA50、TSGF、TPA表达水平取2组患者治疗前后静脉血5 mL,1 000 g离心10 min,收集上清液,采用化学发光法检测CA50、TSGF、TPA水平,试剂盒购自上海雅吉生物。
1.4 统计学分析采用SPSS 25.0软件处理数据。计数资料用率(%)表示,采用χ2检验进行比较。计量资料行Shapiro-Wilk正态性检验和Levene法方差齐性检验,方差齐性且近似服从呈正态分布的计量资料以x±s表示,采用t检验进行比较。采用LASSO回归、logistic回归分析中晚期乳腺癌放化疗敏感性的影响因素,运用R语言rms包构建列线图模型,采用校准曲线、受试者操作特征(receiver operating characteristic,ROC)曲线、决策曲线评估列线图模型的预测效能和临床效用。双侧P < 0.05为差异有统计学意义。
2 结果 2.1 2组一般资料的比较2组比较,肿瘤直径、脉管侵犯、TNM分期、淋巴结转移、分化程度的差异有统计学意义(P < 0.05),年龄、月经状态、BMI、淋巴结转移数量、孕激素受体、雌激素受体、病理类型、肿瘤位置的差异无统计学意义(P > 0.05)。见表 1。
| Clinical data | Insensitive group(n = 25) | Sensitive group(n = 57) | t/χ2 | P |
| Age(year) | 55.82±5.46 | 57.01±4.37 | 1.050 | 0.297 |
| Tumor diameter(cm) | 4.45±1.03 | 3.85±1.26 | 2.092 | 0.040 |
| BMI(kg/m2) | 21.52±1.33 | 21.39±1.42 | 0.389 | 0.698 |
| Menstrual status [n(%)] | 0.326 | 0.568 | ||
| Menopause period | 17(68.00) | 35(61.40) | ||
| Non-menopausal period | 8(32.00) | 22(38.60) | ||
| Vascular invasion [n(%)] | 5.722 | 0.017 | ||
| Yes | 10(40.00) | 9(15.79) | ||
| No | 15(60.00) | 48(84.21) | ||
| TNM stage [n(%)] | 2.817 | 0.005 | ||
| Ⅲa | 5(20.00) | 29(50.88) | ||
| Ⅲb | 8(32.00) | 17(29.82) | ||
| Ⅳ | 12(48.00) | 11(19.30) | ||
| Lymph node metastasis [n(%)] | 4.661 | 0.031 | ||
| Yes | 17(68.00) | 24(42.11) | ||
| No | 8(32.00) | 33(57.89) | ||
| Number of metastatic lymph node [n(%)] | 0.120 | 0.729 | ||
| ≤3 | 13(52.00) | 32(56.14) | ||
| >3 | 12(48.00) | 25(43.86) | ||
| Progesterone receptor [n(%)] | 1.590 | 0.207 | ||
| Positive | 12(48.00) | 19(33.33) | ||
| Negative | 13(52.00) | 38(66.67) | ||
| Estrogen receptor [n(%)] | 1.645 | 0.200 | ||
| Positive | 13(52.00) | 21(36.84) | ||
| Negative | 12(48.00) | 36(63.16) | ||
| Pathological type [n(%)] | 0.284 | 0.777 | ||
| Ductal carcinoma in situ | 18(72.00) | 43(75.44) | ||
| Infiltrating ductal carcinoma | 1(4.00) | 3(5.26) | ||
| Infiltrating lobular carcinoma | 6(24.00) | 11(19.30) | ||
| Degree of differentiation [n(%)] | 4.355 | 0.037 | ||
| Moderately | 11(44.00) | 39(68.42) | ||
| Poorly | 14(56.00) | 18(31.58) | ||
| Tumor location [n(%)] | 0.106 | 0.745 | ||
| Outer quadrant | 15(60.00) | 32(56.14) | ||
| Central or inner quadrant | 10(40.00) | 25(43.86) |
2.2 2组治疗前后血清CA50、TSGF、TPA表达水平的比较
治疗前2组比较,血清CA50、TSGF、TPA表达水平的差异无统计学意义(P > 0.05);治疗后不敏感组血清CA50、TSGF、TPA表达水平高于敏感组,三者差值低于敏感组(P < 0.05)。见表 2。
| Group | n | CA50(U/mL) | TSGF(U/L) | TPA(U/L) | ||||||||
| Before treatment | After treatment | Difference* | Before treatment | After treatment | Difference* | Before treatment | After treatment | Difference* | ||||
| Insensitive | 25 | 44.96±5.52 | 35.52±4.08 | 9.44±3.05 | 90.02±8.44 | 70.72±6.62 | 19.30±3.44 | 72.90±6.46 | 65.48±5.95 | 7.42±1.33 | ||
| Sensitive | 57 | 45.78±4.69 | 19.91±3.32 | 25.87±4.44 | 88.96±9.56 | 40.35±4.42 | 48.61±5.89 | 74.11±5.02 | 50.52±4.77 | 23.59±3.56 | ||
| t | 0.690 | 18.253 | 16.816 | 0.478 | 24.445 | 23.159 | 0.918 | 12.104 | 21.983 | |||
| P | 0.492 | <0.001 | <0.001 | 0.634 | <0.001 | <0.001 | 0.361 | <0.001 | <0.001 | |||
| * The difference is considered an absolute value. | ||||||||||||
2.3 预测因素初筛
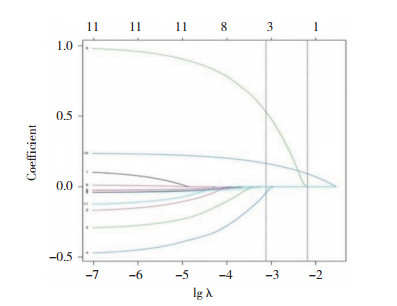
应用R语言glmnet程序包,以中晚期乳腺癌放化疗敏感性(不敏感=1,敏感=0)为因变量,肿瘤直径(实测值)、脉管侵犯(有=1,无=0)、TNM分期(Ⅲa期=1,Ⅲb期=2,Ⅳ期=3)、淋巴结转移(有=1,无=0)、分化程度(低分化=1,中分化=2)、治疗后血清CA50、TSGF、TPA表达水平及差值(实测值)纳入LASSO回归分析。随着惩罚系数λ变化,模型初始纳入影响因素系数被压缩,达到最佳影响因素选择的效果图(图 1)。采用交叉验证法绘制均方误差随lgλ的变化图(图 2),寻找到可使模型性能优良且影响因素最少的最佳惩罚系数λ,即图中虚线对应的λ=3。依据此λ值,选出3个预测变量,分别为TSGF差值、CA50差值、TPA差值。
|
| 图 1 LASSO回归筛选变量动态过程图 Fig.1 Dynamic graph of screening variable using LASSO regression |
|
| 图 2 交叉验证最佳参数λ的选择过程图 Fig.2 Selection process of the optimal parameter λ for cross-validation |
2.4 中晚期乳腺癌放化疗敏感性影响因素的多因素logistic回归分析
以中晚期乳腺癌放化疗敏感性(不敏感=1,敏感=0)为因变量,以LASSO回归筛选指标作为自变量,纳入多因素logistic回归分析,结果显示,CA50、TSGF、TPA差值是中晚期乳腺癌放化疗敏感性的影响因素(P < 0.05),见表 3。
| Independent variable | β | SE | Wald χ2 | OR | 95%CI | P |
| Constant | 12.456 | |||||
| Difference in CA50 | -0.596 | 0.171 | 12.149 | 0.551 | 0.345-0.888 | <0.001 |
| Difference in TSGF | -2.561 | 0.496 | 26.663 | 0.077 | 0.011-0.542 | <0.001 |
| Difference in TPA | -0.718 | 0.165 | 18.955 | 0.488 | 0.346-0.687 | <0.001 |
2.5 列线图模型的构建
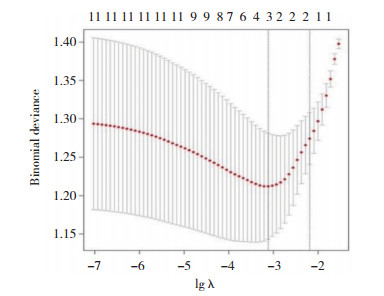
基于多因素logistic回归分析结果(CA50、TSGF、TPA差值),运用R语言rms包构建中晚期乳腺癌放化疗敏感性列线图模型。见图 3。
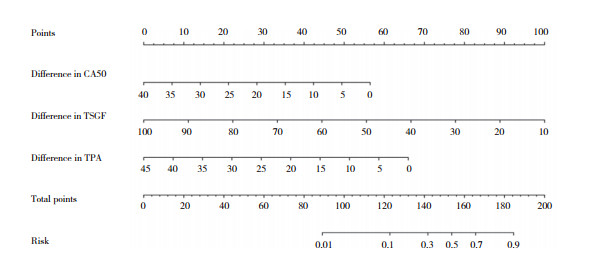
|
| 图 3 中晚期乳腺癌放化疗敏感性列线图模型 Fig.3 Nomogram model for predicting sensitivity to radiochemotherapy in middle- and advanced-stage breast cancers |
2.6 列线图模型的验证与评价
采用Hosmer-Lemeshow检验检测列线图模型拟合度,结果显示,该列线图预测模型具有良好拟合度(P = 0.482)。采用Bootstrap自抽样法及计算预测模型区分度(C-index),展开内部验证,结果显示,该列线图模型C-index为0.839,校准曲线趋向于理想曲线,见图 4A。ROC曲线显示,列线图模型的AUC为0.839(95%CI:0.814~0.871),灵敏度和特异度分别为80.00%和75.74%,说明基于CA50、TSGF、TPA差值构建的列线图模型具有良好的预测精准度,见图 4B。决策曲线显示,在0.1~1.0范围内,该列线图模型预测净获益值较高,说明该列线图模型临床预测效能良好,见图 4C。
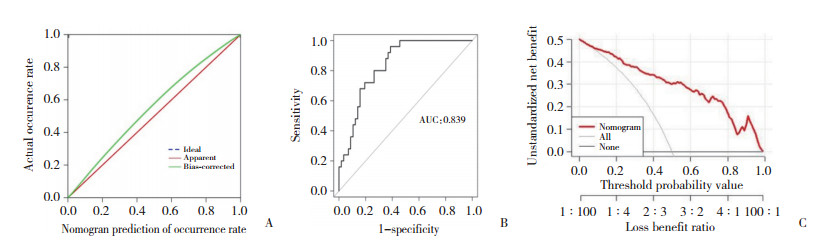
|
| A, calibration curve; B, ROC curve; C, decision curve. 图 4 中晚期乳腺癌放化疗敏感性列线图模型的校准曲线、ROC曲线和决策曲线 Fig.4 Calibration, ROC, and decision curves of the nomogram model for predicting sensitivity to radiochemotherapy in middle- and advanced-stage breast cancer |
3 讨论
研究[7]显示,中晚期乳腺癌患者采取同步放化疗治疗,可提高临床缓解率,缩小肿瘤体积,并改善生活质量和免疫功能。但部分患者未获得临床缓解,导致预后不佳,还易出现复发、转移等。因此,寻找一些特异度和灵敏度高的无创分子标志物,对预测中晚期乳腺癌患者放化疗敏感性具有重要作用。
肿瘤标志物主要以激素、抗原、酶等形式存在于肿瘤细胞和宿主体液中,可准确反映肿瘤细胞的生化性质和代谢情况,临床主要用于鉴别良恶性肿瘤、筛选肿瘤高危人群、判断肿瘤进展、评估肿瘤预后等[8]。血清肿瘤标志物检测是一种非介入性检查措施,操作简单、检测快捷。目前临床发现,血清CA153、癌胚抗原、CA125等在乳腺癌患者组织和血清中差异表达,且与远处转移、分化程度、血管形成等密切相关,可用于乳腺癌的早期诊断、预后评估等[9-10]。CA50、TSGF、TPA是临床常见肿瘤标志物。CA50是一种非特异性肿瘤抗原,与癌抗原19-9具有交叉性。CA50在人正常组织中含量较低,细胞癌变后可激活糖基化酶,引起细胞膜表层糖基结构改变,导致大量CA50进入血液或组织中[11]。已有研究[12]显示,CA50在多种恶性肿瘤中高表达,肿瘤分期越高、分化程度越低、直径越小,CA50阳性率越高。TSGF是国际公认的与恶性肿瘤有关的广谱肿瘤标志物,是由多种小分子代谢产物、氨基酸和糖类物质形成的一种多肽因子,TSGF分泌增多可刺激恶性肿瘤细胞新血管形成,促进肿瘤恶性生长分化、转移和毛细血管增生;TSGF在多种肿瘤中表达升高,具有较高灵敏度和特异度,可用于良恶性肿瘤鉴别、预后评估等[13]。TPA是一种与角蛋白8、18、19有关的一种多肽物质,主要在多种恶性肿瘤组织和胎盘组织中表达;研究[14]发现,TPA水平升高与恶性肿瘤直径、分化程度、浸润程度等有关。本研究发现,治疗后不敏感组血清CA50、TSGF、TPA表达水平高于敏感组,CA50、TSGF、TPA差值低于敏感组,提示血清CA50、TSGF、TPA表达水平与中晚期乳腺癌患者放化疗敏感性有关。笔者推测:患者肿瘤直径越大、脉管发生侵犯、TNM分期越高、淋巴结发生转移可促进肿瘤细胞恶性生长,进而促进新血管形成;放化疗治疗后,约30.49%的患者不敏感,影响治疗效果,故不敏感组患者治疗前后血清CA50、TSGF、TPA差值变化较小。
本研究使用LASSO回归筛选的有统计学差异的11个变量,发现最佳惩罚系数λ=3,筛选出3个预测变量为治疗前后TSGF、CA50、TPA差值。logistic回归也证实三者差值是中晚期乳腺癌放化疗敏感性的影响因素,进一步说明血清CA50、TSGF、TPA与中晚期乳腺癌放化疗敏感性有关。根据logistic回归结果构建中晚期乳腺癌放化疗敏感性列线图模型,提示三者差值越小,中晚期乳腺癌放化疗不敏感风险越高。采用C-index对该模型进行内部验证,发现C-index为0.839,具有较高的准确度,且校准曲线趋向于理想曲线;ROC曲线和决策曲线显示,该模型具有良好的预测精准度和临床预测效能。
综上所述,在中晚期乳腺癌放化疗不敏感患者中血清CA50、TSGF、TPA表达水平较高,且血清CA50、TSGF、TPA差值是患者放化疗敏感性的影响因素,基于以上因素构建的列线图预测模型,具有较好的参考价值和临床适用性。
| [1] |
WILKINSON L, GATHANI T. Understanding breast cancer as a global health concern[J]. Br J Radiol, 2022, 95(1130): 20211033. DOI:10.1259/bjr.20211033 |
| [2] |
WANG X, WANG SS, HUANG H, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial[J]. JAMA, 2021, 325(1): 50-58. DOI:10.1001/jama.2020.23370 |
| [3] |
张雁, 刘松岭, 周爱凤, 等. 乳腺癌患者血清因子水平与疾病的相关性研究[J]. 中国肿瘤临床与康复, 2022, 29(4): 412-415. DOI:10.13455/j.cnki.cjcor.2022.04.07 |
| [4] |
陈鹏, 马德寿, 赵海宁. 保乳术治疗对乳腺癌患者疗效、血清肿瘤标志物的影响及预后分析[J]. 湖南师范大学学报(医学版), 2019, 16(3): 51-54. DOI:10.3969/j.issn.1673-016X.2019.03.016 |
| [5] |
中国抗癌协会乳腺癌专业委员会. 中国抗癌协会乳腺癌诊治指南与规范(2017年版)[J]. 中国癌症杂志, 2017, 27(9): 695-759. DOI:10.19401/j.cnki.1007-3639.2017.09.004 |
| [6] |
杨学宁, 吴一龙. 实体瘤治疗疗效评价标准——RECIST[J]. 循证医学, 2004, 4(2): 85-90. DOI:10.3969/j.issn.1671-5144.2004.02.012 |
| [7] |
张叶青, 孙成晖, 卢柳岑. 心理护理干预在多西他赛+表柔比星方案化疗中晚期乳腺癌患者中的应用效果[J]. 中国医药科学, 2020, 10(19): 143-146. DOI:10.3969/j.issn.2095-0616.2020.19.035 |
| [8] |
IWAMOTO T, KAJIWARA Y, ZHU Y, et al. Biomarkers of neoadjuvant/adjuvant chemotherapy for breast cancer[J]. Chin Clin Oncol, 2020, 9(3): 27. DOI:10.21037/cco.2020.01.06 |
| [9] |
LI H, WANG S, LI X, et al. Dual-channel detection of breast cancer biomarkers CA15-3 and CEA in human serum using dialysis-silicon nanowire field effect transistor[J]. Int J Nanomedicine, 2022, 17: 6289-6299. DOI:10.2147/IJN.S391234 |
| [10] |
LI J, LIU L, FENG Z, et al. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study[J]. Breast Cancer, 2020, 27(4): 621-630. DOI:10.1007/s12282-020-01058-3 |
| [11] |
LI H, LI L, SUN J, et al. Value of TCT combined with serum CA153 and CA50 in early diagnosis of cervical cancer and precancerous lesions[J]. Pak J Med Sci, 2022, 38(6): 1471-1476. DOI:10.12669/pjms.38.6.5503 |
| [12] |
ZHANG Q, DONG G, WANG F, et al. Correlation between the changes of serum COX-2, APE1, VEGF, TGF-β and TSGF levels and prognosis in patients with osteosarcoma before and after treatment[J]. J Cancer Res Ther, 2020, 16(2): 335-342. DOI:10.4103/jcrt.JCRT_11_20 |
| [13] |
YU C, SUN C. Diagnostic value of multislice spiral computed tomography combined with serum AFP, TSGF, and GP73 assay in the diagnosis of primary liver cancer[J]. Evid Based Complement Alternat Med, 2022, 2022: 6581127. DOI:10.1155/2022/6581127 |
| [14] |
CHEN Z, LIU X, SHANG X, et al. The diagnostic value of the combination of carcinoembryonic antigen, squamous cell carcinoma-related antigen, CYFRA 21-1, neuron-specific enolase, tissue polypeptide antigen, and progastrin-releasing peptide in small cell lung cancer discrimination[J]. Int J Biol Markers, 2021, 36(4): 36-44. DOI:10.1177/17246008211049446 |
 2024, Vol. 53
2024, Vol. 53