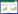文章信息
- 赵蔓嘉, 范世光, 赵爱农, 梁再赋, 傅炜昕
- ZHAO Manjia, FAN Shiguang, ZHAO Ainong, LIANG Zaifu, FU Weixin
- 神经肽P物质通过Ca2+和CREB诱导自然杀伤细胞的活化作用
- The neuropeptide substance P activates natural killer cells through Ca2+ and CREB
- 中国医科大学学报, 2022, 51(6): 502-507
- Journal of China Medical University, 2022, 51(6): 502-507
-
文章历史
- 收稿日期:2021-06-23
- 网络出版时间:2022-06-01 17:34
P物质(substance P,SP) 最初被认为是感觉神经递质,广泛分布于中枢和外周神经系统,可参与机体多种生理病理过程,包括疼痛感知,免疫细胞增殖、趋化及炎症等,并在心血管、呼吸和消化系统中发挥作用[1-3]。许多免疫细胞也可产生SP,SP在免疫系统中以旁分泌和自分泌的形式调节免疫细胞功能,如促进免疫细胞增殖和分化,影响免疫球蛋白的合成及细胞因子分泌等[4-5]。SP还通过调节趋化因子和黏附分子的表达,对免疫细胞的迁移发挥重要调节作用[5-6]。SP的生物学功能由3种受体NK-1R、NK-2R、NK-3R介导,其中NK-1R与SP的亲和力最高,故NK-1R为主要介导者[7-8]。研究[9]表明,NK-1R信号转导通路是T细胞活化的最佳Ca2+通量所必需的。CD4和CD8 T细胞都表达功能性NK-1R,在同源T细胞活化过程中自分泌/旁分泌的SP激活NK-1R信号转导通路,从而增加T细胞受体(T cell receptor,TCR) 信号转导后的Ca2+通量,这种作用对于后续的启动白细胞介素-2 (interleukin-2,IL-2) 合成、T细胞存活以及辅助性T (helper T,Th) 细胞1和Th17细胞极化的下游信号通路是必需的。
自然杀伤(natural killer,NK) 细胞是固有免疫的主要承担者,在机体早期抗感染、抗肿瘤免疫中发挥重要作用[10-11]。NK细胞识别靶细胞后激活,受控于自身共表达的活化性受体和抑制性受体的动态信号平衡[11-13]。研究[14-15]证明,SP对NK细胞功能具有调节作用,SP可刺激NK细胞迁移及细胞毒活性[6, 15]。另有研究[16]发现,某些病理状态下,循环SP浓度升高可损伤NK细胞功能,抑制NK细胞杀伤活性。说明SP在不同条件下对NK细胞的作用不同,提示SP对NK细胞的调控作用具有复杂性和多样性,SP主要通过NK-1R调节NK细胞功能[16-17],但确切的信号通路及机制还不十分明确。本研究选用NK92-MI细胞作为研究体系,体外研究SP对NK92-MI细胞增殖、杀伤活性的影响,并探讨SP受体NK-1R在SP调控NK细胞活性中的作用及可能的信号分子机制。
1 材料与方法 1.1 材料NK92-MI细胞为非IL-2依赖的NK92细胞株,购自中科院上海细胞库。SP购自美国Sigma公司。抗NK-1R单克隆抗体、抗环磷酸腺苷反应元件结合蛋白(cyclic adenosine monophosphate response element binding protein,CREB)/p-CREB单克隆抗体购自美国abcam公司。FITC标记的二抗购自北京鼎国昌盛生物技术有限公司。
1.2 方法 1.2.1 细胞培养用含12.5%胎牛血清和12.5%马血清的α-MEM培养基(不含RNA和DNA),在37 ℃、5%CO2培养箱中传代培养NK92-MI细胞。
1.2.2 MTT释放法检测SP对NK92-MI细胞增殖的影响NK92-MI细胞(1×105/mL) 接种于96孔培养板(100 µL/孔),加入10-12 mol/L的SP (100 µL/孔),于37 ℃、5%CO2培养箱中孵育;分别于孵育24、48和72 h加入MTT液,继续孵育4 h;加入DMSO和甘氨酸缓冲液溶解甲臜结晶,用酶标仪于570 nm处测定吸光度(optical density,OD) 值。用特异性NK-1R拮抗剂[D-Arg1,D-Phe5,D-Trp7,D-Trp9,Leu11] SP (10-7 mol/L) 预处理NK92-MI细胞30 min,再用10-12 mol/L SP作用48 h,MTT法检测NK92-MI细胞的增殖活性。
1.2.3 MTT释放法检测SP对NK92-MI杀伤活性的影响(1) 将4×105/mL的NK92-MI细胞作为效应细胞接种至96孔培养板(100 µL /孔),加入10-12 mol/L SP (100 µL /孔) 作用24 h;将1×105/mL的K562细胞作为靶细胞,接种至实验孔(100 µL /孔),使效靶细胞比为4∶1;效靶细胞共同孵育4 h后,加入MTT液孵育4 h;加入DMSO和甘氨酸缓冲液,振荡15~20 min后,用酶标仪测定570 nm OD值。计算NK-92MI细胞对K562细胞的杀伤率,杀伤率(%) = [1-(杀伤实验组OD值-效应细胞对照组OD值) /靶细胞对照组OD值]×100。(2) 用特异性NK-1R拮抗剂预处理NK92-MI细胞30 min,再用10-12 mol/L SP作用24 h,MTT法检测NK92-MI细胞对K562细胞的杀伤活性。
1.2.4 荧光定量PCR采用TRIzol法提取NK92-MI细胞总RNA,经37 ℃、15 min反转录,反应体系10 μL [5×Prime ScriptTM Buffer 4.0 μL,Prime ScriptTM Enzyme MixⅠ 0.5 μL,50 μmol/L Oligo dT primer 0.5 μL,100 μmol/L random 6 mers 0.5 μL,total RNA (﹤500 ng) 1.0 μL,RNase Free dH2O 3.5 μL]。PCR反应为两步法,95 ℃30 s预变性;95 ℃5 s,60 ℃34 s,40个循环。PCR反应体系20 μL [SYBY Primix Ex TaqTM (2×) 10 μL,PCR上下游引物(10 μmol/L) 各0.4 μL,ROX Reference Dye Ⅱ (50×) 0.4 μL,模版cDNA 2.0 μL,dH2O 6.8 μL]。用Primer Premier 5.0软件设计NK-1R引物,由金思特科技有限公司(南京) 合成。引物序列,正向5’-tccactaacacctcggaacc-3’,反向5’-acaggccgtagtaccattgg-3’。应用ABI PRISM 7500 Real-Time PCR System进行检测,以18SrRNA作为参照基因,用2-ΔΔCt法比较实验组相对表达量与对照组的倍数差异[18],ΔΔCt =实验组ΔCt-对照组ΔCt。
1.2.5 流式细胞术检测NK-1R的膜表达用10-12 mol/L SP处理NK-92MI细胞24 h,收集、洗涤细胞,加入无标记的抗NK-1R单克隆抗体,4 ℃孵育40 min;洗涤细胞后,再加入FITC标记的二抗,4 ℃继续孵育40 min,洗涤细胞后应用FACScan流式细胞仪进行检测分析。
1.2.6 Fura-2/AM荧光探针法检测NK92-MI细胞胞质钙浓度用10-12 mol/L SP处理NK-92MI细胞1 h,收集、洗涤细胞,加入Fura-2/AM/DMSO液(终浓度为5 μmol/L),37 ℃避光振荡孵育30 min;洗涤细胞后于25 ℃放置30 min,使Fura-2/AM完全去酯化;上机测定荧光强度F,加入10%Triton X-100 (10 μL),测定饱和Ca2+溶液的荧光强度Fmax;各管加入EGTA 10 μL,测定零Ca2+溶液的荧光强度Fmin。按下列双波长探针测定的计算公式计算胞质Ca2+浓度,钙含量[Ca2+] = Kd× (R-Rmin) / (R-Rmax)×F2min/F2max,R=Fλ1/ Fλ2,Rmin = F1min/F2min,Rmax= F1max/F2max。Fλ1和Fλ2分别为在波长λ1与λ2时的荧光强度;F1min和F2min分别为零Ca2+溶液在波长λ1与λ2时的荧光强度;F1max和F2max分别为饱和Ca2+溶液在波长λ1与λ2时的荧光强度。
1.2.7 Western blotting测定CREB磷酸化水平用10-12 mol/L SP作用NK-92MI细胞1 h,收集1×107个细胞,提取细胞总蛋白并定量,行聚丙烯酰胺凝胶电泳。室温、50 V (100 mA) 转印PVDF膜3 h。转印膜漂洗后,加入封闭液(10%牛奶) 于4 ℃摇床封闭过夜。加入抗CREB/p-CREB单克隆抗体(1∶1 000稀释),室温孵育2.5 h;漂洗后,加入二抗(1∶2 000稀释) 孵育1 h。以β-actin作为内参照。用ECL试剂显影、检测。
1.3 统计学分析采用SPSS 13.0软件进行统计学分析。数据以x±s表示,组间比较采用单因素方差分析。P < 0.05为差异有统计学意义。
2 结果 2.1 SP促进NK92-MI细胞增殖活性MTT结果显示,10-12 mol/L SP可促进NK92-MI细胞的增殖活性,且作用48 h促增殖活性最强(图 1A)。NK-1R拮抗剂可完全阻断SP的促增殖活性(图 1B)。提示SP通过NK-1R发挥其对NK细胞的促增殖活性。
|
| A, NK92-MI cells were treated by SP at concentrations of 10-12 mol/L for 24, 48 and 72 h, NK92-MI cell viability (OD value) was measured using MTT test; B, effects of NK1R antagonist on SP increases proliferation of NK92-MI cells. * P < 0.05 vs control. 图 1 SP对NK-92MI细胞增殖活性的影响 Fig.1 Effects of SP on NK-92MI cell proliferation |
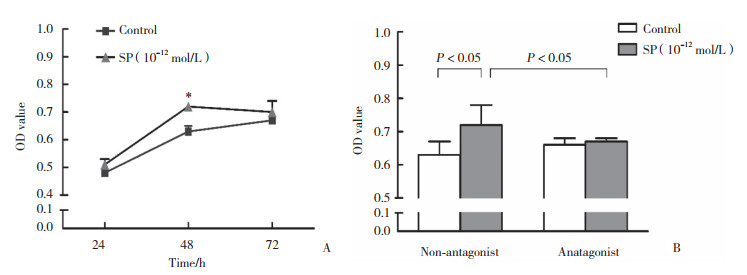
2.2 SP对NK92-MI细胞杀伤活性的影响
MTT结果显示,经10-12 mol/L SP作用24 h后,NK92-MI细胞对K562细胞的杀伤活性(效靶细胞比为4∶1) 明显增强(P < 0.01),且NK-1R拮抗剂可大部分阻断SP的增强作用(图 2)。
|
| 图 2 SP对NK92-MI细胞杀伤活性的影响 Fig.2 Effects of SP on NK-92MI cell killing activity |
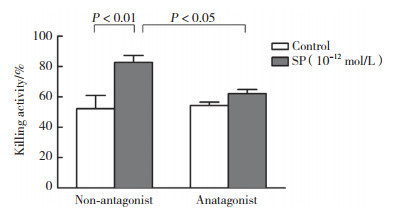
2.3 SP启动NK-1R受体及Ca2+信号通路
荧光定量PCR结果显示,10-12 mol/L SP作用1 h即可促进NK92-MI细胞的NK-1R mRNA表达,作用4 h使NK-1R mRNA表达上调至最高水平;至24 h时,NK-1R mRNA表达水平回落至正常(图 3A)。10-12 mol/L SP作用1~4 h,NK92-MI细胞的NK-1R膜表达无明显变化,作用至24 h,NK-1R的膜表达明显增加(图 3B、3D)。同时发现,SP作用1 h即可引起NK92-MI细胞胞质Ca2+浓度明显升高,而作用至24 h,Ca2+浓度回落至基础水平(图 3C)。上述结果表明,SP通过增加NK-1R的表达及激活Ca2+信号通路,发挥对NK92-MI细胞活性的调节作用。
|
| A, mRNA expression of NK-1R in NK92-MI cells; B, expression of NK-1R on NK92-MI cells treated by SP for 24 h; C, concentrations of intracellular Ca2+ in NK92-MI cells treated by SP for 1 h. **P < 0.01, * P < 0.05 vs control. 图 3 SP对NK-1R及Ca2+信号通路的作用 Fig.3 Effects of SP on NK-1R and Ca2+ signaling pathway |
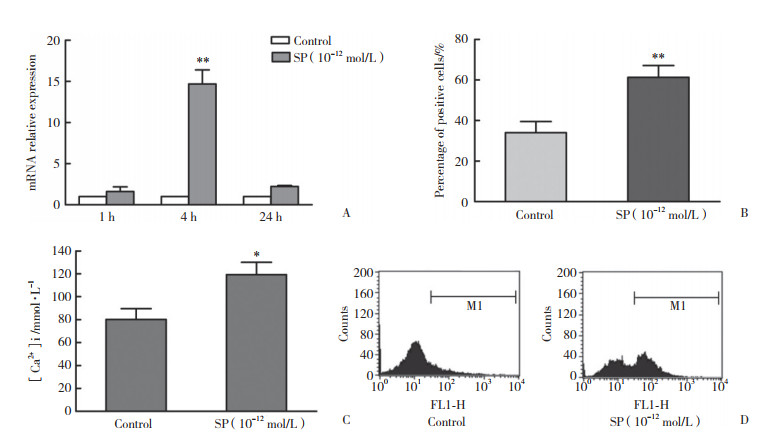
|
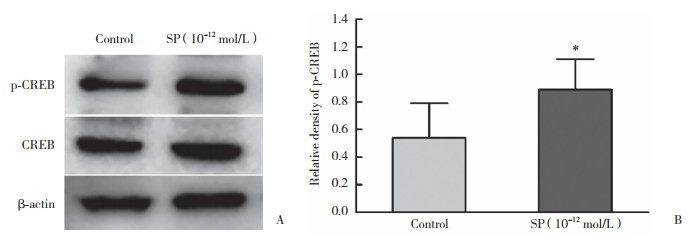
| A,expressions of CREB and p-CREB were detected by Western blotting;B,relative quantification of p-CREB. * compared with control,P < 0.05. 图 4 SP诱导NK-92MI细胞CREB磷酸化 Fig.4 SP induces CREB phosphorylation in NK-92MI cells |
2.4 SP诱导NK92-MI细胞CREB磷酸化
选择NK-1R mRNA表达水平和Ca2+浓度均增高的时间点,检测CREB的磷酸化状态。结果显示,未受SP作用的NK92-MI细胞p-CREB水平较低,10-12 mol/L SP作用1 h后p-CREB的水平明显增高,表明SP可诱导CREB磷酸化而活化CREB。
3 讨论本研究发现,SP可有效促进NK92-MI细胞的增殖和杀伤活性,且该促进作用可被SP受体(NK-1R) 拮抗剂完全阻断和大部分阻断。另一方面,SP可诱导NK92-MI细胞NK-1R的表达增加,说明SP可通过NK-1R的表达来介导其对NK92-MI细胞活性的促进作用。
NK-1R广泛分布于神经系统和免疫系统,且在免疫系统中主要参与介导调节功能[6, 19]。NK-1R为G蛋白耦联受体(G protein-coupled receptors,GPCRs),与高亲和性配体相互作用,通过不同的G蛋白产生不同的第二信使而发挥作用。主要有2条下游信号转导途径,环磷酸腺苷(cyclic adenosine monophosphate,cAMP) 信号途径和磷脂酰肌醇信号途径。NK-1R与Gαq蛋白作用诱导磷酯酶C活化,导致胞内三磷酸肌醇(inositol triphosphate,IP3) 和甘油二酯迅速产生,并增加胞质Ca2+作为第二信使[9, 11, 20]。cAMP则被与NK-1R结合的Gαs蛋白激活[5, 11]。NK-1R与SP结合活化可激活几种第二信使,包括钙(Ca2+)、IP3、蛋白激酶C、促分裂原活化蛋白激酶和转录因子核因子κB及CREB等[20-21]。SP与NK-1R结合通过Gαs激活腺苷环化酶,使cAMP水平升高,而激活依赖于cAMP的蛋白激酶A (protein kinase A,PAK);活化的PAK进入细胞核内,使转录因子CREB磷酸化,从而具有转录活性,启动下游基因的转录,完成信号转导途径[21-22]。
前期研究[17]结果显示,SP通过NK-1R介导胞质Gαs的上调和Cαi信号通路参与了其增强NK92-MI细胞杀伤活性及对杀伤介质穿孔素和颗粒酶B表达的促进作用。本研究结果表明,SP可促进NK92-MI细胞胞质Ca2+浓度迅速升高,提示Ca2+依赖的信号通路启动了NK-1R,或部分参与了NK-1R介导的SP活化NK92-MI细胞的信号转导。
本研究结果显示,SP通过增加CREB的磷酸化而活化CREB。CREB是一种碱性亮氨酸拉链转录因子,可由细胞外调节蛋白激酶、Ca2+和应急刺激等信号通过Ser133位点的磷酸化来激活,继而选择性活化一系列下游基因[23-24]。前期研究数据表明,SP可在转录水平调节NK细胞的活性,即上调杀伤介质穿孔素、颗粒酶B以及受体NCRs、NKG2D和NKG2A的mRNA水平。
综上所述,本研究发现cAMP通路参与了NK-1R介导的SP对NK92-MI细胞的活化,且Ca2+和CREB是关键信号分子,这些结果有助于更好地了解SP调控NK细胞功能的作用机制。
| [1] |
GRAEFE S, MOHIUDDIN SS. Biochemistry, substance P[M]. Treasure Island (FL): StatPearls Publishing, 2022.
|
| [2] |
MISTROVA E, KRUZLIAK P, CHOTTOVA DVORAKOVA M. Role of substance P in the cardiovascular system[J]. Neuropeptides, 2016, 58: 41-51. DOI:10.1016/j.npep.2015.12.005 |
| [3] |
WEINSTOCK JV. Substance P and the regulation of inflammation in infections and inflammatory bowel disease[J]. Acta Physiol (Oxf), 2015, 213(2): 453-461. DOI:10.1111/apha.12428 |
| [4] |
SUVAS S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis[J]. J Immunol, 2017, 199(5): 1543-1552. DOI:10.4049/jimmunol.1601751 |
| [5] |
MASHAGHI A, MARMALIDOU A, TEHRANI M, et al. Neuropeptide substance P and the immune response[J]. Cell Mol Life Sci, 2016, 73(22): 4249-4264. DOI:10.1007/s00018-016-2293-z |
| [6] |
LANG K, DRELL TL, NIGGEMANN B, et al. Neurotransmitters regu-late the migration and cytotoxicity in natural killer cells[J]. Immunol Lett, 2003, 90(2/3): 165-172. DOI:10.1016/j.imlet.2003.09.004 |
| [7] |
PENNEFATHER JN, LECCI A, CANDENAS ML, et al. Tachykinins and tachykinin receptors: a growing family[J]. Life Sci, 2004, 74(12): 1445-1463. DOI:10.1016/j.lfs.2003.09.039 |
| [8] |
DOUGLAS SD, LEEMAN SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation[J]. Ann N Y Acad Sci, 2011, 1217: 83-95. DOI:10.1111/j.1749-6632.2010.05826.x |
| [9] |
MORELLI AE, SUMPTER TL, ROJAS-CANALES DM, et al. Neurokinin-1 receptor signaling is required for efficient Ca2+ flux in T-cell-receptor-activated T cells[J]. Cell Rep, 2020, 30(10): 3448-3465. DOI:10.1016/j.celrep.2020.02.054 |
| [10] |
ABEL AM, YANG C, THAKAR MS, et al. Natural killer cells: development, maturation, and clinical utilization[J]. Front Immunol, 2018, 9: 1869. DOI:10.3389/fimmu.2018.01869 |
| [11] |
MEZA GUZMAN LG, KEATING N, NICHOLSON SE. Natural killer cells: tumor surveillance and signaling[J]. Cancers, 2020, 12(4): 952. DOI:10.3390/cancers12040952 |
| [12] |
SIVORI S, OLIVE D, LÓPEZ-BOTET M, et al. NK receptors: tools for a polyvalent cell family[J]. Front Immunol, 2014, 5: 617. DOI:10.3389/fimmu.2014.00617 |
| [13] |
MARRAS F, BOZZANO F, DE MARIA A. Involvement of activating NK cell receptors and their modulation in pathogen immunity[J]. J Biomed Biotechnol, 2011, 2011: 152430. DOI:10.1155/2011/152430 |
| [14] |
FU WX, QIN B, ZHOU AP, et al. Regulation of NK92-MI cell cytotoxicity by substance P[J]. Scand J Immunol, 2011, 74(2): 107-113. DOI:10.1111/j.1365-3083.2011.02550.x |
| [15] |
FEISTRITZER C, CLAUSEN J, STURN DH, et al. Natural killer cell functions mediated by the neuropeptide substance P[J]. Regul Pept, 2003, 116(1/3): 119-126. DOI:10.1016/s0167-0115(03)00193-9 |
| [16] |
MONACO-SHAWVER L, SCHWARTZ L, TULUC F, et al. Substance P inhibits natural killer cell cytotoxicity through the neurokinin-1 receptor[J]. J Leukoc Biol, 2011, 89(1): 113-125. DOI:10.1189/jlb.0410200 |
| [17] |
HOU DD, SUN KF, FU WX, et al. The role of Gαs in activation of NK92-MI cells by neuropeptide substance P[J]. Neuropeptides, 2014, 48(1): 1-5. DOI:10.1016/j.npep.2013.12.001 |
| [18] |
LIVAK KJ, SCHMITTGEN TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-delta delta C (T)) method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [19] |
TULUC F, LAI JP, KILPATRICK LE, et al. Neurokinin 1 receptor isoforms and the control of innate immunity[J]. Trends Immunol, 2009, 30(6): 271-276. DOI:10.1016/j.it.2009.03.006 |
| [20] |
ROELSE M, DE RUIJTER NCA, VROUWE EX, et al. A generic microfluidic biosensor of G protein-coupled receptor activation-monitoring cytoplasmic[Ca (2+) ] changes in human HEK293 cells[J]. Biosens Bioelectron, 2013, 47: 436-444. DOI:10.1016/j.bios.2013.03.065 |
| [21] |
ZACCOLO M, ZERIO A, LOBO MJ. Subcellular organization of the cAMP signaling pathway[J]. Pharmacol Rev, 2021, 73(1): 278-309. DOI:10.1124/pharmrev.120.000086 |
| [22] |
LIU SB, LI Y, KIM S, et al. Phosphodiesterases coordinate cAMP propagation induced by two stimulatory G protein-coupled receptors in hearts[J]. Proc Natl Acad Sci USA, 2012, 109(17): 6578-6583. DOI:10.1073/pnas.1117862109 |
| [23] |
ROSETHORNE EM, NAHORSKI SR, CHALLISS RAJ. Regulation of cyclic AMP response-element binding-protein (CREB) by Gq/11-protein-coupled receptors in human SH-SY5Y neuroblastoma cells[J]. Biochem Pharmacol, 2008, 75(4): 942-955. DOI:10.1016/j.bcp.2007.10.015 |
| [24] |
TAN YW, ZHANG SJ, HOFFMANN T, et al. Increasing levels of wild-type CREB up-regulates several activity-regulated inhibitor of death (AID) genes and promotes neuronal survival[J]. BMC Neurosci, 2012, 13: 48. DOI:10.1186/1471-2202-13-48 |
 2022, Vol. 51
2022, Vol. 51