文章信息
- 石晓萌, 罗颖, 何庭艳, 夏宇, 杨军
- SHI Xiaomeng, LUO Ying, HE Tingyan, XIA Yu, YANG Jun
- 全身型幼年特发性关节炎患儿发生巨噬细胞活化综合征的早期预警指标
- Early warning indicators of systemic juvenile idiopathic arthritis complicated with macrophage activation syndrome
- 中国医科大学学报, 2023, 52(6): 531-537
- Journal of China Medical University, 2023, 52(6): 531-537
-
文章历史
- 收稿日期:2022-06-07
- 网络出版时间:2023-05-25 17:18:08
全身型幼年特发性关节炎(systemic juvenile idiopathic arthritis,sJIA) 是幼年特发性关节炎(juvenile idiopathic arthritis,JIA) 的一种特殊亚型,约占JIA的10%~20%,发病率约为(6.6~15) /100 000[1]。sJIA患儿易发生巨噬细胞活化综合征(macrophage activation syndrome,MAS),严重威胁患儿的生命,因此尽早诊断并治疗尤为重要。为早期预测MAS,欧洲抗风湿病联盟/美国风湿病学会/儿童风湿病国际试验组织于2016年联合制定了关于sJIA合并MAS的新分类标准[2]。疑似MAS患者的临床表现晚于实验室指标异常,且缺乏特异性,因此除发热外未纳入其他临床表现。分类标准中血小板计数(platelet count,PLT)和纤维蛋白原(fibrinogen,FIB)水平的截断值处于实验室参考范围内,甘油三酯(triglyceride,TG)和血清转氨酶的临界值仅稍高于正常范围上限。
同时,强调早期识别sJIA合并MAS,对于确诊/疑诊sJIA患者,监测实验室指标对提示MAS发生更为敏感[2]。研究[2]表明,MAS发病前、发病时PLT、肝功能、乳酸脱氢酶(lactate dehydrogenase,LDH)、TG、D-二聚体和血清铁蛋白(serum ferritin,SF) 增加了50%以上,其中SF变化最大;而红细胞沉降率(erythrocyte sedimentation rate,ESR) 急剧下降是MAS的另一个重要提示。LDH可能是除SF之外的早期识别MAS的敏感指标。以往研究[3]显示,活动性sJIA或sJIA合并MAS患儿白细胞介素(interleukin,IL) -6水平显著升高。MAS患者血清中γ干扰素(inteferon-γ,IFN-γ)、IFN-γ诱导的趋化因子CXC趋化因子配体9 (CXC chemokine ligand 9,CXCL9) 和CXC趋化因子配体10 (CXC chemokine ligand 10,CXCL10) 较活动性sJIA患者显著升高,并且与疾病活动性显著相关[4-5]。因此,找到诊断和预后的生物学标志物可能会使早期干预成为可能。MAS是炎性细胞因子风暴所致,目前国内对sJIA合并MAS患者细胞因子谱的研究鲜有报道。本研究回顾性分析本科室收治的14例sJIA合并MAS患儿临床资料,并与同期收治的单纯sJIA患儿进行比较,探讨sJIA患儿发生MAS的早期预警指标,旨在为早期识别MAS发生,更有效保证医疗安全提供依据。
1 材料与方法 1.1 临床资料及分组选取2015年1月至2021年9月我科收治的14例sJIA合并MAS患者为sJIA-MAS组,选取同期我科36例性别、年龄匹配,临床资料相对齐全,初次诊断、未使用激素的单纯sJIA患者作为对照(sJIA组)。纳入标准:(1) sJIA诊断符合2001年加拿大埃德蒙顿sJIA诊断标准[6];(2) MAS诊断符合2004年国际组织细胞协会制定的噬血细胞综合征标准[7]和(或) 2005年sJIA合并MAS的初步诊断标准[8]和(或) 2016年EULAR/ACR/儿童风湿病国际试验组织标准[排除继发其他疾病(免疫介导的血小板减少症、传染性肝炎、内脏利什曼病、家族性高脂血症等) 所致] [2]。排除标准:患有其他免疫系统疾病、恶性肿瘤等疾病。本研究获得深圳市儿童医院伦理委员会批准[深儿医伦审(科研) 2021048号]并豁免知情同意。
sJIA-MAS组男6例,女8例,发病年龄(6.3±3.6)岁,sJIA病程1.0 (0.4~16.1) 个月,住院时间(28.9±18.8)d;sJIA组男23例,女13例,发病年龄(8.2±3.7) 岁,sJIA病程1.0 (0.4~2.2) 个月,住院时间(14.0±5.8) d。2组性别、年龄、病程比较差异均无统计学意义(均P > 0.05),具有可比性;但住院时间比较差异有统计学意义(t = 2.912,P = 0.011)。
1.2 检测指标收集患者电子病历资料,主要包括:(1) 一般资料,年龄、性别、病程、住院时间;(2) 首次确诊及规律激素治疗前MAS的主要临床特征,发热、肝/脾/淋巴结肿大、出血倾向、外周血细胞减少、中枢神经系统受累表现、肝功能损害;(3) 实验室检查数据,血细胞、谷氨酸氨基转移酶(alanine aminotransferase,ALT)、天冬氨酸氨基转移酶(aspartate aminotransferase,AST)、白蛋白(albumin,ALB)、ESR、FIB、TG、SF、LDH、骨髓噬血阳性率、细胞因子[IL-2、IL-4、IL-6、肿瘤坏死因子(tumor necrosis factor,TNF)-α和IFN-γ) 等]。
1.3 统计学分析采用SPSS 25.0软件进行统计分析,正态分布的计量资料以x±s表示,组间比较采用t检验,非正态分布的计量资料以M (P25~P75) 表示,组间比较采用Mann-Whitney U检验。计数资料以绝对数和百分比(%) 表示,组间比较采用χ2检验或Fisher’s确切概率法。采用受试者操作特征(receiver operating characte-ristic,ROC) 曲线分析sJIA合并MAS发生的最佳截断值。P < 0.05为差异有统计学意义。
2 结果 2.1 2组临床表现比较sJIA-MAS组中,1例合并急性脑病,1例颅内静脉窦血栓形成,1例心肌炎。sJIA组中1例心肌损害,1例肝损伤。
结果显示,与sJIA组比较,sJIA-MAS组患儿肝脾大、转氨酶升高、中枢神经系统障碍、骨髓嗜血现象、儿科重症监护病房(pediatric intensive care unit,PICU) 入住发生率均显著增高(均P < 0.05)。而2组发热、热程、关节痛、皮疹、皮肤黄染、淋巴结肿大、出血倾向、血细胞减少、心包积液、心肌损害、胸腔积液、肺炎、胸膜炎、腹腔积液发生率比较,差异无统计学意义(均P > 0.05)。见表 1。
| Item | sJIA-MAS group (n = 14) | sJIA grpup (n = 36) | Z/χ2 | P |
| Fever [n (%)] | 14 (100) | 36 (100) | - | - |
| Duration of fever (d) | 25 (19-37) | 20 (11-31) | -1.114 | 0.265 |
| Arthralgia/arthritis [n (%)] | 13 (92.9) | 36 (100) | - | 0.280 |
| Rash [n (%)] | 11 (78.6) | 18 (50.0) | 3.378 | 0.066 |
| Jaundice [n (%)] | 1 (7.1) | 0 (0) | - | 0.280 |
| Hepatomegaly [n (%)] | 11 (78.6) | 13 (36.1) | 7.281 | 0.007 |
| Splenomegaly [n (%)] | 7 (50.0) | 7 (19.4) | - | 0.042 |
| Lymphadenopathy [n (%)] | 11 (78.6) | 24 (66.7) | - | 0.507 |
| hemorrhagic diathesis [n (%)] | 1 (7.1) | 0 (0) | - | 0.280 |
| high level of the serum transaminase [n (%)] | 11 (78.6) | 1 (2.8) | - | < 0.001 |
| Hematocytopenia [n (%)] | 9 (64.3) | 13 (36.1) | 3.247 | 0.072 |
| Pericardial effusion [n (%)] | 0 (0) | 4 (11.1) | - | 0.566 |
| Pleural effusion [n (%)] | 5 (35.7) | 5 (13.9) | - | 0.118 |
| Interstitial pneumonia [n (%)] | 6 (42.9) | 11 (30.6) | - | 0.511 |
| Pleurisy [n (%)] | 5 (35.7) | 6 (16.7) | - | 0.252 |
| Pyoperitoneum [n (%)] | 1 (7.1) | 2 (5.6) | - | 1.000 |
| CNS involvement *[n (%)] | 6 (42.9) | 2 (5.6) | - | 0.004 |
| Bone marrow aspirate [n (%)] | 7 (50.0) | 6 (16.7) | - | 0.033 |
| PICU occupancy rate [n (%)] | 3 (21.4) | 0 (0) | - | 0.019 |
| -,Fisher’s exact test;* CNS involvement include seizures,dizziness,headache,irritability,lethargy. CNS,central nervous system. | ||||
2.2 2组实验室检查指标比较
结果显示,sJIA-MAS组2例完善sCD25检测,提示均升高,分别为18 944 pg/mL、65 510 pg/mL。与sJIA组比较,sJIA-MAS组白细胞计数(white blood cell count,WBC)、中性粒细胞计数(neutrophil count,NY)、淋巴细胞计数(lymphocyte count,L)、PLT、ALB、FIB均显著降低,降钙素原(procalcitonin,PCT)、SF、ALT、AST、LDH、TG、SF/ESR、LDH/ESR、IL-10、IFN-γ均显著升高(均P < 0.05)。而2组IL-2、IL-4、IL-6水平比较差异无统计学意义(均P > 0.05)。见表 2。
| Item | sJIA-MAS group (n = 14) | sJIA group (n = 36) | t/Z | P |
| WBC (×109/L) | 9.0±5.3 | 18.0±7.7 | -4.063 | < 0.001 |
| NY (×109/L) | 5.7 (2.1-9.7) | 15.1 (8.5-20.2) | -3.219 | 0.001 |
| L (×109/L) | 1.3 (0.9-2.3) | 2.6 (1.8-3.5) | -2.885 | 0.004 |
| HB (g/L) | 99.3±19.1 | 105.2±12.0 | -1.312 | 0.196 |
| PLT (×109/L) | 226.3±111.0 | 470.7±142.4 | -5.741 | < 0.001 |
| PCT (ng/mL) | 0.8 (0.2-2.0) | 0.2 (0.1-0.5) | -2.660 | 0.008 |
| CRP (mg/L) | 76.7 (51.8-116.1) | 73.2 (56.3-112.0) | -0.065 | 0.948 |
| ESR (mm/h) | 48.5 (14.8-103.3) | 91.5 (71.8-105.0) | -1.967 | 0.049 |
| SF (ng/mL) | 12 986.7 (8 194.8-22 020.8) | 685.0 (287.7-2 274.7) | -5.056 | < 0.001 |
| ALB (g/L) | 32.0±4.7 | 34.9±4.4 | -2.024 | 0.049 |
| ALT (U/L) | 78.5 (29.3-220.8) | 14.5 (10.3-21.8) | -4.272 | < 0.001 |
| AST (U/L) | 142.0 (71.0-241.3) | 25.0 (17.3-36.8) | -5.069 | < 0.001 |
| LDH (U/L) | 757 (625.2-1047.5) | 273 (214.3-318.3) | -5.316 | < 0.001 |
| TG (mmol/L) | 2.2 (1.4-3.2) | 1.0 (0.8-1.2) | -4.711 | < 0.001 |
| FIB (g/L) | 3.2±1.5 | 6.3±1.4 | -6.957 | < 0.001 |
| PT (S) | 13.8±1.7 | 14.0±1.4 | -0.387 | 0.700 |
| APTT (S) | 34.5 (30.0-40.1) | 36.4 (32.6-39.0) | -0.735 | 0.463 |
| INR | 1.1±0.2 | 1.2±0.1 | -0.529 | 0.599 |
| Na+ (mmol/L) | 135.2±3.6 | 135.8±2.1 | -0.558 | 0.584 |
| K+ (mmol/L) | 3.7±0.5 | 4.0±0.4 | -1.767 | 0.084 |
| CL- (mmol/L) | 101.8 (97.6-104.2) | 99.9 (98.3-103.1) | -0.756 | 0.449 |
| SF/ESR | 265.7 (142.4-722.3) | 8.3 (2.9-29.7) | -5.358 | < 0.001 |
| LDH/ESR | 18.6 (6.9-68.9) | 3.1 (2.2-4.5) | -5.164 | < 0.001 |
| IL-2 (pg/mL) | 4.4 (0.5-12.7) | 3.0 (1.3-10.0) | -0.184 | 0.854 |
| IL-4 (pg/mL) | 3.5 (1.5-5.7) | 2.1 (1.1-3.6) | -1.102 | 0.270 |
| IL-6 (pg/mL) | 48.4 (10.9-96.4) | 113.0 (50.5-139.9) | -1.607 | 0.108 |
| IL-10 (pg/mL) | 13.4 (8.0-31.9) | 6.0 (4.2-9.2) | -2.143 | 0.032 |
| TNF-α (pg/mL) | 1.7 (0.7-5.6) | 1.1 (0.2-2.1) | -0.950 | 0.342 |
| IFN-γ (pg/mL) | 138.3 (19.0-322.9) | 3.7 (1.8-5.6) | -3.062 | 0.002 |
| HB,hemoglobin;CRP,C-reactive protein;PT,prothrombin time;APTT,activated partial thromboplastin time;INR,international normalized ratio. | ||||
2.3 sJIA合并MAS患者实验室指标的ROC曲线分析
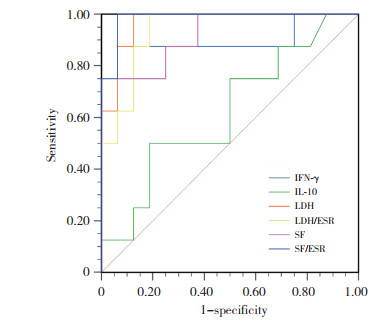
选择2组比较有统计学差异(P < 0.05) 且排除MAS诊断标准(铁蛋白) 的实验室指标进行ROC曲线分析,结果显示,SF/ESR、LDH、LDH/ ESR、SF的曲线下面积(area under curve,AUC) 分别为0.992、0.988、0.974、0.964,表明SF/ESR、LDH、LDH/ ESR及SF对sJIA合并MAS诊断具有较高价值。细胞因子中IFN-γ、IL-10的AUC分别为0.891、0.773,灵敏度分别为0.875、0.750,特异度分别为0.937、0.812,可见也是sJIA合并MAS的重要诊断指标。见表 3、图 1。
| Item | AUC | P | Cut-off | Sensitivity | specificity | YI | 95%CI |
| SF/ESR | 0.992 | < 0.001 | 65.23 | 1.000 | 0.944 | 0.944 | 0.976-1.000 |
| LDH (U/L) | 0.988 | < 0.001 | 448.50 | 1.000 | 0.917 | 0.917 | 0.966-1.000 |
| LDH/ ESR | 0.974 | < 0.001 | 5.22 | 1.000 | 0.889 | 0.889 | 0.938-1.000 |
| SF (ng/mL) | 0.964 | < 0.001 | 6 900.00 | 0.857 | 1.000 | 0.857 | 0.911-1.000 |
| IFN-γ (pg/mL) | 0.891 | 0.002 | 12.32 | 0.875 | 0.937 | 0.812 | 0.714-1.000 |
| IL-10 (pg/mL) | 0.773 | 0.032 | 10.13 | 0.750 | 0.812 | 0.562 | 0.575-0.972 |
| ESR (mm/h) | 0.681 | 0.049 | 44.50 | 0.944 | 0.500 | 0.444 | 0.488-0.873 |
|
| 图 1 sJIA-MAS组实验室指标的ROC曲线 Fig.1 Receiver operating characteristic curve of laboratory indexes of patients in the sJIA-MAS group |
3 讨论
sJIA主要临床表现有驰张热、皮疹、关节炎和全身性炎症。约7%~13%sJIA患儿合并MAS [8]。目前认为MAS的发病与遗传缺陷、背景炎症、感染触发均有关,即“疾病阈值模型”。一些遗传损伤可能与某些风湿性疾病特有的炎症状态、诱因相结合,共同驱使巨噬细胞和T细胞过度激活[9]。固有免疫系统如单核/巨噬细胞、中性粒细胞和自然杀伤细胞(NK细胞) 在MAS的启动中起着核心作用[10],MAS患者NK细胞功能减低及(或) 穿孔素蛋白表达减少,对CD8+T细胞抑制减低,淋巴细胞与其靶抗原提呈细胞持续相互作用,T细胞和巨噬细胞持续扩张,炎症反应持续活化。
国外学者[11]对sJIA患者遗传背景研究发现,超过30%患者有pHLH单等位基因突变(PRF1、UNC13D、STX11、STXBP2和RAB27A)。此外,MAS患者中UNC13D、STXBP2和LYST罕见蛋白变异的频率(36%) 高于非MAS患者(14%) [12]。VASTERT等[13]认为pHLH单基因杂合变异(主要为错义突变) 对穿孔素介导的溶细胞功能有部分或全部显性负效应,可能是MAS的危险因素,PRF1杂合变异对sJIA患儿发生MAS起一定作用。对于pHLH相关基因杂合变异是否导致蛋白功能部分缺失,对风湿性疾病发生MAS易感性的影响有待多中心大样本的研究。
本研究中sJIA-MAS组患者肝功能损伤是突出表现,78.6%患儿合并肝功能损害,发生率显著高于sJIA组(P < 0.05)。sJIA-MAS组中ALT、AST、LDH水平显著高于sJIA组,与MAS引起的肝损害有关,是细胞裂解死亡的信号。sJIA-MAS组中1例患儿以黄疸首发起病,肝功能损害表现早,临床上需与重症肝炎、药物性肝损伤、自身免疫性肝炎、嗜肝病毒感染、肝衰竭等相鉴别。本研究sJIA-MAS组中患儿肝脾大、肝功能损害、中枢神经系统症状、骨髓嗜血现象、PICU入住发生率均较sJIA组增高(均P < 0.05)。因此认为当sJIA患者出现肝和(或)脾大、肝功能损害、中枢神经系统症状、骨髓嗜血现象时,需警惕MAS发生。而sJIA-MAS组胸膜炎(35.7%)、胸腔积液(35.7%)、心包积液(0%)、腹腔积液(7.1%) 比例较小,浆膜炎表现2组比较无统计学差异(P > 0.05);而sJIA本身也可以出现浆膜炎表现,故不能鉴别MAS的发生。
本研究结果显示,sJIA-MAS组多项实验室指标异常,SF升高(100%) 最常见,是MAS的高度敏感和特异性指标。本研究中sJIA-MAS组SF显著高于sJIA组,中位值为12 986.7 μg /L,显著高于2004年HPS诊断标准[7]中的阈值(500 μg/L),并且sJIA组SF也明显升高(685.0 μg/L)。可见SF及SF/ESR可作为预测sJIA合并MAS的重要指标,且SF/ESR可能比SF的预测价值更大。ZOU等[14]发现ALB降低、AST、LDH、SF及SF/ESR比值升高可能预测sJIA合并MAS发生,其中SF≥12 217.5 μg/L、SF/ESR≥267.5对sJIA合并MAS诊断的灵敏度(0.800、0.905) 和特异度(0.882、0.867) 均高。GORELIK等[8]提出SF/ESR比值可能优于SF,SF/ESR > 80可有效区分MAS和sJIA,灵敏度和特异度均达100%。GUO等[3]研究得出LDH > 596.0 U/L预测sJIA合并MAS的灵敏度和特异度分别为84.0%、88.4%。本研究中SF/ESR≥65.23、SF≥6 900.00 μg/L的灵敏度为1.000、0.857,特异度为0.944、1.000,且SF/ESR (0.992) 比SF (0.964) 具有更大的AUC,与以往研究结果类似。本研究中获得的截断值更低,可能与样本量及实验室检测误差有关。KOSTIK等[15]通过研究发现LDH≥882 U/L诊断sJIA合并MAS的灵敏度为75%,特异度为100%,本研究还发现LDH≥448.50 U/L、LDH/ ESR≥5.224对于预测sJIA合并MAS的灵敏度(1.000、1.000) 和特异度(0.917、0.889) 也较高。
IL-1、IL-6、IL-10、IL-18、IFN-γ等细胞因子和炎症小体的激活在MAS的发病中起主要作用[16],MAS中高水平IL-6 (sJIA的高炎症状态) 与遗传背景的特殊病理生理状态可能影响对TLR配体的反应强度,导致感染触发后的高炎症反应。IL-10产生缺陷和IFN-γ过度产生在MAS的发病机制中起重要作用。IL-10是一种抑炎性细胞因子,负向调节IFN-γ,在sJIA患者中,血浆IL-10水平较低,sJIA患者CD19+ B细胞在体外刺激后显示IL-10产生减少[17]。IL-10产生不足也可能与MAS有关。IL-10基因多态性与IL-10活性降低相关[18]。反复刺激TLR9后小鼠出现了MAS样表现,TLR/IL-1β信号过度活跃与IL-10功能降低,两者共同导致的免疫学损伤作用下引起MAS易感性增加[19-20]。
IFN-γ是调控MAS病理生理状态的主要细胞因子之一。CD8+T细胞分泌IFN-γ,继而激活巨噬细胞。有研究[19-20]报道MAS患者的新蝶呤水平升高,提示IFN-γ的激活在MAS发病中起一定作用。此外,抗IFN-γ抗体治疗原发性噬血细胞淋巴组织细胞增多症和MAS的疗效已取得初步证实。本研究中与sJIA组相比,sJIA-MAS组IL-10、IFN-γ水平均显著升高(均P < 0.05);2组IL-6水平均较正常值明显升高,但差异无统计学意义,与GUO等[3]及姜丽娇等[21]研究结果相同,提示sJIA患儿在IL-6升高的基础上,出现IL-10和IFN-γ明显升高可能是MAS发生的预警信号。本研究结果显示,IFN-γ≥12.32 pg/mL、IL-10≥10.13 pg/mL时可区分sJIA疾病活动与sJIA合并MAS。
sJIA-MAS组WBC、NY、L、PLT、Fib均较sJIA组明显下降,但中位数或均值均在正常范围内,血红蛋白均值略下降。由于sJIA本身炎症活动WBC、PLT及Fib升高,而合并MAS时这些指标可能“相对下降”而呈现出正常范围,与RAVELLI等[2]提出的理论相同。由Fib消耗造成的ESR急剧下降对MAS发生也是有用的提示。本研究sJIA-MAS组中的ESR中位数为48.5 mm/h,与sJIA组比较显著下降(P < 0.05),但中位数仍高于正常值上限。因此,当sJIA患者疾病活动时出现了WBC、PLT、ESR、FIB指标处于正常范围,应需警惕MAS的发生。
综上所述,sJIA较其他风湿免疫性疾病更易发生MAS,也常以MAS为首发表现,对于确诊或疑诊sJIA患者出现肝和(或) 脾大、肝功能损害、中枢神经系统症状、骨髓嗜血现象时,需高度警惕MAS发生。SF、SF/ESR、LDH、LDH/ESR、IL-10及IFN-γ均是早期诊断MAS的生物学标志物。随着对MAS的深入研究,近年来发现IL-18、CXCL9、新蝶呤和sTNFRⅡ等生物标志物可能有助于预测MAS的发生发展[22-24],因此有待更多的临床及基础研究进一步论证。
| [1] |
PETTY RE, SOUTHWOOD TR, MANNERS P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001[J]. J Rheumatol, 2004, 31(2): 390-392. |
| [2] |
RAVELLI A, MINOIA F, DAVÌ S, et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European league against rheumatism/American college of rheumatology/paediatric rheumatology international trials organisation collaborative initiative[J]. Arthritis Rheumatol, 2016, 68(3): 566-576. DOI:10.1002/art.39332 |
| [3] |
GUO L, XU Y, QIAN X, et al. Sudden hypotension and increased serum interferon-γ and interleukin-10 predict early macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis[J]. J Pediatr, 2021, 235: 203-211. DOI:10.1016/j.jpeds.2021.02.008 |
| [4] |
BRACAGLIA C, DE GRAAF K, PIRES MARAFON D, et al. Eleva-ted circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis[J]. Ann Rheum Dis, 2017, 76(1): 166-172. DOI:10.1136/annrheumdis-2015-209020 |
| [5] |
TAKAKURA M, SHIMIZU M, IRABU H, et al. Comparison of serum biomarkers for the diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis[J]. Clin Immunol, 2019, 208: 108252. DOI:10.1016/j.clim.2019.108252 |
| [6] |
KIM KH, KIM DS. Juvenile idiopathic arthritis: diagnosis and differen-tial diagnosis[J]. Korean J Pediatr, 2010, 53(11): 931-935. DOI:10.3345/kjp.2010.53.11.931 |
| [7] |
HENTER JI, HORNE A, ARICÓ M, et al. HLH-2004:diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis[J]. Pediatr Blood Cancer, 2007, 48(2): 124-131. DOI:10.1002/pbc.21039 |
| [8] |
GORELIK M, FALL N, ALTAYE M, et al. Follistatin-like protein 1 and the ferritin/erythrocyte sedimentation rate ratio are potential biomarkers for dysregulated gene expression and macrophage activation syndrome in systemic juvenile idiopathic arthritis[J]. J Rheumatol, 2013, 40(7): 1191-1199. DOI:10.3899/jrheum.121131 |
| [9] |
STRIPPOLI R, CAIELLO I, DE BENEDETTI F. Reaching the thresho-ld: a multilayer pathogenesis of macrophage activation syndrome[J]. J Rheumatol, 2013, 40(6): 761-767. DOI:10.3899/jrheum.121233 |
| [10] |
RAVELLI A, GROM AA, BEHRENS EM, et al. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment[J]. Genes Immun, 2012, 13(4): 289-298. DOI:10.1038/gene.2012.3 |
| [11] |
BRACAGLIA C, SIENI E, DA ROS M, et al. Mutations of familial hemophagocytic lymphohistiocytosis (FHL) related genes and abnormalities of cytotoxicity function tests in patients with macrophage activation syndrome (MAS) occurring in systemic juvenile idiopathic arthritis (sJIA)[J]. Pediatr Rheumatol Online J, 2014, 12(suppl 1): P53. DOI:10.1186/1546-0096-12-s1-p53 |
| [12] |
KAUFMAN KM, LINGHU BL, SZUSTAKOWSKI JD, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis[J]. Arthritis Rheumatol, 2014, 66(12): 3486-3495. DOI:10.1002/art.38793 |
| [13] |
VASTERT SJ, VAN WIJK R, D'URBANO LE, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis[J]. Rheumatology (Oxford), 2010, 49(3): 441-449. DOI:10.1093/rheumatology/kep418 |
| [14] |
ZOU LX, ZHU Y, SUN L, et al. Clinical and laboratory features, treatment, and outcomes of macrophage activation syndrome in 80 children: a multi-center study in China[J]. World J Pediatr, 2020, 16(1): 89-98. DOI:10.1007/s12519-019-00256-0 |
| [15] |
KOSTIK MM, DUBKO MF, MASALOVA VV, et al. Identification of the best cutoff points and clinical signs specific for early recognition of macrophage activation syndrome in active systemic juvenile idiopathic arthritis[J]. Semin Arthritis Rheum, 2015, 44(4): 417-422. DOI:10.1016/j.semarthrit.2014.09.004 |
| [16] |
MELLINS ED, MACAUBAS C, GROM AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions[J]. Nat Rev Rheumatol, 2011, 7(7): 416-426. DOI:10.1038/nrrheum.2011.68 |
| [17] |
IMBRECHTS M, AVAU A, VANDENHAUTE J, et al. Insufficient IL-10 production as a mechanism underlying the pathogenesis of systemic juvenile idiopathic arthritis[J]. J Immunol, 2018, 201(9): 2654-2663. DOI:10.4049/jimmunol.1800468 |
| [18] |
HERSH AO, PRAHALAD S. Genetics of juvenile idiopathic arthritis[J]. Rheum Dis Clin North Am, 2017, 43(3): 435-448. DOI:10.1016/j.rdc.2017.04.007 |
| [19] |
LOCATELLI F, JORDAN MB, ALLEN C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis[J]. N Engl J Med, 2020, 382(19): 1811-1822. DOI:10.1056/NEJMoa1911326 |
| [20] |
GABR JB, LIU E, MIAN S, et al. Successful treatment of secondary macrophage activation syndrome with emapalumab in a patient with newly diagnosed adult-onset Still's disease: case report and review of the literature[J]. Ann Transl Med, 2020, 8(14): 887. DOI:10.21037/atm-20-3127 |
| [21] |
姜丽娇, 陈玲玲, 张梅娟. 细胞因子失衡在全身型幼年特发性关节炎合并巨噬细胞活化综合征中的意义[J]. 中国医师进修杂志, 2021, 44(11): 987-990. DOI:10.3760/cma.j.cn115455-20200310-00263 |
| [22] |
WEISS ES, GIRARD-GUYONVARC'H C, HOLZINGER D, et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome[J]. Blood, 2018, 131(13): 1442-1455. DOI:10.1182/blood-2017-12-820852 |
| [23] |
PASCARELLA A, BRACAGLIA C, CAIELLO I, et al. Monocytes from patients with macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis are hyperresponsive to interferon gamma[J]. Front Immunol, 2021, 12: 663329. DOI:10.3389/fimmu.2021.663329 |
| [24] |
MIZUTA M, SHIMIZU M, IRABU H, et al. Comparison of serum cytokine profiles in macrophage activation syndrome complicating different background rheumatic diseases in children[J]. Rheumatolo-gy (Oxford), 2021, 60(1): 231-238. DOI:10.1093/rheumatology/keaa299 |
 2023, Vol. 52
2023, Vol. 52