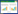文章信息
- 邢玉娇, 衣曼, 富建华, 薛辛东
- XING Yujiao, YI Man, FU Jianhua, XUE Xindong
- 高-低氧双向诱导制备新生大鼠支气管肺发育不良并发肺动脉高压模型
- A novel neonatal rat model of bronchopulmonary dysplasia complicated by pulmonary hypertension established by high-low oxygen bidirectional induction
- 中国医科大学学报, 2020, 49(9): 846-853
- Journal of China Medical University, 2020, 49(9): 846-853
-
文章历史
- 收稿日期:2019-06-25
- 网络出版时间:2020-09-11 10:55
2. 加拿大曼尼托巴大学儿童医院研究所生理系, Winnipeg R3E 3P4
2. Department of Pathology, Children's Hospital Research Insititute of Manitoba, University of Manitoba, Winnipeg, R3E 3P4, Canada
支气管肺发育不良(bronchopulmonary dysplasia,BPD)常见于早产儿,是一种慢性肺损伤疾病,在接受呼吸支持治疗和氧暴露的超低出生体重儿(extremely low birth weight,ELBW)中,BPD发病率高达60%~83%,由于该病缺乏有效的防治手段,目前已经成为在儿童医疗费用消耗中仅次于哮喘的第二大疾病[1]。BPD的主要病理变化为肺泡发育障碍和肺微血管的发育不良,以往研究[2-5]多集中于BPD的肺泡发育障碍,近年来发现,BPD患儿合并肺动脉高压(pulmonary hypertension,PH)高达25%~40%,BPD-PH患儿不仅死亡率高达14%~38%,存活患儿的生存质量也受到严重影响。
实验模型是研究疾病发生机制及治疗方法的基础,目前临床有关早产儿BPD的模型制备包括吸入高浓度氧气、机械通气、LPS诱导等,而其中最经典、最常用为高浓度氧(30%~100%)制备[6],该模型虽然在临床过程和病理改变上符合早产儿BPD,但缺乏PH的病理改变,故探讨加用短暂低氧刺激来模拟临床早产儿生后短暂血氧饱和度下降,有助于更好研究BPD-PH疾病的发生机制。本研究采用60%~13%高-低氧双向诱导方法建立模型,并通过影像学、组织学证实所建模型符合临床BPD-PH患儿表现。
1 材料与方法 1.1 实验动物足月新生Sprague-Dawley大鼠,由曼尼托巴大学儿童医院研究所动物中心提供。
1.2 分组将大鼠随机分为4组,每组12只,分组饲养于不同氧浓度的玻璃箱内。
1.3 模型制备方法空气组(A组),饲养于21% O2环境,BPD组(H组)饲养于60% O2环境,低氧组(AL组)每日23 h置于21% O2环境,1 h置于13% O2环境,BPD-PH组(HL组)每日23 h置于60% O2,1 h置于13% O2环境,A组与AL组作为对照,每日分别与H组和HL组母鼠进行交换,以保证母鼠的哺乳能力及防止氧中毒。各组按不同条件持续饲养至生后14 d。
1.4 实验设备及试剂 1.4.1 主要仪器动物氧箱(美国BioSpherix ProOx P110高/低氧可控氧箱),水浴箱(Thermo ScientificTM PrecisionTM water baths),切片机(ShandonTM FinesseTM 325 Manual Microtome),过缸机(Leica TP1020半自动台式组织脱水机),包埋机(Shandon HistoCentre 2 Embedding Center),光学显微镜(ZEISS Axio Lab A1;AxioCam 105 color成像系统),60℃烤箱(Techne HB-1D Hybridiser Hybridisation Oven/Incubator),心脏超声仪[Vevo 2100 High Resolution Imaging System(Visualsonics,Canada)],肺功能仪[Flexi-Vent Small Animal Ventilator(Scireq,Canada)],摇床(Lab-Line Model 4631 Maxi Rotator),电子秤[TR-8102D Toploading Balances(大),METTLER TOLEDO AL104 Analytical Balance(小)]。
1.4.2 主要试剂HE染色试剂购自美国Sigma公司,免疫组化试剂购自美国Vector Laboratories公司,弹性蛋白染色试剂购自美国Rowley Biochemical公司,α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)抗体(Sigma A2547,Mouse monoclonal Anti-α Smooth Muscle Actin)。
1.5 功能测定 1.5.1 二维超声心动图和多普勒检查分别于生后7 d和14 d对各组大鼠进行体质量称量,应用vevo 2100高分辨成像系统(VisualSonics,Toronto canada),40 MHz探头(MS 550D)进行超声心动图检查以评价肺血流动力学。异氟烷吸入麻醉诱导后,将大鼠仰卧位置于加热的ECG板上,保持体温并获得心电图数据,同时面罩持续给予适当浓度O2和异氟烷吸入维持麻醉状态。将探头温和置于胸部,在胸骨旁主动脉根部短轴切面肺动脉瓣口处测量肺动脉加速时间(pulmonary arterial acceleration time,PAAT)和右心室射血时间(right ventricular ejection time,RVET)。PAAT为从肺动脉收缩开始到达到流出速度峰值的时间,RVET为从肺动脉射血开始到完成收缩期的时间。
1.5.2 肺功能测定应用flexi-Vent小动物呼吸机(Scireq,Montreal,Quebec)测定14 d大鼠肺功能[7]。大鼠接受90 mg/kg剂量的戊巴比妥钠腹腔内注射。建立麻醉后,行颈正中气管切开术,并在气管内插入聚乙烯导管(1.1 mm×25 mm),用外科缝线固定导管位置。导管另一端连接到小动物呼吸机,呼气末正压(positive end-expiratory pressure,PEEP)维持在3 cmH2O。呼吸机根据大鼠体质量向大鼠肺部传送10 mL/kg的潮气量,呼吸频率150次/min。大鼠通过导管接受不同浓度的醋甲胆碱(35 μL,3~50 mg/mL,配制成生理盐水溶液)激发,收缩气道平滑肌以放大模型效果,进行肺功能测定,基础肺功能测定则仅使用等量生理盐水。每次激发前,肺载气量由总肺活量校准。使用机器预设的flexiVent Prime-3低频强制振荡程序获得呼吸机械输入阻抗,机器测得参数后通过阻抗校准,输出肺气道阻力,肺组织阻力和肺组织总体弹性等结果。
1.6 动物组织标本检测指标 1.6.1 标本的留取及组织切片制备生后14 d大鼠接受肺功能测定后,打开胸腔,剪开左心耳,用无菌输液针刺入右心室,4 ℃ PBS溶液以18 cm H2O压力进行灌洗,直至肺循环血液灌出。将左肺叶浸泡于4% PFA中固定72 h。心脏减去心房和游离大血管,沿室间隔剪下右心室壁,分别称量右心室及左心室+室间隔的质量,计算右心室质量/(左心室质量+室间隔质量)[right ventricular/(left ventricle + ventricle septum),RV/(LV+VS)]比值。
组织浸泡于4% PFA中固定72 h后,用Leica TP1020半自动台式组织脱水机,顺序浸泡于70%乙醇,80%乙醇,95%乙醇,100%乙醇,二甲苯,60℃石蜡进行脱水,应用Shandon HistoCentre 2 Embedding Center包埋机对脱水后的组织进行石蜡包埋,制作蜡块。应用ShandonTM FinesseTM 325 Manual Microtome切片机,制作5 μm组织切片。
1.6.2 HE染色及辐射状肺泡计数(radical alveolar counts,RAC)值计数切片脱蜡水化,应用苏木素(Sigma HHS 16-500 mL)及伊红(Sigma HT110116-500 mL)对切片进行HE染色,封片干燥后由光学显微镜200×采集图像,每张切片选取10个视野,应用Emery和Mithal的方法[8]计数RAC值,并进行组间比较。
1.6.3 α-SMA免疫组化染色组织切片脱蜡水化后浸泡于PBS中:柠檬酸盐缓冲液煮沸30 min,室温冷却20 min,Avidin封闭液(Vector sp-2001)及Biotin封闭液(Vector wp-2001)封闭后4℃过夜孵育一抗:α-SMA(Sigma A2547,mouse monoclonal Anti-α Smooth Muscle Actin;1:800封闭液稀释)次日孵育二抗:驴抗小鼠,1:400封闭液稀释,DAB法显色(Thermo Scientific-34002),苏木素(Vector H-3404)复染,脱水,封片,干燥后光学显微镜200×采集图像。
1.6.4 弹性蛋白特殊染色Hart’s弹性蛋白染色法(染剂购自Rowley Biochemical,F-397)对切片进行染色,分别经过0.25%高锰酸钾水溶液(F-379-3)氧化,雷锁辛-品红(F-397-1)工作液初染,5%草酸水溶液(F-397-4)洗片,Van Gieson’s溶液(F-397-2)复染后,脱水,封片干燥后光学显微镜(200×)采集图像。
1.7 统计学分析应用SPSS 19.0统计软件进行统计学处理,数据以x±s表示,两样本均数间比较采用完全随机设计t检验。P < 0.05为差异有统计学意义。
2 结果 2.1 肺动脉压力改变各组大鼠生后14 d测定PAAT和RVET,A组、AL组、H组间RVET、PAAT及RVET/PAAT无统计学差异,HL组相较于A组,RVET延长但不显著,PAAT明显缩短(P < 0.05),RVET/PAAT明显升高(P < 0.001)。见图 1、表 1。

|
| A, group A; B, group AL; C, group H; D, group HL. Pulmonary arterial acceleration time (PAAT) and right ventricular ejection time (RVET) were measured and labeled with blue lines. 图 1 各组大鼠生后14 d二维心脏超声多普勒结果 Fig.1 Two-dimensional echocardiography and doppler examination on 14 d after birth |
| Group | RVET(s) | PAAT(s) | RVET/PAAT |
| A | 64.31±7.53 | 24.47±5.63 | 2.75±0.68 |
| AL | 62.80±8.17 | 24.60±7.20 | 2.73±0.87 |
| H | 62.40±2.85 | 21.72±1.53 | 2.88±0.14 |
| HL | 73.52±12.04 | 18.23±3.281) | 4.07±0.482) |
| 1)P < 0.05,2)P < 0.001 compared with group A. | |||
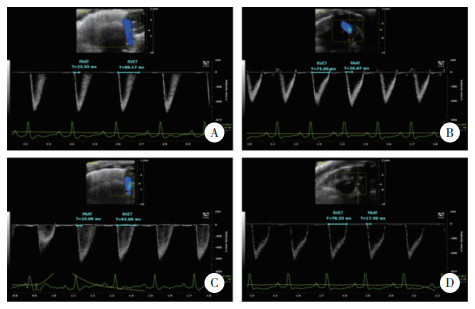
分别测定生后7 d时A组与HL组大鼠PAAT和RVET,并与生后14 d比较(图 2、表 2),7 d时HL组与A组各项均无统计学差异,14 d时PAAT及RVET/PAAT出现统计学差异。
|
| A, group A7 d; B, group A14 d; C, group HL7 d; D, group HL14 d. Pulmonary arterial acceleration time (PAAT) and right ventricular ejection time (RVET) were measured and labeled with blue lines. 图 2 A组和HL组大鼠生后7 d和14 d二维心脏超声多普勒对比 Fig.2 Comparison between HL and A groups using two-dimensional echocardiography and doppler findings from examination on 7 d and 14 d after birth |
| Group | RVET(s) | PAAT(s) | RVET/PAAT |
| A7 d | 82.57±4.43 | 19.86±2.17 | 4.19±0.38 |
| HL7 d | 82.75±8.86 | 20.88±2.02 | 3.97±0.21 |
| A14 d | 64.31±7.53 | 24.47±5.63 | 2.75±0.68 |
| HL14 d | 73.52±12.04 | 18.24±3.281) | 4.07±0.482) |
| 1)P < 0.05,2)P < 0.001 compared with group A14 d. | |||
2.2 肺功能改变
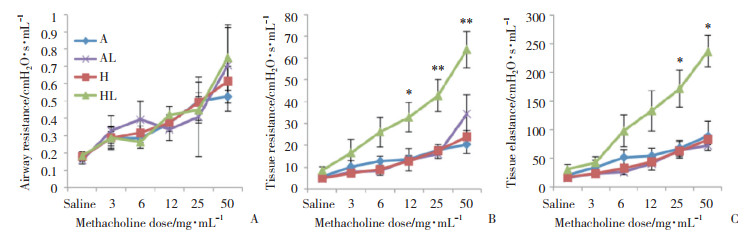
各组肺气道阻力无统计学差异。HL组肺组织阻力较A组高,从Mch 12 mg/mL刺激时开始出现统计学差异(P < 0.05),随着Mch计量增加差异更加明显(P < 0.01)。HL组织弹性阻力从Mch 25 mg/mL刺激开始出现统计学差异(P < 0.05)。H组,AL组与A组无统计学差异。见图 3。
|
| A, airway resistance; B, tissue resistance; C, tissue elastance. *P < 0.05, **P < 0.01 compared with group A. 图 3 各组大鼠肺功能变化 Fig.3 Change in the lung function in each group |
2.3 新生大鼠BPD-PH模型的大体变化
各组大鼠在生后14 d时体质量情况(表 3),A组与AL组无统计学差异,H组与A组相比体质量明显下降(P < 0.05),HL组体质量与A组相比,差异更加显著(P < 0.001),HL组与H组相比,体质量也显著下降(P < 0.001)。RV/(LV+S)比值(表 3),HL组较A组显著增高(P < 0.001),提示发生右心室肥厚,AL组,H组与A组无统计学差异。
| Group | Body weight(g) | RV/(LV+S) |
| A | 34.20±1.32 | 0.22±0.06 |
| AL | 33.90±0.97 | 0.22±0.06 |
| H | 33.30±1.661) | 0.25±0.07 |
| HL | 30.50±1.372),3) | 0.37±0.092) |
| 1)P < 0.05,2)P < 0.001 compared with group A;3)P < 0.001 compaed with group H. | ||
2.4 肺组织形态学改变(图 4)
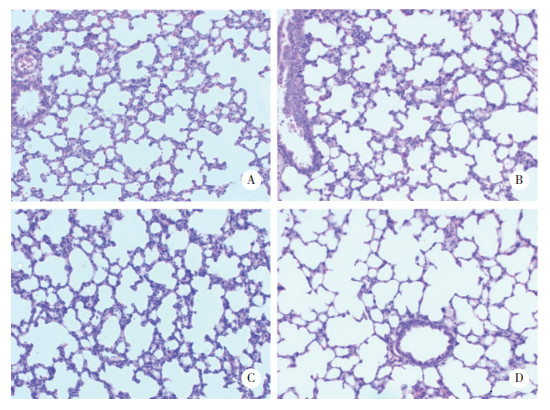
肺组织切片HE染色后可见A组和AL组为肺泡正常发育。H组肺泡体积变大,次级分隔减少,肺间质堆积增厚,HL组表现较H组更为严重:肺泡数量明显下降,肺泡大而结构简单,次级分隔少。
|
| A, group A; B, group H; C, group AL; D, group HL. The nuclei were stained bluish-purple, and the cytoplasm was stained pink. 图 4 各组大鼠肺组织切片HE染色×200 Fig.4 HE staining of lung sections of the groups ×200 |
各组HE切片进行RAC值计数结果显示,A组为9.45±0.78,AL组为9.75±0.88,H组为7.475±1.19,HL组为6±1.68,A组与AL组无统计学差异,H组较A组差异有统计学意义(P < 0.05),RAC值明显降低,HL组与A组比较RAC值进一步降低,差异更为显著(P < 0.01)。
2.5 α-SMA表达变化将各组肺组织切片进行α-SMA免疫组织化学染色(图 5),A组可见染成棕色的α-SMA于次级分隔即将形成处浓聚(红色箭头标出),肺血管壁α-SMA分布菲薄(黄色箭头标出)。AL组表现与A组大致相同。H组可见肺泡次级分隔减少,次级分隔积聚的α-SMA也减少,但有α-SMA呈线性分布于没有形成次级分隔的肺泡壁(红色箭头标出),血管壁染色较A组增多(黄色箭头标出)。HL组表现较H组更加明显,且肺血管腔直径增大。
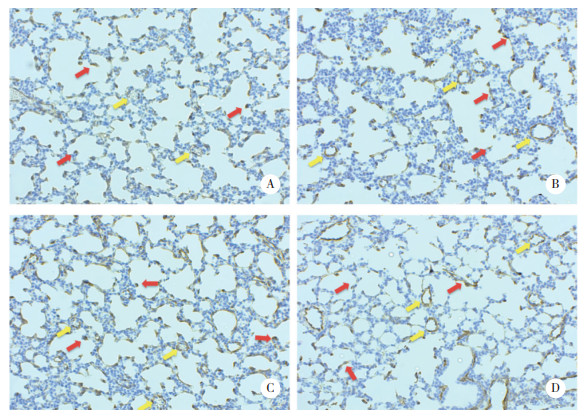
|
| A, group A; B, group H; C, group AL; D, group HL. α-SMA was stained brown, and the nuclei were stained bluish-purple. Red arrow, secondary septation; yellow arrow, small artery. 图 5 各组大鼠肺组织切片α-SMA免疫组织化学染色×200 Fig.5 Immunohistochemical staining of α-SMA in lung sections of the groups ×200 |
2.6 Elastin表达变化
根据Hart’s弹性蛋白染色法对各组肺组织切片进行特殊染色(图 6)。A组可见染成黑色的Elastin于次级分隔即将形成处呈点状浓聚(红色箭头标出),肺血管壁Elastin分布菲薄(蓝色箭头标出)。AL组表现与A组大致相同。H组可见肺泡次级分隔减少,次级分隔积聚的Elastin也减少,有些浓聚处散乱如毛刷,但有Elastin呈线性分布于没有形成次级分隔的肺泡壁(红色箭头标出),血管壁染色较A组增多(蓝色箭头标出)。HL组表现较H组更加明显,且肺血管腔直径增大。

|
| A, group A; B, group H; C, group AL; D, group HL. Elastin was stained black, and the other cell components were stained golden-brown. Red arrow, secondary septation; blue arrow, small artery. 图 6 各组大鼠肺组织切片Hart’s染色×200 Fig.6 Hart's staining of elastin in lung sections of the groups ×200 |
3 讨论
自从上世纪Northway提出BPD至今已50余年,随着早产儿救治技术提高,生存率亦随之提高,BPD的定义在几十年间被逐渐修改完善。早产儿BPD的病因多种多样,包括产前宫内发育迟缓,胎盘功能不良,绒毛膜羊膜炎,细菌感染,孕母高血压/子痫前期,孕母吸烟/滥用药物,遗传因素等,以及生后接受机械通气,氧疗,感染,动脉导管开放,营养不良等因素[9]。
啮齿动物和人类的肺发育分为5个阶段:胚胎期,假腺管期,微管期,囊泡期,肺泡期。足月新生大鼠出生时肺处于囊泡期,类似于人类胎龄26~28周的早产儿[10],因此大鼠模型被广泛应用于BPD研究。目前,研究中常见构建动物BPD研究模型方法包括高氧、机械通气、LPS诱导等,其中最常用的BPD动物模型建立方法为新生大鼠或小鼠,暴露于一定浓度的氧气,持续几天或几周[6]。随着新生儿氧疗的日渐规范,以往研究者建立动物模型应用的高浓度氧(75%[11]、85%[12]、95%[13]、100%[14])条件,目前在临床已不多见。大鼠造模的优点在于繁殖快,廉价。高浓度氧气使新生大鼠肺囊泡分隔形成肺泡的过程延迟,肺间质增厚,持续炎症反应,远端微血管发育不良[15]。这些表现与临床上早产儿BPD类似[16]。高氧模型简便易得,且能还原BPD的主要病理生理特点。
临床上已发现BPD患儿合并PH的几率高达25%~40%,虽然长期接受氧疗及机械通气,中枢神经系统及呼吸系统发育的不完善,仍使早产儿存在血氧饱和度下降的情况,目前关于PH的动物研究模型中,低氧为主要造模手段。前期研究[17-24]发现,新生大鼠暴露于60%O2 14 d,大鼠出现肺发育停滞。肺泡数减少,肺泡体积增大,肺间质增厚,即表现出早产儿BPD的相关特点,但并未出现PH,因此,本研究设想,在经典的BPD造模方法(生后长期吸入高浓度氧气)的基础上,增加短时低氧刺激,以模拟临床中早产儿因缺氧致血氧饱和度下降的事件,建立BPD-PH模型。
本研究中,模型组新生大鼠每日吸入60% O2 23 h,13% O2 1 h,连续14 d后进行心脏超声检测,发现RVET/ PAAT值明显升高,间接说明PH出现。肺功能测定发现HL组组织阻力及弹性阻力显著升高。肺组织切片HE染色,RAC值测定反映模型组出现BPD典型病理改变。表现出肺泡数量减少,结构简单,次级分隔数量减少等肺发育停滞特点。肺泡次级分隔形成的关键成分α-SMA及弹性蛋白表达及分布均发生改变。以上结果说明,应用60%~13%高-低氧双向诱导方法建立模型,不仅肺组织的病理变化符合经典BPD模型表现,而且通过心脏超声和肺功能验证,模型同时形成了PH,存在肺功能受累,与经典的模型相比,更贴近临床早产儿BPD并发PH的表现,故更适用于该疾病相关研究。
综上,本研究应用高-低氧双向诱导建立BPD-PH模型,经鉴定模型存在BPD及PH的典型表现,可以用于今后早产儿BPD-PH的相关研究。
| [1] |
PATEL RM, KANDEFER S, WALSH MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011[J]. N Engl J Med, 2015, 372(4): 331-340. DOI:10.1056/nejmoa1403489 |
| [2] |
MOURANI PM, ABMAN SH. Pulmonary vascular disease in bronchopulmonary dysplasia:pulmonary hypertension and beyond[J]. Curr Opin Pediatr, 2013, 25(3): 329-337. DOI:10.1097/MOP.0b013e328360a3f6 |
| [3] |
SLAUGHTER JL, PAKRASHI T, JONES DE, et al. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation[J]. J Perinatol, 2011, 31(10): 635-640. DOI:10.1038/jp.2010.213 |
| [4] |
KIM D, KIM HS, CHOI CW, et al. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia[J]. Neonatology, 2012, 101(1): 40-46. DOI:10.1159/000327891 |
| [5] |
BERKELHAMER SK, MESTAN KK, STEINHORN RH. Pulmonary hypertension in bronchopulmonary dysplasia[J]. Semin Perinatol, 2013, 37(2): 124-131. DOI:10.1053/j.semperi.2013.01.009 |
| [6] |
HILGENDORFF A, REISS I, EHRHARDT H, et al. Chronic lung disease in the preterm infant:lessons learned from animal models[J]. Am J Respir Cell Mol Biol, 2013, 130911135746008. DOI:10.1165/rcmb.2013-0014tr |
| [7] |
KADKHODA K, WANG SH, FAN YJ, et al. ICOS ligand expression is essential for allergic airway hyperresponsiveness[J]. Int Immunol, 2011, 23(4): 239-249. DOI:10.1093/intimm/dxq476 |
| [8] |
COONEY TP, THURLBECK WM. The radial alveolar count method of Emery and Mithal:a reappraisal 2:intrauterine and early postnatal lung growth[J]. Thorax, 1982, 37(8): 580-583. DOI:10.1136/thx.37.8.580 |
| [9] |
ABMAN SH, BANCALARI E, JOBE A. The evolution of bronchopulmonary dysplasia after 50 years[J]. Am J Respir Crit Care Med, 2017, 195(4): 421-424. DOI:10.1164/rccm.201611-2386ED |
| [10] |
SMITH LJ, MCKAY KO, VAN ASPEREN PP, et al. Normal development of the lung and premature birth[J]. Paediatr Respir Rev, 2010, 11(3): 135-142. DOI:10.1016/j.prrv.2009.12.006 |
| [11] |
HUMMLER SC, RONG M, CHEN SY, et al. Targeting glycogen synthase kinase-3β to prevent hyperoxia-induced lung injury in neonatal rats[J]. Am J Respir Cell Mol Biol, 2013, 48(5): 578-588. DOI:10.1165/rcmb.2012-0383oc |
| [12] |
LEE HJ, CHOI CW, KIM BI, et al. Serial changes of lung morphology and biochemical profiles in a rat model of bronchopulmonary dysplasia induced by intra-amniotic lipopolysaccharide and postnatal hyperoxia[J]. J Perinat Med, 2010, 38(6): 675-681. DOI:10.1515/JPM.2010.091 |
| [13] |
ALPHONSE RS, VADIVEL A, COLTAN L, et al. Activation of Akt protects alveoli from neonatal oxygen-induced lung injury[J]. Am J Respir Cell Mol Biol, 2011, 44(2): 146-154. DOI:10.1165/rcmb.2009-0182oc |
| [14] |
DE VISSER YP, WALTHER FJ, LAGHMANI EH, et al. Apelin attenuates hyperoxic lung and heart injury in neonatal rats[J]. Am J Respir Crit Care Med, 2010, 182(10): 1239-1250. DOI:10.1164/rccm.200909-1361oc |
| [15] |
WARNER BB, STUART LA, PAPES RA, et al. Functional and pathological effects of prolonged hyperoxia in neonatal mice[J]. Am J Physiol, 1998, 275(1): L110-L117. DOI:10.1152/ajplung.1998.275.1.L110 |
| [16] |
JOBE AJ. The new BPD:an arrest of lung development[J]. Pediatr Res, 1999, 46(6): 641-643. DOI:10.1203/00006450-199912000-00007 |
| [17] |
BELIK J, JANKOV RP, PAN J, et al. Chronic O2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostane[J]. J Appl Physiol (1985), 2004, 96(2): 725-730. DOI:10.1152/japplphysiol.00825.2003 |
| [18] |
LAU M, MASOOD A, YI M, et al. Long-term failure of alveologenesis after an early short-term exposure to a PDGF-receptor antagonist[J]. Am J Physiol Lung Cell Mol Physiol, 2011, 300(4): L534-L547. DOI:10.1152/ajplung.00262.2010 |
| [19] |
LI J, MASOOD A, YI M, et al. The IGF-I/IGF-R1 pathway regulates postnatal lung growth and is a nonspecific regulator of alveologenesis in the neonatal rat[J]. Am J Physiol Lung Cell Mol Physiol, 2013, 304(9): L626-L637. DOI:10.1152/ajplung.00198.2012 |
| [20] |
MASOOD A, YI M, LAU M, et al. Cyclooxygenase-2 inhibition partially protects against 60% O2-mediated lung injury in neonatal rats[J]. Pediatr Pulmonol, 2014, 49(10): 991-1002. DOI:10.1002/ppul.22921 |
| [21] |
MASOOD A, YI M, LAU M, et al. Therapeutic effects of hypercapnia on chronic lung injury and vascular remodeling in neonatal rats[J]. Am J Physiol Lung Cell Mol Physiol, 2009, 297(5): L920-L930. DOI:10.1152/ajplung.00139.2009 |
| [22] |
YI M, JANKOV RP, BELCASTRO R, et al. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat[J]. Am J Respir Crit Care Med, 2004, 170(11): 1188-1196. DOI:10.1164/rccm.200402-215oc |
| [23] |
YI M, MASOOD A, ZIINO A, et al. Inhibition of apoptosis by 60% oxygen:a novel pathway contributing to lung injury in neonatal rats[J]. Am J Physiol Lung Cell Mol Physiol, 2011, 300(3): L319-L329. DOI:10.1152/ajplung.00126.2010 |
| [24] |
DI FIORE JM, WALSH M, WRAGE L, et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia[J]. J Pediatr, 2012, 161(6): 1047-1052. DOI:10.1016/j.jpeds.2012.05.046 |
 2020, Vol. 49
2020, Vol. 49