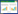文章信息
- 武俊波, 马亮, 许楠, 张慧生, 杜可仆, 乔英, 赵军
- WU Junbo, MA Liang, XU Nan, ZHANG Huisheng, DU Kepu, QIAO Ying, ZHAO Jun
- 原发性纵隔支气管囊肿、胸腺囊肿与心包囊肿CT影像学特征比较
- Comparison of CT imaging features of primary mediastinal bronchial, thymic, and pericardial cysts
- 中国医科大学学报, 2022, 51(2): 163-168
- Journal of China Medical University, 2022, 51(2): 163-168
-
文章历史
- 收稿日期:2021-07-20
- 网络出版时间:2021-12-30 19:03
2. 郑州大学第一附属医院放射与核医学科, 郑州 450003;
3. 山西医科大学附属第一医院放射科, 太原 030001
2. Department of Radiation and Nuclear Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450003, China;
3. Department of Radiation, The First Affiliated Hospital of Shanxi Medical University, Taiyuan 030001, China
纵隔空间狭小且内含气管、食管、心脏及大血管等重要脏器,当纵隔内肿瘤压迫或侵犯周围脏器时会对机体产生严重影响,因此早期诊断纵隔肿瘤具有重要意义。原发性支气管、胸腺及心包囊肿约占纵隔先天性囊肿的90%[1],其临床症状隐匿且无特异性,诊断往往依赖影像学检查。随着胸部CT检查的普及,原发性支气管囊肿、胸腺囊肿及心包囊肿检出率逐年增高[2]。本研究对原发性支气管囊肿、胸腺囊肿及心包囊肿的影像学特征进行比较,并与临床症状的相关性进行分析,旨在为临床上尽早诊断、治疗提供依据。
1 材料与方法 1.1 研究对象收集山西医科大学附属长治市人民医院及郑州大学第一附属医院2015年1月至2020年12月经手术病理证实且临床、影像资料完整的原发性支气管囊肿、胸腺囊肿及心包囊肿患者的临床资料。纳入标准:(1)临床及CT检查资料完整,包括平扫、增强图像资料;(2)所有患者均经手术病理确诊。排除标准:(1)病历资料不完整;(2)病理诊断无法明确;(3)患有基础疾病。
本研究共纳入74例。男38例,女36例;年龄5~87岁,平均年龄(44.84±15.67)岁。有临床症状的患者18例,其中胸痛6例,呼吸困难4例,胸闷3例,咳嗽2例,干咳1例,发热1例,心悸1例,病史4 d~5年。体检发现(无临床症状)53例。所有患者均经手术治疗,术后病理结果显示支气管囊肿28例,胸腺囊肿21例,心包囊肿25例。
1.2 CT检查使用西门子128层螺旋CT扫描仪,管电压120 kV,管电流100 mA,螺距0.6,转速0.5 s,重建层厚0.75 mm,间隔0.5 mm。扫描范围从膈面以下至胸廓入口处,扫描层厚和层间距均为5 mm,先行平扫,经肘静脉穿刺,再高压注射器注入非离子型对比剂碘海醇(350 mg/mL),流率3~4 mL/s,延迟30 s行动脉期扫描,延迟60 s行静脉期扫描。食管钡餐造影检查3例(支气管囊肿2例、胸腺囊肿1例)。心脏彩超检查6例(胸腺囊肿2例,心包囊肿3例,支气管囊肿1例)。
1.3 图像分析所得影像资料均由2位具有副高级职称医师分析判断,内容包括:病变部位、大小(最大径、最小径)、密度、分隔、钙化等,最终结果为2位医师测量结果的平均值,根据囊肿最大径进行分组[3],分为最大径≥5 cm组和最大径 < 5 cm组。
1.4 统计学分析采用SPSS 22.0软件进行统计学处理。符合正态分布计量资料采用x±s表示,组间比较采用方差分析,同组不同处理比较采用配对t检验;计数资料采用率(%)表示,组间比较采用χ2检验,对于理论频数 < 1或样本量 < 40采用Fisher精确概率法。P < 0.05为差异有统计学意义。
2 结果 2.1 支气管囊肿、胸腺囊肿、心包囊肿患者临床资料比较结果显示,支气管囊肿、胸腺囊肿、心包囊肿患者年龄及性别比较差异无统计学意义(F = 1.341,P = 0.268;F = 0.705,P = 0.405),囊肿所处纵隔位置差异有统计学意义(F = 29.815,P < 0.001),囊肿囊壁有无钙化差异有统计学意义(F = 26.228,P = 0.036),而囊肿囊内有无分隔无统计学差异(F = 5.360,P = 0.171),见表 1。
| Item | Bronchogenic cyst group(n = 28) | Thymic cyst group(n = 21) | Pericardial cyst group(n = 25) |
| Age(year) | 41.29±14.47 | 48.48±17.80 | 45.76±14.84 |
| Sex [n (%)] | |||
| Male | 15(53.6) | 12(57.1) | 11(44.0) |
| Female | 13(46.4) | 9(42.9) | 14(56.0) |
| Location(mediastinum)[n (%)] | |||
| Anterior | 6(21.4) | 16(76.2) | 4(16.0) |
| Medial | 18(64.3) | 2(9.5) | 21(84.0) |
| Posterior | 4(14.3) | 3(14.3) | 0(0) |
| Calcification of cyst wall [n (%)] | |||
| Exist | 6(21.4) | 3(14.3) | 0(0) |
| None | 22(78.6) | 18(85.7) | 25(100) |
| Division of cyst [n (%)] | |||
| Exist | 1(3.6) | 3(14.3) | 5(20.0) |
| None | 27(96.4) | 18(85.7) | 20(80.0) |
支气管囊肿患者典型CT检查结果可见囊肿位于左后纵隔,大小约1.8 cm×1.6 cm,平扫病灶CT值约19.4 Hu,增强扫描无强化。病理结果显示,肉眼可见灰白灰黄样组织,大小约3.5 cm×2.0 cm ×0.5 cm,内壁厚约0.1~0.2 cm,内壁灰白、灰红稍粗糙。镜下可见囊肿被覆支气管腺上皮细胞,细胞呈单层立方状,其下可见平滑肌,见图 1。
|
| A, plain scan; B, enhanced scan; C, pathological results (HE×10). 图 1 支气管囊肿患者典型CT图像及病理结果 Fig.1 CT imaging and pathological results of bronchogenic cysts |
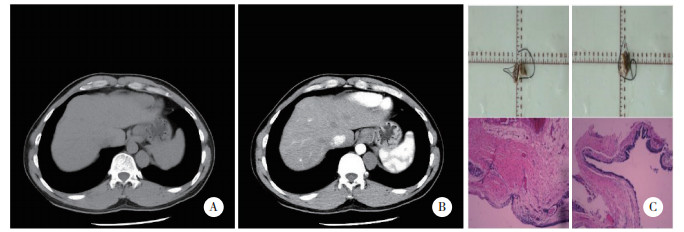
胸腺囊肿患者典型CT检查结果可见囊肿位于右前纵隔,大小约2.0 cm×1.3 cm,平扫CT值约45.2 Hu,增强扫描无强化。病理结果显示,肉眼可见灰黄组织,大小约5.5 cm×3.5 cm×2.1 cm,切开可见大量豆腐渣样物。镜下可见纤维囊壁组织,内衬单层上皮,部分区域见胸腺小体,见图 2。
|
| A, plain scan; B, enhanced scan; C, pathological results (HE×10). 图 2 胸腺囊肿患者典型CT图像及病理结果 Fig.2 CT imaging and pathological results of thymic cysts |
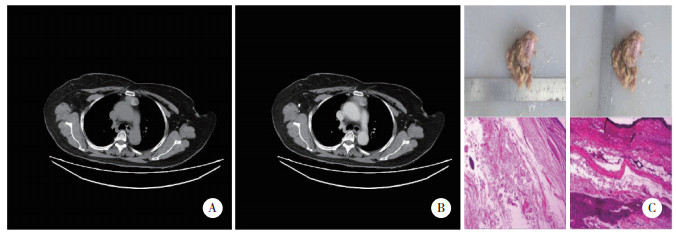
心包囊肿患者典型CT检查结果可见囊肿位于右中纵隔,大小约2.5 cm×1.9 cm,平扫CT值约18.2 Hu,增强扫描无强化。病理结果显示,肉眼可见灰白淡黄组织,其内可见囊性肿物,大小约4 cm×4 cm×3.5 cm,切开内为清亮液体。镜下可见囊肿壁被覆单层间皮细胞,外层为疏松结缔组织。见图 3。
|
| A, plain scan; B, enhanced scan; C, pathological results (HE×10). 图 3 胸腺囊肿患者典型CT图像及病理结果 Fig.3 CT imaging and pathological results of pericardial cyst |
2.2 支气管囊肿、胸腺囊肿、心包囊肿患者囊肿直径、CT值比较
结果显示,支气管囊肿、胸腺囊肿、心包囊肿患者囊肿最大径、最小径及其比值,平扫CT值,增强CT值比较均无统计学差异(均P > 0.05)。而支气管囊肿、心包囊肿、胸腺囊肿患者组内比较结果显示,增强CT值均高于平扫,差异有统计学意义(t分别为-3.990、-4.561、-3.093,P分别为 < 0.001、< 0.001、0.006),见表 2。
| Item | Bronchogenic cyst group | Thymic cyst group | Pericardial cyst group | F | P |
| Max(cm) | 3.56±2.01 | 3.33±1.50 | 4.28±2.73 | 1.234 | 0.297 |
| Min(cm) | 2.55±1.47 | 2.12±0.76 | 2.66±1.26 | 1.173 | 0.315 |
| Min/Max | 0.72±0.18 | 0.68±0.18 | 0.69±0.22 | 0.342 | 0.712 |
| CT value(plain scan,Hu) | 19.67±10.70 | 13.83±8.37 | 14.67±9.50 | 2.820 | 0.066 |
| CT value(enhanced scan,Hu) | 21.69±11.92 | 16.88±11.83 | 15.66±8.94 | 2.231 | 0.115 |
| Max,maximum diameter of cyst;min,minimum diameter of cyst. | |||||
2.3 囊肿直径与临床症状的相关性分析
结果显示,囊肿最大径≥5 cm组14例,其中有临床症状者7例。囊肿最大径 < 5 cm组60例,有临床症状者11例,2比较差异有统计学意义(χ2=4.583,P = 0.032)。
3 讨论支气管囊肿是一种先天畸形,约占纵隔病变的20%,是由于胚胎发育过程中肺实质和肺芽脱落所致,一般分为肺内及纵隔支气管囊肿[4],异位支气管囊肿也可以发生在膈肌、腹部、颈部、腹膜后、皮肤等[5]。纵隔支气管囊肿中79%位于中纵隔,17%位于后纵隔,3%位于前纵隔[6],以气管右旁和隆突下多见[7]。大约90%纵隔支气管囊肿患者无症状,导致诊断延迟和并发症的发生率增加[8]。STEWART等[9]发现支气管囊肿最常见的症状是咳嗽、呼吸急促、喘息等,囊肿的延迟诊断甚至可以导致危及生命的并发症(囊肿破裂引起出血、囊肿过大压迫气管导致窒息、癌变等),因此早期的诊断和手术十分重要[10]。支气管囊肿在CT上通常呈现类似于水的均匀密度,有时囊肿壁也会有钙化,若囊肿和气管支气管的分支结构相连,则可能出现空气或液气平面,可能误诊为肺脓肿、癌或结核性空洞[9, 11]。本研究中支气管囊肿64.3%位于中纵隔,CT平扫60.7%为水样密度(CT值> 20 Hu),78.6%患者无症状,有症状患者中胸痛3例,胸闷1例,呼吸困难1例,干咳1例。
心包囊肿约占纵隔囊肿的33%,是间叶腔融合失败所致[12]。心包囊肿大多数位于中纵隔心膈脚,以右侧多见[13]。ALKHARABSHEH等[14]研究发现心包囊肿女性多发,且囊内会存在分隔。囊内容物通常为液体,也会伴有出血或感染。心包囊肿患者通常没有症状,囊肿的临床表现可能与囊肿的位置、大小有关,主要症状以胸痛、呼吸困难和心悸为主,此外患者心电图还可呈非特异性的ST段改变[15]。心包囊肿在CT上通常表现为边界清楚的无强化结构,密度呈水样,囊内可以有分隔[14, 16]。一些患者心包囊肿可以自行消退,当囊肿体积较大、临床症状明显时则需手术治疗。欧洲心脏协会推荐经皮穿刺和乙醇硬化作为一线治疗[17],但术后复发率较高。本研究中心包囊肿多位于中纵隔,占84.0%,以右侧多见;患者女性居多,占56.0%;76.0%CT平扫为水样密度;72.0%无症状;有症状的患者中胸痛2例,胸闷2例,呼吸困难2例,心悸伴心律失常1例。
胸腺囊肿约占纵隔病变的2%~3%[18],是由于胚胎时期咽部鳃囊组织在下降过程中出现异常所致,一般位于前纵隔[19]。异位胸腺囊肿可以位于颈部、后纵隔、支气管根部等[20],患者一般无明显的临床症状,但随着病灶增大,会压迫周围组织并产生多种症状,以胸痛、呼吸困难、心律失常、咳嗽等为主,典型的胸腺囊肿CT表现呈单房性,亦可有分隔,囊内大多数为清亮的浆液性液体,当囊壁钙化时难以与胸腺瘤鉴别[21]。有症状的胸腺囊肿是明确的手术指征,但对于无明显症状的胸腺囊肿,考虑到其有增大、自然破裂、并发症及恶变等可能性[22],且有时影像学无法做出定性诊断,因此,一旦发现胸腺囊肿,应考虑手术切除;对于体积小、诊断明确且无症状胸腺囊肿可随访观察。本研究中76.2%胸腺囊肿位于前纵隔,85.7%CT平扫为水样密度,76.2%无症状,有症状的患者中咳嗽2例,胸痛1例,呼吸困难1例,发热1例。
支气管囊肿、胸腺囊肿、心包囊肿的好发部位、是否有钙化等特点存在差异,这与以往研究[6-7, 13, 18]结果一致。另外,囊肿是否有内部分隔和囊肿的CT值不能作为3种囊肿的特有属性。本研究结果显示囊肿大小和患者的临床表现可能存在相关性,推测可能是直径大的囊肿可以通过压迫邻近组织产生相应的症状,这与以往研究[8, 14]结果一致。
综上所述,原发性支气管囊肿、胸腺囊肿及心包囊肿的影像学表现各有特点,但利用CT平扫、CT增强扫描和囊内有无分隔无法区分。囊肿所处位置和囊壁有无钙化可能为临床鉴别纵隔囊肿提供信息。囊肿大小和临床症状之间可能存在相关性,提示肿瘤大小可作为临床干预的指标。但直径小、无邻近结构的囊肿的部分患者也有临床症状,具体的机制仍需进一步研究。由于纵隔囊肿的发病率较低,本研究收集到的样本量有限,且患者体格检查和实验室检查一般无异常,临床症状主要通过询问病史获得,因此容易产生选择偏倚。此外,由于缺乏随访资料,本研究也未能反映疾病的发生、发展和转归。
| [1] |
孙红, 蔡祖龙, 高元桂, 等. 原发性纵隔囊肿的影像学表现[J]. 中华放射学杂志, 2005, 39(10): 1055-1059. DOI:10.3760/j.issn:1005-1201.2005.10.010 |
| [2] |
潘忠. 前纵隔占位性病变的多层螺旋CT影像学表现和诊断意义[J]. 中国误诊学杂志, 2020, 15(10): 455-457. |
| [3] |
WANG X, LI Y, CHEN K, et al. Clinical characteristics and management of primary mediastinal cysts: a single-center experience[J]. Thorac Cancer, 2020, 11(9): 2449-2456. DOI:10.1111/1759-7714.13555 |
| [4] |
ARUN S, KUMAR M, ROSS BJ. Mediastinal bronchogenic cyst mimicking congenital lobar emphysema[J]. BMJ Case Rep, 2016, 2016: bcr2016216704. DOI:10.1136/bcr-2016-216704 |
| [5] |
TROSSMAN CM. Push-up stridor caused by a bronchogenic cyst[J]. Am J Dis Child, 1964, 107: 293-296. DOI:10.1001/archpedi.1964.02080060295013 |
| [6] |
ALI S, RAUF A, MENG LB, et al. Case report: severe back pain, epigastric distress and refractory nausea; an unusual presentation of mediastinal bronchogenic cyst[J]. F1000Res, 2018, 1(7): 960. DOI:10.12688/f1000research.15128.1 |
| [7] |
沈训泽, 王华. 纵隔支气管囊肿的CT表现[J]. 中国医学影像学杂志, 2014, 22(11): 820-823. DOI:10.3969/j.issn.1005-5185.2014.11.005 |
| [8] |
WENIG BL, ABRAMSON AL. Tracheal bronchogenic cyst: a new clinical entity?[J]. Ann Otol Rhinol Laryngol, 1987, 96(1): 58-60. DOI:10.1177/000348948709600114 |
| [9] |
STEWART B, COCHRAN A, IGLESIA K, et al. Unusual case of stridor and wheeze in an infant: tracheal bronchogenic cyst[J]. Pediatr Pulmonol, 2002, 34(4): 320-323. DOI:10.1002/ppul.10129 |
| [10] |
GOSWAMY J, DE KRUIJF S, HUMPHREY G, et al. Bronchogenic cysts as a cause of infantile stridor: case report and literature review[J]. J Laryngol Otol, 2011, 125(10): 1094-1097. DOI:10.1017/s0022215111001502 |
| [11] |
DURELL J, LAKHOO K. Congenital cystic lesions of the lung[J]. Early Hum Dev, 2014, 90(12): 935-939. DOI:10.1016/j.earlhumdev.2014.09.014 |
| [12] |
PARK M, KIM GJ. Prenatal sonographic diagnosis of a pericardial cyst[J]. J Ultrasound Med, 2015, 34(4): 739-741. DOI:10.7863/ultra.34.4.739 |
| [13] |
PATEL J, PARK C, MICHAELS J, et al. Pericardial cyst: case reports and a literature review[J]. Echocardiography, 2004, 21(3): 269-272. DOI:10.1111/j.0742-2822.2004.03097.x |
| [14] |
ALKHARABSHEH S, GENTRY Ⅲ JL, KHAYATA M, et al. Clinical features, natural history, and management of pericardial cysts[J]. Am J Cardiol, 2019, 123(1): 159-163. DOI:10.1016/j.amjcard.2018.09.009 |
| [15] |
SOROUR AA, MALESZEWSKI JJ, SCHAFF HV, et al. A symptomatic calcified pericardial cyst[J]. Mayo Clin Proc, 2019, 94(2): 367-369. DOI:10.1016/j.mayocp.2018.11.013 |
| [16] |
KLEIN AL, ABBARA S, AGLER DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography[J]. J Am Soc Echocardiogr, 2013, 26(9): 965-1012. DOI:10.1016/j.echo.2013.06.023 |
| [17] |
ADLER Y, CHARRON P, IMAZIO M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS)[J]. Eur Heart J, 2015, 36(42): 2921-2964. DOI:10.1093/eurheartj/ehv318 |
| [18] |
KONDOV G, KONDOV B, SRCEVA MJ, et al. Giant mediastinal thymic cyst[J]. Pril (Makedon Akad Nauk Umet Odd Med Nauki), 2017, 38(2): 139-145. DOI:10.1515/prilozi-2017-0032 |
| [19] |
MINNITI S, VALENTINI M, PINALI L, et al. Thymic masses of the middle mediastinum: report of 2 cases and review of the literature[J]. J Thorac Imag, 2004, 19(3): 192-195. DOI:10.1097/01.rti.0000120060.90202.7a |
| [20] |
ZIELIŃSKI M, KUZDZAŁ J, SZLUBOWSKI A, et al. Transcervical-subxiphoid-videothoracoscopic "maximal" thymectomy: operative technique and early results[J]. Ann Thorac Surg, 2004, 78(2): 404-409. DOI:10.1016/j.athoracsur.2004.02.021 |
| [21] |
王宏伟, 杨军浩, 王天君. 胸腺瘤的CT表现及其临床应用价值[J]. 中国医科大学学报, 2004, 33(6): 553-555. DOI:10.3969/j.issn.0258-4646.2004.06.028 |
| [22] |
ORAMAS DM, MORAN CA. Multilocular thymic cyst (MTC) and other tumors with MTC features: pitfalls in diagnosis[J]. Semin Diagn Pathol, 2021. DOI:10.1053/j.semdp.2021.06.004 |
 2022, Vol. 51
2022, Vol. 51