文章信息
- 秦思文, 姜红
- QIN Siwen, JIANG Hong
- 单侧输尿管梗阻大鼠肾组织中过氧化物酶2的表达
- Expression of peroxiredoxin 2 in renal tissues of unilateral ureteral obstruction rats
- 中国医科大学学报, 2021, 50(10): 940-943
- Journal of China Medical University, 2021, 50(10): 940-943
-
文章历史
- 收稿日期:2020-11-26
- 网络出版时间:2021-09-29 19:34
衰老和慢性肾脏疾病都会影响肾脏功能,最终导致肾纤维化。肾纤维化是一种慢性、进行性过程,目前尚无减缓肾纤维化的靶向疗法[1]。过氧化物酶2(peroxiredoxin2,Prx2)是哺乳动物细胞中常见的抗氧化酶,主要存在于细胞质和细胞膜中,参与凋亡、增殖、分化、炎症、癌症和先天免疫缺陷等多种细胞过程[2]。Prx2在肺脏[3]、肝脏[4-5]等实质器官纤维化疾病过程中都有特异性表达。然而,肾纤维化组织中Prx2表达情况尚未见报道。本研究拟探讨Prx2在肾纤维化大鼠肾组织中的表达情况,旨在为阐明其与肾脏纤维化的关系提供依据。
1 材料与方法 1.1 实验动物及分组将24只8周龄、体质量180~200 g的雄性SD大鼠(购自北京维通利华公司)随机分为假手术组(n = 8)和单侧输尿管梗阻(unilateral ureteral obstruction,UUO)组(n = 16,UUO组)。假手术组大鼠戊巴比妥钠麻醉后沿腹中线开腹,自中下1/3交界处至剑突,暴露左侧肾脏及左输尿管,游离左侧输尿管而不结扎。UUO组大鼠入路方式同假手术组,暴露左侧肾脏及左输尿管结扎输尿管上下两端,中间剪断。假手术组术后3 d处死大鼠并获取肾组织,UUO组分别于术后3、7、14、21 d处死大鼠并获取肾组织。一部分肾组织4%多聚甲醛固定24 h,制作石蜡切片,另一部分保存于-80 ℃冰箱待用。
1.2 方法 1.2.1 HE染色2组术后3 d的石蜡切片(2 μm)染色,按照染色试剂盒(中国索莱宝公司)说明书操作,光镜下观察肾脏组织形态。
1.2.2 免疫组化染色检测大鼠肾脏组织中Prx2的表达2组术后3 d的石蜡切片(4 μm)常规脱蜡、水化,浸入柠檬酸缓冲液,微波炉加热,修复抗原冷却后洗涤,血清封闭;一抗Prx2(英国Abcam公司,1∶1 000)4 ℃过夜。二抗37 ℃温箱30 min,DAB染色后苏木素轻度复染,常规脱水、透明、封片、光镜观察。阴性对照组用PBS代替一抗进行反应。采用Image J软件对Prx2结果进行半定量分析,每张切片在光镜(400倍)下观察20个不同的肾间质区域,测定光密度并计算平均值。
1.2.3 实时PCR检测Prx2、α-SMA和vimentin mRNA的表达使用Trizol试剂从大鼠肾脏组织中分离提取总RNA。按照试剂盒说明书操作进行反转录,合成cDNA,配制20 μL反应体系,取反转录后的cDNA产物2 μL,使用ChamQ Universal SYBR qPCR Master Mix(Q3定量PCR仪,美国Thermo fisher公司)进行PCR反应。引物序列:Prx2,上游引物5’- GACCTACCTGTGGGACGCTCTG-3’,下游引物5’- TCCAGCCAGCAGGACAGACTTC -3’;α-SMA,上游引物5’- AGCCAGTCGCCATCAGGAAC -3’,下游引物5’- CCGGAGCCATTGTCACACAC -3’;Vimentin,上游引物5’- CGTTTCCAAGCCTGACCTCACC-3’,下游引物5’- GCCATCTTTACATTGAGCAGGT-3’;GAPDH,上游引物5’- TGATGCTGGTGCTGAGTAT G-3’,下游引物5’- AGATGATGACCCTTTTGGC-3’。反应条件预变性95 ℃30 s,循环反应95 ℃10 s,62 ℃30 s;溶解曲线95 ℃15 s,60 ℃60 s,95 ℃15 s,共40次循环。以GAPDH为内参,采用2-ΔΔCt方法计算。
1.2.4 Western blotting检测Prx2、α-SMA和vimentin蛋白的表达提取肾组织蛋白,浓度定量后加入1/4体积上样缓冲液95 ℃10 min变性,每个上样孔道20 μL裂解蛋白经十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)还原分离,转移至PVDF膜。将膜浸泡在无蛋白快速封闭液(中国雅酶公司)中,室温置于摇床15 min,然后4 ℃一抗[Prx2(英国Abcam公司)、α-SMA(美国Affinity公司)、Vimentin(英国Abcam公司)和GAPDH(美国Affinity公司)]孵育过夜。第2天洗膜3次后加入HRP二抗(英国Abcam公司)室温摇床孵育1 h,去除二抗并洗膜3次后曝光显影。使用Image J软件对条带进行定量分析。
1.3 统计学分析采用SPSS 26.0进行统计分析,计量资料采用x±s表示。多组间比较采用单因素方差分析,P < 0.05为差异有统计学意义。
2 结果 2.1 2组大鼠肾组织HE染色结果结果显示,假手术组未见肾脏病理改变;UUO组肾间质水肿炎性渗出,肾小管扩张,表明大鼠肾纤维化模型构建成功。见图 1。
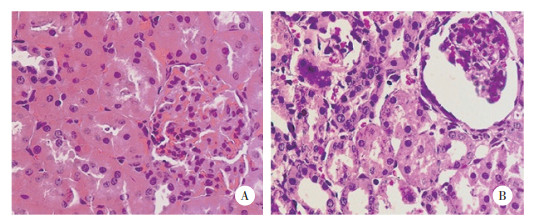
|
| A, sham group; B, UUO group. 图 1 2组肾组织HE染色结果×400 Fig.1 HE staining of the kidney tissue between sham group and UUO group ×400 |
2.2 2组大鼠免疫组化结果
结果显示,假手术组(0.31±0.02)肾小管上皮可见Prx2少量表达;而UUO组(0.47±0.01)肾小管上皮Prx2表达明显升高(P < 0.01)。见图 2。
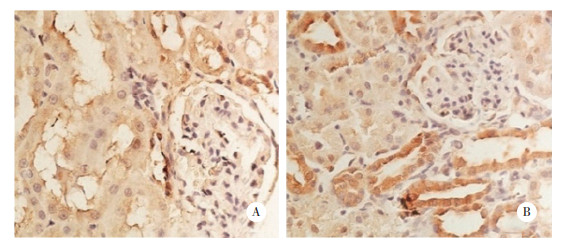
|
| A, sham group; B, UUO group. 图 2 2组肾组织Prx2表达的免疫组化学结果×400 Fig.2 Prx2 expression in renal tissues between sham group and UUO group by immunohistochemical staining ×400 |
2.3 UUO组大鼠实时PCR检测结果
结果显示,UUO组大鼠肾组织Prx2、α-SMA和Vimentin mRNA表达水平随着时间延长明显增加(F分别为261.412、43.291、1247.781,均P < 0.01)。见表 1。
| Group | n | Prx2 mRNA | α-SMA mRNA | Vimentin mRNA |
| 3 d after surgery | 4 | 1.29±0.07 | 1.23±0.10 | 1.11±0.02 |
| 7 d after surgery | 4 | 2.48±0.08 | 2.10±0.54 | 1.42±0.01 |
| 14 d after surgery | 4 | 2.90±0.10 | 4.56±0.94 | 4.68±0.11 |
| 21 d after surgery | 4 | 3.21±0.22 | 5.47±1.02 | 5.54±0.19 |
2.4 UUO组大鼠Western blotting检测结果
结果显示,UUO组大鼠肾组织Prx2、α-SMA和Vimentin蛋白的表达水平随着时间延长明显增加(F分别为538.66、330.167、500.706,均P < 0.01),与实时PCR结果一致。见表 2、图 3。
| Group | n | Prx2 protein | α-SMA protein | Vimentin protein |
| 3 d after surgery | 4 | 0.46±0.01 | 0.52±0.02 | 0.23±0.03 |
| 7 d after surgery | 4 | 0.79±0.07 | 1.03±0.04 | 0.69±0.05 |
| 14 d after surgery | 4 | 0.98±0.04 | 1.19±0.08 | 1.22±0.08 |
| 21 d after surgery | 4 | 1.07±0.04 | 1.34±0.03 | 1.29±0.04 |
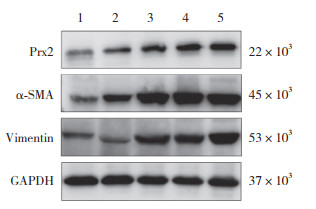
|
| 1, sham group; 2, 3 d after surgery in UUO group; 3, 7 d after surgery in UUO group; 4, 14 d after surgery in UUO group; 5, 21 d after surgery in UUO group. 图 3 Western blotting检测Prx2、α-SMA和Vimentin蛋白表达 Fig.3 Protein expression of Prx2, α-SMA, and Vimentin by Western blotting |
3 讨论
肾纤维化是慢性肾脏疾病的共同特征。肾小球和肾间质区域过多的细胞外基质蛋白沉积是肾纤维化的典型标志[6]。氧化应激在肾纤维化的病因学中起着至关重要的作用。Prx2是抗氧化酶,对氧化反应敏感,可作为氧化应激标志物[7-8]。因此,Prx2对于维持哺乳动物体内的氧化还原平衡起重要作用,在氧化应激的发展过程中,Prx2的表达水平增加[9-10]。另外,过氧化物浓度增加后,过氧化形式Prx2向其寡聚形式(具有分子伴侣活性)转化,发挥分子伴侣功能,参与调节细胞间信号传导[11-12];Prx2还与许多转录因子(NF-κB、HIF-1α、HIF-2α、STAT3、P53、AP-1、c-MYC等)、血小板衍化生长因子受体和激酶(ASK1、JNK、p38、MAPK等)相互作用,影响细胞的生长、分化和凋亡[13-14]。研究显示Prx2在多种纤维化疾病(肺纤维化[15]、肝纤维化[16]等)中发挥作用,因此推测Prx2与肾纤维化的发生发展也存在联系。
UUO是公认的梗阻性肾病实验模型,Vimentin和α-SMA是检测肾纤维化发生的有效指标。本研究成功建立了UUO大鼠模型,免疫组化结果显示Prx2主要表达于肾小管上皮细胞的胞质中,间质也有少量表达。Western blotting和实时PCR证实了UUO组大鼠肾组织Prx2的表达水平随着时间延长呈增加趋势,因此推测Prx2与肾纤维化存在密切联系。
综上所述,Prx2在UUO大鼠肾组织中高表达,并呈现时间依赖性,其表达增高可能与肾纤维化密切相关。Prx2可能参与了肾纤维化发病机制,今后还需进一步研究论证。
| [1] |
HUMPHREYS BD. Mechanisms of renal fibrosis[J]. Annu Rev Physiol, 2018, 80: 309-326. DOI:10.1146/annurev-physiol-022516-034227 |
| [2] |
KIM MH, KIM JY, KIM JH, et al. Peroxiredoxin 2 deficiency reduces white adipogenesis due to the excessive ROS generation[J]. Cell Biol Int, 2020, 44(10): 2086-2093. DOI:10.1002/cbin.11417 |
| [3] |
FEDERTI E, MATTE A, GHIGO A, et al. Data demonstrating the role of peroxiredoxin 2 as important anti-oxidant system in lung homeostasis[J]. Data Brief, 2017, 15: 376-381. DOI:10.1016/j.dib.2017.09.062 |
| [4] |
LU D, WANG W, LIU J, et al. Peroxiredoxins in inflammatory liver diseases and ischemic/reperfusion injury in liver transplantation[J]. Food Chem Toxicol, 2018, 113: 83-89. DOI:10.1016/j.fct.2018.01.025 |
| [5] |
CZARNY P, WIGNER P, GALECKI P, et al. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression[J]. Prog Neuropsycho-pharmacol Biol Psychiatry, 2018, 80: 309-321. DOI:10.1016/j.pnpbp.2017.06.036 |
| [6] |
NOGUEIRA A, PIRES MJ, OLIVEIRA PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies[J]. In Vivo, 2017, 31(1): 1-22. DOI:10.21873/invivo.11019 |
| [7] |
PORTILLO-LEDESMA S, RANDALL LM, PARSONAGE D, et al. Differential kinetics of two-cysteine peroxiredoxin disulfide formation reveal a novel model for peroxide sensing[J]. Biochemistry, 2018, 57(24): 3416-3424. DOI:10.1021/acs.biochem.8b00188 |
| [8] |
DALLA RIZZA J, RANDALL LM, SANTOS J, et al. Differential parameters between cytosolic 2-Cys peroxiredoxins, PRDX1 and PRDX2[J]. Protein Sci, 2019, 28(1): 191-201. DOI:10.1002/pro.3520 |
| [9] |
AREVALO J, VÁZQUEZ-MEDINA J. The role of peroxiredoxin 6 in cell signaling[J]. Antioxidants, 2018, 7(12): 172. DOI:10.3390/antiox7120172 |
| [10] |
RADYUK SN, ORR WC. The multifaceted impact of peroxiredoxins on aging and disease[J]. Antioxid Redox Signal, 2018, 29(13): 1293-1311. DOI:10.1089/ars.2017.7452 |
| [11] |
SCHRÖDER E, BRENNAN JP, EATON P. Cardiac peroxiredoxins undergo complex modifications during cardiac oxidant stress[J]. Am J Physiol Heart Circ Physiol, 2008, 295(1): 425-433. DOI:10.1152/ajpheart.00017.2008 |
| [12] |
RHEE SG. Overview on peroxiredoxin[J]. Mol Cells, 2016, 39(1): 1-5. DOI:10.14348/molcells.2016.2368 |
| [13] |
KIM Y, JANG HH. Role of cytosolic 2-cys Prx1 and Prx2 in redox signaling[J]. Antioxidants, 2019, 8(6): 169-183. DOI:10.3390/antiox8060169 |
| [14] |
KANG S, LEE S, LEE J. Cancer-associated function of 2-cys peroxiredoxin subtypes as a survival gatekeeper[J]. Antioxidants, 2018, 7(11): 161-170. DOI:10.3390/antiox7110161 |
| [15] |
张钊, 刘英宇, 刘岩, 等. 硫氧环蛋白过氧化物酶-2对转化生长因子β1诱导的人胚肺成纤维细胞增殖及胶原合成的影响[J]. 中华劳动卫生职业病杂志, 2020, 38(1): 7-12. DOI:10.3760/cma.j.issn.1001-9391.2020.01.002 |
| [16] |
LU Y, LIU J, LIN C, et al. Peroxiredoxin 2:a potential biomarker for early diagnosis of hepatitis B virus related liver fibrosis identified by proteomic analysis of the plasma[J]. BMC Gastroenterol, 2010, 10: 115-127. DOI:10.1186/1471-230x-10-115 |
 2021, Vol. 50
2021, Vol. 50




