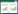文章信息
- 冯子轩, 栾军军, 周华
- FENG Zixuan, LUAN Junjun, ZHOU Hua
- RNA甲基化在非肿瘤肾脏疾病中的研究进展
- Progress in research on RNA methylation in kidney diseases
- 中国医科大学学报, 2023, 52(3): 263-268
- Journal of China Medical University, 2023, 52(3): 263-268
-
文章历史
- 收稿日期:2021-10-29
- 网络出版时间:2023-03-16 09:18:48
RNA修饰种类丰富,在编码RNA和非编码RNA中约有170余种[1]。目前,虽然已知mRNA甲基化N6-甲基腺嘌呤(N6-methyladenosine,m6A)、C5-甲基胞嘧啶(C5-methylcytosine,m5C)等修饰形式的存在,但对其如何产生功能尚不清楚[2]。
近年来,随着高通量测序技术的发展,甲基化研究快速进展,发现了多种能影响RNA修饰的相关蛋白[3]。研究[4]发现,RNA甲基化通过改变细胞生物学功能影响癌症的进展。在非肿瘤肾脏疾病领域内,这些修饰同样也扮演重要角色,参与了肾脏疾病的发生和发展。本文从最常见的mRNA甲基化m6A、m5C等修饰入手,对其在非肿瘤肾脏疾病领域中发挥的作用进行综述。
1 m6A RNA甲基化m6A修饰是在RNA腺嘌呤上第6个氮原子上进行甲基化修饰,是目前为止真核生物中最丰富和研究最多的mRNA修饰[5]。随着甲基化酶“writer”(如METTL3-METTL4复合体)、去甲基化酶“eraser”(如FTO、ALKBH5),以及结合蛋白“reader”(YTH结构域蛋白、METTL3、IGF2BP2)等的发现,证明了m6A是一种动态修饰过程,影响包括mRNA的稳定、核输出、翻译和剪接等,参与疾病的发生和发展[6]。在非肿瘤肾脏疾病领域内,m6A甲基化修饰同样扮演着重要角色,参与了肾脏疾病的发生和发展(表 1)。
| Disease | Materials | Enzymes | Mechanism |
| Acute kidney disease | Cisplatin-induced AKI mice | METTL3/14↑ | p53↑ |
| Cisplatin-treated HK-2 cells | FTO↓ | apoptosis↑ | |
| I/R-induced AKI mice | METTL3↑ | foxd1 mRNA↑ | |
| H/R-treated NRE-52K cells | |||
| I/R-induced AKI mice | METTL14↑ | YAP1↓ | |
| H/R-treated HK-2 cells | |||
| Chronic kidney disease | UUO-induced CKD mice | METTL3/METTL14↓FTO↑ | - |
| UUO-induced CKD mice | ALKBH5↓ | Snail↑ | |
| TGF-β-treated HK-2 cells | EMT↑ | ||
| obstructive nephropathy patients’ renal biopsies | METLL3/METTL14/WTAP↑ | lncRNA MALAT1↑→miR145/FAK | |
| UUO-induced CKD mice | |||
| TGF-β-treated HK-2 cells | |||
| CKD patients’ peripheral blood mononuclear cells | - | m6A↑ | |
| Diabetic kidney disease | HG-treated MPC5 cells | METTL3↓ | PTEN↑→PI3K/Akt Pyroptosis↑ |
| HG-treated HK-2 cells | METTL14↓ | PTEN↑→PI3K/Akt→HDAC5↑ | |
| STZ-induced DKD mice | EMT↑ | ||
| Drug-induced kidney injury | Colistin-treated mTECs | METTL3↓ | DCGR8→miR-873-5p↓→Keap↑/Nrf2↓ Regulate oxidative stress |
| Alcohol-induced kidney injury | Alcohol-treated mice | FTO↓ | PPAR-α↓→NLRP3↑ |
| Alcohol-treated HK-2 cells | YTHDF2↑ | ||
| Autosomal dominant polycystic kidney disease | ADPKD transgenesis mice | METTL3↑ | c-myc/cAMP |
| mIMCD3,Pkd1RC/-,and Pkd1RC/+ cells | cyst growth |
1.1 m6A RNA甲基化在急性肾损伤(acute kidney injury,AKI)中的作用
AKI是常见的临床综合征,能导致其他器官功能衰竭,死亡率高达50%~80%,每年导致全球170万人死亡[7]。AKI的特征是肾功能短时间内突然降低至正常值以下,同时伴随尿量减少,血肌酐、血尿素氮水平显著升高和水电解质酸碱平衡紊乱[8]。AKI的发病机制仍不完全清楚,缺少有效的治疗方法。近年的研究发现,m6A RNA甲基化修饰参与了AKI的发病机制。在顺铂诱导的AKI模型中,去甲基化酶FTO表达降低,导致m6A修饰增加。在顺铂处理的HK-2细胞中,通过甲氯芬酸抑制FTO,可引起p53表达增加,促进凋亡,加重AKI [9]。在缺血再灌注模型中,甲基转移酶METTL3表达增加,从而增高 foxd1 mRNA m6A甲基化水平[10]。在肾脏缺血再灌注模型的研究[11]中发现,增加的METTL14通过升高YAP1 mRNA m6A甲基化水平,抑制YAP1表达,从而促进肾脏损伤。
1.2 m6A RNA甲基化在慢性肾脏病(chronic kidney disease,CKD)中的作用CKD是十大疾病死亡原因之一,在我国发病率为10.8%[12]。越来越多的CKD患者逐渐发展成为终末期肾脏病(end-stage renal disease,ESRD),需要肾脏替代治疗。肾脏间质纤维化导致细胞外基质的沉积和瘢痕形成是CKD的重要病理特征[13]。研究[14]发现,m6A参与肾间质纤维化形成,如在单侧输尿管梗阻(unilateral ureteral obstruction,UUO)模型中,m6A甲基化水平以及相关酶METTL3、METTL14的表达水平随着模型时间进程逐渐下降,FTO表达逐渐升高。另一项关于UUO模型的研究[13]证明,m6A去甲基化酶ALKBH5降低,导致m6A水平升高,增加间质标志物Snail表达,从而促进了上皮-间质转化。在TGF-β处理的人肾小管上皮细胞中,m6A表达水平升高,甲基转移酶METTL3、METLL14、WTAP水平升高,METTL3能调控MALAT1表达,参与MALAT1/miR-145/FAK介导的肾脏纤维化过程[15]。CKD患者更易发生血管钙化,并发心血管疾病的概率更高,最终导致最严重的CKD并发症,增加了CKD的死亡率[16]。RNA m6A甲基化不仅参与CKD的发病,同时也参与了CKD并发症心血管疾病的发生[17]。研究[18]发现,在吲哚硫酸盐诱导的人平滑肌细胞血管钙化模型中,血管保护基因Klotho mRNA m6A甲基化水平升高,且该改变受METTL14调节,最终使Klotho表达下降。还有研究[19]发现,在CKD患者外周血中性粒细胞中,RNA m6A甲基化水平下降。RNA m6A甲基化通过多种不同途径,影响不同基因的表达,参与CKD的发病机制,以上研究结果提供了靶向RNA m6A甲基化治疗CKD的一个新策略。
1.3 m6A RNA甲基化在其他非肿瘤肾脏疾病中的作用糖尿病肾病(diabetic kidney disease,DKD)是糖尿病最常见的微血管并发症之一。近半数糖尿病患者可能出现肾脏损害[20-21]。DKD主要表现为肾小球硬化、肾小管间质纤维化和肾血管损伤[22]。足细胞是肾小球中的重要细胞类型,在维持肾小球滤过屏障正常功能的过程中扮演重要角色,足细胞的损伤被认为参与DKD的发生。在高糖处理的小鼠足细胞中发现足细胞焦亡参与DKD的发展,黄蜀葵提取物能够通过增加METTL3表达,提高m6A甲基化水平,降低PTEN表达,调节PI3K/Akt通路,改善足细胞焦亡和损伤[23]。早期的DKD以肾小球病变为主,而随着病情发展肾小管也受累,肾小管发生纤维化,最终发展成为ESRD,造成这种后果的病因中也包括m6A甲基化。在高糖处理的HK-2细胞中,METTL14表达下降,同时m6A甲基化水平降低,PTEN表达增加,影响PI3K/Akt信号通路,HDAC5表达上调,引起肾小管细胞上皮间质转化,参与DKD进展[24]。提示在相同疾病影响的不同细胞亚型中,不同的甲基转移酶能影响相同基因表达改变。
除了DKD,一些产生肾毒性的药物和毒物同样可通过m6A甲基化影响肾脏疾病的发展进程。在鼠肾小管上皮细胞中,甲基转移酶METTL3能够以m6A依赖性方式与DGCR8结合,上调miRNA-873-5p,调节Keap1/Nrf2通路,减轻黏菌素介导的肾损伤[25]。在乙醇介导的肾损伤中,去甲基化酶FTO表达降低,导致PPAR-α m6A甲基化水平升高,同时YTHDF2表达增加,识别m6A位点,下调PPAR-α,进而活化NLRP3炎性小体,加重肾损伤[26]。
m6A RNA甲基化作为一种表观遗传学机制,也参与遗传性肾病的发生发展。有研究[27]发现,METTL3能通过增加促囊肿形成的c-myc和Avpr2 mRNA m6A修饰,激活c-myc和cAMP通路,参与常染色体显性遗传性多囊肾的发生发展。
2 m5C RNA甲基化m5C是在RNA胞嘧啶第5位碳原子上进行甲基化修饰,是最早被发现存在于多个物种的修饰之一。m5C可控制mRNA出核[28],调控翻译,影响蛋白质合成[29]。在m5C发挥作用的过程中,也会发生动态的修饰过程,该过程受甲基转移酶“writer”、去甲基酶“eraser”、结合蛋白“reader”等多种酶调控。“writer”包括NSUN1-7、DNMT2、TRM4A/B,“eraser”主要包括TET2、ALKBH1,“reader”包括ALYREF、YBX1。见图 1。

|
| 图 1 RNA m5C相关酶 Fig.1 RMA m5C related enzymes |
2.1 m5C RNA甲基转移酶
与m5C相关的甲基转移酶包括DNA甲基转移酶同系物DNMT2(又名TRDMT1)以及NSUN家族的7个成员(NSUN1,NSUN2,NSUN3,NSUN4,NSUN5,NSUN6和NSUN7)[30]。以上酶中,目前只有NSUN2和DNMT2参与mRNA的代谢过程[31]。NSUN2是NOP2/SUN结构域家族第2个成员,是核仁甲基转移酶,在某些肿瘤中高表达,通过抑制NSUN2表达,抑制胆囊癌细胞的增殖[32]。NSUN2介导的m5C能促进膀胱尿路上皮癌发生[33]。这些过程都与NSUN2可作为m5C的甲基转移酶密切相关。但NSUN2在肾脏病领域的作用依然未知。TRDMT1最早被认为是DNA的甲基转移酶,后来发现TRDMT1对DNA的作用很小,而对tRNA有甲基化酶的作用[34]。最近的研究[31]发现,在HEK293T细胞中敲低TRDMT1能降低UGP2 mRNA m5C水平。
2.2 m5C RNA去甲基化酶TET2是依赖于α-酮戊二酸和Fe2+的双加氧酶[35],能够作为m5C去甲基化酶将DNA的m5C催化成为hm5C。TETs也能作用于HEK293T细胞中RNA的m5C,过表达的TETs能够增加RNA hm5C水平[36]。TET2催化产生的RNA hm5C参与RNA的降解,提示了其在转录后调控的作用[37]。TET2与肾脏疾病有关,在缺血再灌注诱导的AKI模型中,TET2敲除可加重肾损伤[38],盐酸多柔比星诱导的局灶节段性肾小球硬化模型中TET2表达降低[39]。然而,在高糖诱导的足细胞模型以及DKN动物模型中TET2表达升高[40]。这些不同的结果可能与TET2既能作用于DNA m5C修饰,也能作用于RNA m5C修饰,以及作用的靶基因不同等原因有关。TET2在肾脏疾病中的作用仍需进一步研究探讨。
2.3 m5C RNA甲基化结合蛋白目前发现的RNA m5C结合蛋白有ALYREF和YBX1。ALYREF是mRNA输出蛋白复合体TREX的关键成分[41]。ALYREF受其靶标mRNA甲基化水平的影响,能够在核内与靶基因的m5C位点结合,促进mRNA出核[28]。在Hela细胞中敲除ALYREF后发现,具有m5C甲基化的mRNA滞留在核内,而缺少m5C修饰的mRNA未发现有出核现象[28]。此外,近期研究[42]发现,在膀胱癌中,ALYREF能够以m5C依赖的方式与PKM2 mRNA结合,稳定mRNA,促进糖酵解,最终导致肿瘤细胞增殖。
与ALYREF不同的是,YBX1常在细胞质内发挥其潜在的m5C结合蛋白功能。YBX1是一种DNA/RNA结合蛋白,由多种结构域组成,能够在细胞核内作为转录因子与目标基因启动子Y盒结合,还能通过其冷休克结构域(cold shock domain,CSD)在细胞质结合RNA,同时也存在于细胞外。YBX1通过其众多的角色参与肿瘤细胞的增殖和侵袭[43]、药物耐受[44]、DNA修复[45],以及RNA剪接、转录、翻译和细胞外信号应激反应等生物学过程[46]。YBX1在肾脏疾病中同样发挥重要作用。纤维化是肾脏疾病常见病理表现,YBX1参与纤维化相关因子的调节,如YBX1在系膜细胞中通过增加COLA1 mRNA稳定性促进翻译,介导促纤维化作用[47],在肾小管上皮细胞中通过与TGF-β1 mRNA 5’-UTR结合,促进TGF-β1翻译,参与慢性肾脏疾病的发生发展[48]。然而,当YBX1存在于细胞核中,则能通过抑制COL1a1、α-SMA/Acta2转录[49],促进MMP2转录,发挥抗纤维化的作用,这些均与YBX1能够作为转录因子发挥作用有关[50]。有研究认为,在正常情况下,肾小管的YBX1通常在细胞质表达,肾小球的YBX1主要在细胞核表达。YBX1的作用主要取决于其亚细胞定位,在细胞质中,YBX1具有致纤维化作用,在细胞核中,则具有抗纤维化作用。研究[51-52]发现,通过药物强制增加YBX1细胞核内水平,减少异位,可减轻UUO肾脏模型的纤维化程度。YBX1除参与纤维化的调节,也是调节炎症信号的重要枢纽,YBX1在肝、肾损伤中可抑制Cxcl1表达,减轻肾脏损害[53]。内源性细胞的YBX1参与炎症细胞的浸润,单核-巨噬细胞YBX1通过产生白细胞介素-10(interlukin-10,IL-10)等抗炎因子调节炎症过程[54]。同时,在狼疮肾炎中,也发现了新的YBX1修饰形式,即胍基化的YBX1通过Notch3增加IL-10 mRNA的转录,发挥抗炎作用[55]。此外,在CKD中,YBX1被发现作为转录因子参与细胞周期的调节[56]。综上,YBX1在肾脏疾病中扮演着多种角色,在细胞核内作为转录因子发挥作用,而在细胞质内则能调节翻译。最近的研究[33]发现,YBX1可做为m5C的“reader”发挥作用,YBX1通过靶向结合癌基因HDGF mRNA m5C位点,并募集mRNA稳定因子ELAVL1,促进膀胱癌的增殖和转移。细胞质中的YBX1是否是通过甲基化依赖的方式增加mRNA稳定性,促进翻译过程,需要进一步考证。
3 结论及展望RNA甲基化修饰参与多种癌症的发生、发展,而在非肿瘤疾病中究竟扮演何种角色仍有待更多的研究。m6A RNA甲基化可能在非肿瘤肾脏疾病的发病机制中发挥重要功能,而m5C、N1-甲基腺嘌呤(N1-methyladenosine,m1A)、N7-甲基鸟嘌呤(N7-methylguanosine,m7G)等众多RNA修饰的作用尚有待发掘。RNA甲基化修饰是否也能同长链非编码RNA一样成为疾病诊断的标志物以及治疗的靶点,是研究者未来需要攻克的难题。
| [1] |
BOCCALETTO P, MACHNICKA MA, PURTA E, et al. MODOMICS: a database of RNA modification pathways. 2017 update[J]. Nucleic Acids Res, 2018, 46(D1): D303-D307. DOI:10.1093/nar/gkx1030 |
| [2] |
CHEN X, SUN YZ, LIU H, et al. RNA methylation and diseases: experimental results, databases, Web servers and computational mo-dels[J]. Brief Bioinform, 2019, 20(3): 896-917. DOI:10.1093/bib/bbx142 |
| [3] |
SHI HL, WEI JB, HE C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers[J]. Mol Cell, 2019, 74(4): 640-650. DOI:10.1016/j.molcel.2019.04.025 |
| [4] |
MA S, CHEN C, JI X, et al. The interplay between m6A RNA methy-lation and noncoding RNA in cancer[J]. J Hematol Oncol, 2019, 12(1): 121. DOI:10.1186/s13045-019-0805-7 |
| [5] |
SUN T, WU RY, MING L. The role of m6A RNA methylation in cancer[J]. Biomed Pharmacother, 2019, 112: 108613. DOI:10.1016/j.biopha.2019.108613 |
| [6] |
PAN YT, MA P, LIU Y, et al. Multiple functions of m6A RNA methylation in cancer[J]. J Hematol Oncol, 2018, 11(1): 48. DOI:10.1186/s13045-018-0590-8 |
| [7] |
BOUCHARD J, ACHARYA A, CERDA J, et al. A prospective international multicenter study of AKI in the intensive care unit[J]. Clin J Am Soc Nephrol, 2015, 10(8): 1324-1331. DOI:10.2215/CJN.04360514 |
| [8] |
KELLUM JA, et al. The definition of acute kidney injury[J]. Lancet, 2018, 391(10117): 202-203. DOI:10.1016/S0140-6736(17)31630-6 |
| [9] |
ZHOU PH, WU M, YE CY, et al. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m6A abrogation in RNA[J]. J Biol Chem, 2019, 294(45): 16908-16917. DOI:10.1074/jbc.RA119.011009 |
| [10] |
MENG FH, LIU YG, CHEN QY, et al. METTL3 contributes to renal ischemia-reperfusion injury by regulating Foxd1 methylation[J]. Am J Physiol Renal Physiol, 2020, 319(5): F839-F847. DOI:10.1152/ajprenal.00222.2020 |
| [11] |
XU Y, YUAN XD, WU JJ, et al. The N6-methyladenosine mRNA methylase METTL14 promotes renal ischemic reperfusion injury via suppressing YAP1[J]. J Cell Biochem, 2020, 121(1): 524-533. DOI:10.1002/jcb.29258 |
| [12] |
ZHANG LX, WANG F, WANG L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey[J]. Lancet, 2012, 379(9818): 815-822. DOI:10.1016/S0140-6736(12)60033-6 |
| [13] |
NING YC, CHEN J, SHI YQ, et al. Genistein ameliorates renal fibrosis through regulation snail via m6A RNA demethylase ALKBH5[J]. Front Pharmacol, 2020, 11: 579265. DOI:10.3389/fphar.2020.579265 |
| [14] |
LI XY, FAN X, YIN XM, et al. Alteration of N6-methyladenosine epitranscriptome profile in unilateral ureteral obstructive nephropathy[J]. Epigenomics, 2020, 12(14): 1157-1173. DOI:10.2217/epi-2020-0126 |
| [15] |
LIU PH, ZHANG B, CHEN Z, et al. m6A-induced lncRNA MALAT1 aggravates renal fibrogenesis in obstructive nephropathy through the miR-145/FAK pathway[J]. Aging, 2020, 12(6): 5280-5299. DOI:10.18632/aging.102950 |
| [16] |
REISS AB, MIYAWAKI N, MOON J, et al. CKD, arterial calcification, atherosclerosis and bone health: inter-relationships and controversies[J]. Atherosclerosis, 2018, 278: 49-59. DOI:10.1016/j.atherosclerosis.2018.08.046 |
| [17] |
HUNG SC, KUO KL, WU CC, et al. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease[J]. J Am Heart Assoc, 2017, 6(2): e005022. DOI:10.1161/JAHA.116.005022 |
| [18] |
CHEN J, NING YC, ZHANG H, et al. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate[J]. Life Sci, 2019, 239: 117034. DOI:10.1016/j.lfs.2019.117034 |
| [19] |
WANG CY, LIN TA, HO MY, et al. Regulation of autophagy in leukocytes through RNA N6-adenosine methylation in chronic kidney disease patients[J]. Biochem Biophys Res Commun, 2020, 527(4): 953-959. DOI:10.1016/j.bbrc.2020.04.138 |
| [20] |
PARVING HH, LEWIS JB, RAVID M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of typeⅡdiabetic patients: a global perspective[J]. Kidney Int, 2006, 69(11): 2057-2063. DOI:10.1038/sj.ki.5000377 |
| [21] |
THOMAS MC, BROWNLEE M, SUSZTAK K, et al. Diabetic kidney disease[J]. Nat Rev Dis Primers, 2015, 1: 15018. DOI:10.1038/nrdp.2015.18 |
| [22] |
ALICIC RZ, ROONEY MT, TUTTLE KR. Diabetic kidney disease: challenges, progress, and possibilities[J]. Clin J Am Soc Nephrol, 2017, 12(12): 2032-2045. DOI:10.2215/CJN.11491116 |
| [23] |
LIU BH, TU Y, NI GX, et al. Total flavones of Abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-dependent m6A modification-mediated NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling[J]. Front Pharmacol, 2021, 12: 667644. DOI:10.3389/fphar.2021.667644 |
| [24] |
XU ZX, JIA KQ, WANG H, et al. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease[J]. Cell Death Dis, 2021, 12(1): 32. DOI:10.1038/s41419-020-03312-0 |
| [25] |
WANG J, ISHFAQ M, XU L, et al. METTL3/m6A/miRNA-873-5p attenuated oxidative stress and apoptosis in colistin-induced kidney injury by modulating Keap1/Nrf2 pathway[J]. Front Pharmacol, 2019, 10: 517. DOI:10.3389/fphar.2019.00517 |
| [26] |
YU JT, HU XW, CHEN HY, et al. DNA methylation of FTO promotes renal inflammation by enhancing m6A of PPAR-α in alcohol-induced kidney injury[J]. Pharmacol Res, 2021, 163: 105286. DOI:10.1016/j.phrs.2020.105286 |
| [27] |
RAMALINGAM H, KASHYAP S, COBO-STARK P, et al. A methionine-Mettl3-N6-methyladenosine axis promotes polycystic kidney disease[J]. Cell Metab, 2021, 33(6): 1234-1247. DOI:10.1016/j.cmet.2021.03.024 |
| [28] |
YANG X, YANG Y, SUN BF, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m5C reader[J]. Cell Res, 2017, 27(5): 606-625. DOI:10.1038/cr.2017.55 |
| [29] |
WANG N, TANG H, WANG X, et al. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes[J]. Biochem Biophys Res Commun, 2017, 493(1): 94-99. DOI:10.1016/j.bbrc.2017.09.069 |
| [30] |
BOHNSACK K, HÖBARTNER C, BOHNSACK M. Eukaryotic 5-methylcytosine (m5C) RNA methyltransferases: mechanisms, cellular functions, and links to disease[J]. Genes, 2019, 10(2): 102. DOI:10.3390/genes10020102 |
| [31] |
XUE SL, XU H, SUN Z, et al. Depletion of TRDMT1 affects 5-methylcytosine modification of mRNA and inhibits HEK293 cell proliferation and migration[J]. Biochem Biophys Res Commun, 2019, 520(1): 60-66. DOI:10.1016/j.bbrc.2019.09.098 |
| [32] |
GAO Y, WANG Z, ZHU YD, et al. NOP2/Sun RNA methyltransferase 2 promotes tumor progression via its interacting partner RPL6 in gallbladder carcinoma[J]. Cancer Sci, 2019, 110(11): 3510-3519. DOI:10.1111/cas.14190 |
| [33] |
CHEN X, LI A, SUN BF, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs[J]. Nat Cell Biol, 2019, 21(8): 978-990. DOI:10.1038/s41556-019-0361-y |
| [34] |
GOLL MG, KIRPEKAR F, MAGGERT KA, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2[J]. Science, 2006, 311(5759): 395-398. DOI:10.1126/science.1120976 |
| [35] |
CIMMINO L, DOLGALEV I, WANG YB, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression[J]. Cell, 2017, 170(6): 1079-1095. DOI:10.1016/j.cell.2017.07.032 |
| [36] |
FU LJ, GUERRERO CR, ZHONG N, et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA[J]. J Am Chem Soc, 2014, 136(33): 11582-11585. DOI:10.1021/ja505305z |
| [37] |
GUALLAR D, BI XJ, PARDAVILA JA, et al. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells[J]. Nat Genet, 2018, 50(3): 443-451. DOI:10.1038/s41588-018-0060-9 |
| [38] |
YAN H, TAN L, LIU YQ, et al. Ten-eleven translocation methyl-cytosine dioxygenase 2 deficiency exacerbates renal ischemia-reperfusion injury[J]. Clin Epigenetics, 2020, 12(1): 98. DOI:10.1186/s13148-020-00892-8 |
| [39] |
WAN F, TANG YW, TANG XL, et al. TET2 mediated demethylation is involved in the protective effect of triptolide on podocytes[J]. Am J Transl Res, 2021, 13(3): 1233-1244. |
| [40] |
YANG LL, ZHANG Q, WU Q, et al. Effect of TET2 on the pathogenesis of diabetic nephropathy through activation of transforming growth factor β1 expression via DNA demethylation[J]. Life Sci, 2018, 207: 127-137. DOI:10.1016/j.lfs.2018.04.044 |
| [41] |
SHI M, ZHANG H, WU XD, et al. ALYREF mainly binds to the 5' and the 3' regions of the mRNA in vivo[J]. Nucleic Acids Res, 2017, 45(16): 9640-9653. DOI:10.1093/nar/gkx597 |
| [42] |
WANG JZ, ZHU W, HAN J, et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer[J]. Cancer Commun (Lond), 2021, 41(7): 560-575. DOI:10.1002/cac2.12158 |
| [43] |
ZHAO XL, ZHAO ZH, XU WC, et al. Circ-SAR1A promotes renal cell carcinoma progression through miR-382/YBX1 axis[J]. Cancer Manag Res, 2020, 12: 7353-7361. DOI:10.2147/CMAR.S245918 |
| [44] |
SHIBATA T, WATARI K, KAWAHARA A, et al. Targeting phosphorylation of Y-box-binding protein YBX1 by TAS0612 and everolimus in overcoming antiestrogen resistance[J]. Mol Cancer Ther, 2020, 19(3): 882-894. DOI:10.1158/1535-7163.MCT-19-0690 |
| [45] |
KIM ER, SELYUTINA AA, BULDAKOV IA, et al. The proteolytic YB-1 fragment interacts with DNA repair machinery and enhances survival during DNA damaging stress[J]. Cell Cycle, 2013, 12(24): 3791-3803. DOI:10.4161/cc.26670 |
| [46] |
BATES M, BOLAND A, MCDERMOTT N, et al. YB-1:the key to personalised prostate cancer management?[J]. Cancer Lett, 2020, 490: 66-75. DOI:10.1016/j.canlet.2020.07.006 |
| [47] |
HANSSEN L, FRYE BC, OSTENDORF T, et al. Y-box binding protein-1 mediates profibrotic effects of calcineurin inhibitors in the kidney[J]. J Immunol, 2011, 187(1): 298-308. DOI:10.4049/jimmunol.1100382 |
| [48] |
FRASER DJ, PHILLIPS AO, ZHANG X, et al. Y-box protein-1 controls transforming growth factor-beta1 translation in proximal tubular cells[J]. Kidney Int, 2008, 73(6): 724-732. DOI:10.1038/sj.ki.5002719 |
| [49] |
ZHANG AW, LIU XY, COGAN JG, et al. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta1 and thrombin during differentiation of human pulmonary myofibroblasts[J]. Mol Biol Cell, 2005, 16(10): 4931-4940. DOI:10.1091/mbc.e05-03-0216 |
| [50] |
MERTENS PR, HARENDZA S, POLLOCK AS, et al. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1[J]. J Biol Chem, 1997, 272(36): 22905-22912. DOI:10.1074/jbc.272.36.22905 |
| [51] |
WANG JL, GIBBERT L, DJUDJAJ S, et al. Therapeutic nuclear shuttling of YB-1 reduces renal damage and fibrosis[J]. Kidney Int, 2016, 90(6): 1226-1237. DOI:10.1016/j.kint.2016.07.008 |
| [52] |
GIBBERT L, HERMERT D, WANG JL, et al. YB-1 increases glomerular, but decreases interstitial fibrosis in CNI-induced nephropathy[J]. Clin Immunol, 2018, 194: 67-74. DOI:10.1016/j.clim.2018.07.002 |
| [53] |
HERMERT D, MARTIN IV, REISS LK, et al. The nucleic acid binding protein YB-1-controlled expression of CXCL-1 modulates kidney damage in liver fibrosis[J]. Kidney Int, 2020, 97(4): 741-752. DOI:10.1016/j.kint.2019.10.024 |
| [54] |
BERNHARDT A, FEHR A, BRANDT S, et al. Inflammatory cell infiltration and resolution of kidney inflammation is orchestrated by the cold-shock protein Y-box binding protein-1[J]. Kidney Int, 2017, 92(5): 1157-1177. DOI:10.1016/j.kint.2017.03.035 |
| [55] |
BREITKOPF DM, JANKOWSKI V, OHL K, et al. The YB-1:Notch-3 axis modulates immune cell responses and organ damage in systemic lupus erythematosus[J]. Kidney Int, 2020, 97(2): 289-303. DOI:10.1016/j.kint.2019.09.031 |
| [56] |
WANG L, ZHU N, JIA JS, et al. Trimethylamine N-oxide mediated Y-box binding protein-1 nuclear translocation promotes cell cycle progression by directly downregulating Gadd45a expression in a cellular model of chronic kidney disease[J]. Life Sci, 2021, 271: 119173. DOI:10.1016/j.lfs.2021.119173 |
 2023, Vol. 52
2023, Vol. 52