文章信息
- 李野, 李斌超, 张雅琳, 刘宁, 刘祖望, 孔娟
- LI Ye, LI Binchao, ZHANG Yalin, LIU Ning, LIU Zuwang, KONG Juan
- 胆维丁乳对急性肺损伤小鼠肺表面活性物质的影响
- Effect of cholecalciterol cholesterol emulsion on pulmonary surfactant in a mouse model of acute lung injury
- 中国医科大学学报, 2020, 49(8): 685-689, 693
- Journal of China Medical University, 2020, 49(8): 685-689, 693
-
文章历史
- 收稿日期:2019-06-25
- 网络出版时间:2020-07-28 15:55
急性肺损伤(acute lung injury,ALI)是肺泡上皮细胞和肺毛细血管内皮细胞受损的急性肺部炎症综合征(acute respiratory distress syndrome,ARDS)[1],是一种高死亡率的急危重症。通过气管滴注脂多糖(lipopolysaccharides,LPS)可建立ALI小鼠模型[2]。
肺表面活性物质由Ⅱ型肺泡上皮细胞分泌,主要成分为二棕榈酰卵磷脂和肺表面活性物质结合蛋白(surfactant protein,SP),分布于肺泡液体分子层表面,能降低肺泡表面张力,维持大小肺泡容量的相对稳定[3],阻止肺泡毛细血管中液体向肺泡内滤出,是治疗ALI的有效手段[4]。目前已知的SP有SP-A、SP-B、SP-C、SP-D 4种。维生素D是一种脂溶性类固醇激素,在钙磷平衡和骨代谢中起重要作用[5-7],并可通过其受体(vitamin D receptor,VDR)调控多种生理反应[2, 8-16]。研究[17-19]发现,维生素D3能调节Ⅱ型肺泡上皮细胞增殖。胆维丁乳(cholecalciterol cholesterol emulsion,CCE)是一种新型的活性维生素D前体,临床上常用于治疗婴幼儿维生素D缺乏性佝偻病。SP在维生素D缺乏小鼠的肺中表达减少[20]。本研究拟探讨CCE对LPS所致ALI肺表面活性物质的影响。
1 材料与方法 1.1 制作动物模型与分组将36只健康雄性BALB/c小鼠随机分为正常对照组、CCE组、LPS组、LPS+CCE组,每组9只。正常对照组和LPS组给予饮用水,CCE组和LPS+CCE组小鼠给予10%CCE水溶液(避光,购自中国医科大学附属盛京医院)。饲喂14 d后,LPS组和LPS+CCE组小鼠行气管切开,滴注LPS(10 mg/kg),24 h后取血清、肺泡灌洗液及肺组织备用。
1.2 检测血清钙、磷的含量从小鼠心脏取血,分离血清,采用钙测试盒(带标准)微板法(C004-2,南京建成生物工程研究所)、无机磷测试盒(C006-1,南京建成生物工程研究所)检测各组小鼠血清钙、磷含量。
1.3 测定小鼠肺组织湿/干质量比取4组小鼠的右下肺,用吸水纸吸干肺组织表面血迹,立即称质量,记为“湿质量”。置于80 ℃恒温干燥箱内烘干,48 h后称质量,记为“干质量”。计算肺湿/干质量比。
1.4 肺组织HE染色4%多聚甲醛固定肺组织,石蜡包埋并切片,脱蜡,HE染色,封片,光镜下观察并拍照。
1.5 实时PCR用TRIzol试剂盒(美国Invitrogen公司)提取肺组织总RNA,按照产品说明书操作。用生物分光光度计测定RNA浓度和纯度。取2 μg RNA样本,用TaKaRa逆转录试剂盒(A2302-1,日本TaKaRa公司)进行逆转录合成cDNA,并以此为模板进行PCR扩增反应。逆转录和PCR反应条件按照试剂盒说明书设置。引物由上海生工生物工程公司合成,见表 1。
| Gene name | Primer sequence |
| VDR | Forward primer 5’- TGACCCTGGAGACTTTGACC -3’ |
| Reverse primer 5’- GTTGAAGGGGCAGGTGAATA -3’ | |
| GAPDH | Forward primer 5’- ACCACAGTCCATGCCATCAC -3’ |
| Reverse primer 5’- TCCACCACCCTGTTGCTGTA -3’ | |
| SP-A | Forward primer 5’- AATGGGAGTCCTCAGCTTGC -3’ |
| Reverse primer 5’- CCGGCTCTGGTACACATCTC -3’ | |
| SP-B | Forward primer 5’- TGCTTGATGTCTACCTGCCC -3’ |
| Reverse primer 5’- AGCAGGAGAACTGTGTAGCG -3’ | |
| SP-C | Forward primer 5’- TGATGGAGAGTCCACCGGAT -3’ |
| Reverse primer 5’- CCACCACAACCACGATGAGA -3’ | |
| SP-D | Forward primer 5’- CGAGCCTGACAAACAGAGGT -3’ |
| Reverse primer 5’- GGAGAGAAAGGGCAGCATGT -3’ |
1.6 Western blotting
取肺组织,研磨匀浆后,蛋白裂解,BCA法检测总蛋白浓度。取50 μg总蛋白,行SDS-PAGE电泳,电转至PVDF膜,用5%脱脂奶粉封闭1 h。加入一抗(VDR抗体1:1 000稀释,购自美国SANTA CRUZ BIOTECHNOLOGY公司;GAPDH抗体1:5 000稀释,购自美国Proteintech公司),4 ℃孵育过夜。TBST洗3次,室温孵育二抗1 h,ECL显色。Image J图像分析软件对条带进行定量分析比较。
1.7 绘制生存曲线另取健康雄性BALB/c小鼠20只,分为LPS组、LPS+CCE组,每组10只,处置同1.1,绘制并分析2组小鼠生存时间曲线。
1.8 统计学分析采用Graph Pad Prism 5.0软件进行统计学分析,计量结果均采用x±s表示,采用方差分析或配对t检验进行组间比较。P < 0.05为差异有统计学意义。
2 结果 2.1 CCE对ALI模型小鼠血清钙、磷含量的影响如表 2所示,喂饲CCE 2周后,4组小鼠血清钙、磷水平无统计学差异。说明CCE对LPS所致ALI模型小鼠血清钙、磷的影响较小,故可排除血清钙、磷水平的高低对实验结果的干扰。
| Item | Control group | CCE group | LPS group | LPS+CCE group | P |
| Serum calcium(mmol/L) | 2.216±0.421 | 2.051±0.219 | 2.256±0.466 | 2.033±0.348 | 0.491 |
| Serum phosphorus(mmol/L) | 2.618±0.490 | 2.854±0.217 | 2.799±0.487 | 2.678±0.210 | 0.535 |
| CCE,cholecalciterol cholesterol emulsion;LPS,lipopolysaccharides. | |||||
2.2 CCE对ALI模型小鼠生存时间的影响
如图 1所示,LPS+CCE组小鼠平均生存时间长于LPS组,小鼠死亡的首发时间晚于LPS组,给予LPS处理后25 h,LPS+CCE组小鼠仍有80%存活,LPS组则有40%小鼠死亡;53 h以内LPS组小鼠全部死亡,2组小鼠生存时间有显著的统计学差异(P < 0.05)。表明CCE能显著延长ALI小鼠的生存时间,对ALI起保护作用。
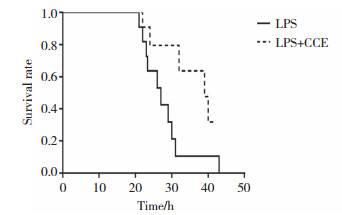
|
| 图 1 Kaplan-Meier法测定LPS诱导的ALI小鼠生存曲线(n = 10) Fig.1 The survival curve of ALI mice induced by LPS determined by Kaplan-Meier method (n = 10) |
2.3 CCE对ALI模型小鼠肺组织及肺水肿的影响
肉眼观察可见,与正常对照组相比,LPS组小鼠双肺肿胀,体积变大,肺表面可见出血区。光镜下(图 2A)可见,正常对照组与CCE组小鼠肺泡结构完整,肺泡腔和间隙内无炎症细胞浸润;LPS组小鼠肺泡和肺间质广泛充血、水肿、大量炎性渗出液,肺泡腔可见破裂的红细胞和渗出物,肺泡结构紊乱,肺泡腔狭窄,肺泡间隔增厚;与LPS组相比,LPS+CCE组小鼠肺泡和肺间质微血管充血扩张程度及炎症细胞浸润减轻,肺泡腔内红细胞减少。
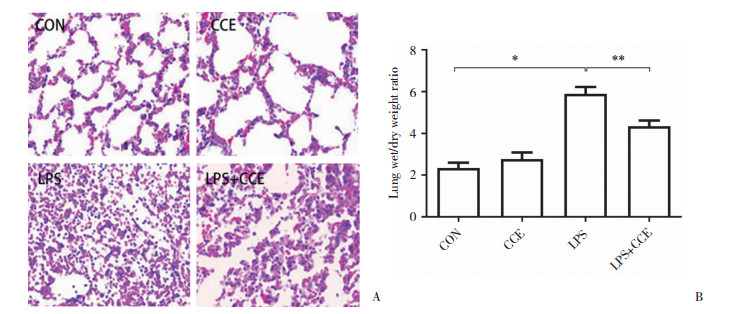
|
| A, the protective effect of CCE on acute lung injury in mice(HE staining×200);B, wet-dry weight ratio of lung in different groups of mice(n = 9). *P < 0.05;**P < 0.01. CON, control group. 图 2 CCE对ALI小鼠肺组织病理变化及水肿程度的影响 Fig.2 The effect of CCE on the pathological changes of lung tissue and the degree of edema in ALI mice |
为了观察ALI时肺泡和肺间质充血、水肿的程度,本研究进一步检测了肺湿/干质量比。结果如图 2B所示,LPS组小鼠肺湿/干质量高于正常对照组,差异有统计学意义(P < 0.01);LPS+CCE组较LPS组显著降低,差异有统计学意义(P = 0.023)。提示CCE能改善ALI肺组织水肿程度。
2.4 CCE对ALI模型小鼠肺中VDR表达的影响Western blotting和实时PCR结果如图 3所示,CCE组小鼠肺组织中VDR蛋白表达水平高于正常对照组,LPS+CCE组VDR蛋白表达水平高于LPS组,差异有统计学意义(P = 0.012,P = 0.005)。提示饲喂CCE能增加小鼠肺组织内VDR表达。
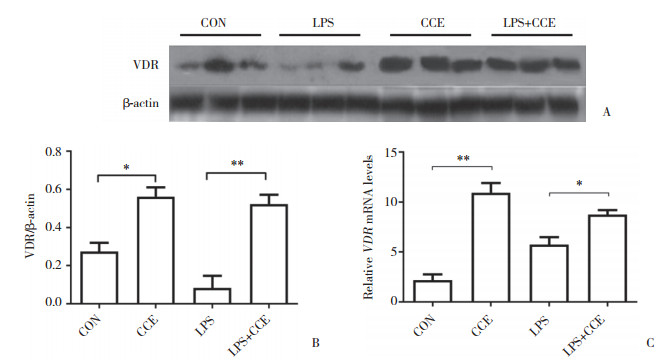
|
| A and B, Western blotting results; C, real-time PCR results. *P < 0.05;**P < 0.01. 图 3 小鼠肺组织VDR蛋白及mRNA表达情况 Fig.3 Expression of VDR protein and mRNA in the lungs of mice |
2.5 CCE对ALI模型小鼠肺组织SP-A、SP-B、SP-C、SP-D mRNA表达的影响
如表 3所示,CCE小鼠肺组织SP-A、SP-C和SP-D的表达水平高于正常对照组,差异有统计学意义(P = 0.007,P = 0.011,P = 0.006);与正常对照组相比,LPS组SP-A、SP-B、SP-C、SP-D的表达水平均显著下降(P = 0.006,P = 0.016,P = 0.001,P = 0.001),而LPS+CCE组SP-A、SP-B、SP-C、SP-D的表达水平均较LPS组显著上升(P = 0.001,P = 0.049,P = 0.001,P = 0.042),差异有统计学意义。表明CCE能促进小鼠肺泡中肺表面活性物质的产生。
| Group | SP-A | SP-B | SP-C | SP-D |
| Control | 2.403±1.551 | 0.003±0.001 | 7.522±1.998 | 3.758±0.511 |
| CCE | 3.175±0.6111) | 0.005±0.002 | 11.233±3.3322) | 5.189±1.2781) |
| LPS | 0.750±0.0011) | 0.001±0.0002) | 1.985±0.8011) | 0.861±0.1271) |
| LPS+CCE | 0.998±0.0311) | 0.002±0.0012) | 3.452±0.0531) | 1.233±0.1302) |
| 1)P < 0.01,2)P < 0.05 vs control group. | ||||
3 讨论
尽管临床研究[21-22]发现,活性维生素D可影响血清钙磷水平,但本研究结果显示CCE并未影响小鼠的血清钙磷水平,因此排除了血清钙磷对后续实验结果的影响。
本研究发现,LPS+CCE组小鼠死亡首发时间晚于LPS组,且平均生存时间长于LPS组,提示维生素D前体CCE有显著延长ALI小鼠生存时间的作用,原因可能与维生素D的抗炎特性有关[23-24]。本研究还发现,CCE具有减轻ALI所致肺组织损伤的作用。HE染色显示,LPS组小鼠肺组织可见肺泡上皮细胞及间质水肿、肺泡腔内渗出、充血、炎症细胞浸润等典型ALI病理改变[25],而LPS+CCE组ALI病理改变较LPS组减轻。小鼠肺湿/干质量比能反映ALI所致肺水肿的程度,ALI时肺水肿严重,肺湿/干质量比升高[26]。本研究结果显示,LPS+CCE组肺湿/干质量比较LPS组降低,提示CCE在一定程度上缓解了LPS对小鼠造成的ALI肺水肿。
本研究发现,与正常对照组和LPS组相比,CCE组和LPS+CCE组小鼠肺组织内VDR表达水平显著升高,表明饲喂CCE能使小鼠体内VDR表达增多。研究[27-29]表明,ALI会造成肺泡不稳定、肺泡去复张及相应的组织重塑,肺表面活性物质可降低肺泡表面张力,从而防止低肺容量时发生肺泡塌陷及水肿[30-31],因此增加肺表面活性物质可治疗ALI [4]。维生素D3可加速胎儿肺成熟和Ⅱ型肺泡上皮细胞分化,并增加大鼠肺组织和Ⅱ型肺泡上皮细胞中肺表面活性物质的表达和分泌[32-34]。有研究[35]表明,维生素D3可直接作用于原代培养的Ⅱ型肺泡上皮细胞,诱导SP-B mRNA的产生,故推测维生素D前体CCE对ALI的保护作用有可能是因为增加了肺表面活性物质,因此本研究检测了各组小鼠肺组织中SP-A、SP-B、SP-C、SP-D的表达情况,结果证实CCE确能增加SP的表达。
综上所述,本研究证实了维生素D前体物质CCE能缓解LPS造成的ALI,改善并延长ALI小鼠的生存时间,改善肺组织病理学变化及肺水肿,增加SP的表达。因此,维生素D前体CCE增加肺表面活性物质可能是缓解ALI的机制之一,为探索ALI治疗方法提供了新的思路。
| [1] |
ZIENTARA A, STEPHAN M, VON HÖRSTEN S, et al. Differential severity of LPS-induced lung injury in CD26/DPP4 positive and deficient F344 rats[J]. Histol Histopathol, 2019, 34(10): 1151-1171. DOI:10.14670/HH-18-117 |
| [2] |
KONG J, ZHU XD, SHI YY, et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system[J]. Mol Endocrinol, 2013, 27(12): 2116-2125. DOI:10.1210/me.2013-1146 |
| [3] |
MARTÍNEZ-CALLE M, OLMEDA B, DIETL P, et al. Pulmonary surfactant protein SP-B promotes exocytosis of lamellar bodies in alveolar typeⅡcells[J]. FASEB J, 2018, 32(8): 4600-4611. DOI:10.1096/fj.201701462rr |
| [4] |
STEFFEN L, RUPPERT C, HOYMANN HG, et al. Surfactant replacement therapy reduces acute lung injury and collapse induration-related lung remodeling in the bleomycin model[J]. Am J Physiol Lung Cell Mol Physiol, 2017, 313(2): L313-L327. DOI:10.1152/ajplung.00033.2017 |
| [5] |
MATSUMOTO Y, PARK IK, KOHYAMA K. B-cell epitope spreading is a critical step for the switch from C-protein-induced myocarditis to dilated cardiomyopathy[J]. Am J Pathol, 2007, 170(1): 43-51. DOI:10.2353/ajpath.2007.060544 |
| [6] |
GROLLEAU-JULIUS A, RAY D, YUNG RL. The role of epigenetics in aging and autoimmunity[J]. Clin Rev Allergy Immunol, 2010, 39(1): 42-50. DOI:10.1007/s12016-009-8169-3 |
| [7] |
TSE G, YEO JM, CHAN YW, et al. What is the arrhythmic substrate in viral myocarditis? insights from clinical and animal studies[J]. Front Physiol, 2016, 7: 308. DOI:10.3389/fphys.2016.00308 |
| [8] |
KONG J, LI YC. Molecular mechanism of 1, 25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells[J]. Am J Physiol Endocrinol Metab, 2006, 290(5): E916-E924. DOI:10.1152/ajpendo.00410.2005 |
| [9] |
XIONG YY, LOU Y, SU H, et al. Cholecalciterol cholesterol emulsion ameliorates experimental colitis via down-regulating the pyroptosis signaling pathway[J]. Exp Mol Pathol, 2016, 100(3): 386-392. DOI:10.1016/j.yexmp.2016.03.003 |
| [10] |
LIU N, SU H, ZHANG YL, et al. Cholecalciterol cholesterol emulsion attenuates experimental autoimmune myocarditis in mice via inhibition of the pyroptosis signaling pathway[J]. Biochem Biophys Res Commun, 2017, 493(1): 422-428. DOI:10.1016/j.bbrc.2017.09.006 |
| [11] |
SU H, LOU Y, FU Y, et al. Involvement of the vitamin D receptor in energy metabolism revealed by profiling of lysine succinylome of white adipose tissue[J]. Sci Rep, 2017, 7(1): 14132. DOI:10.1038/s41598-017-14477-8 |
| [12] |
KONG J, LI YC. Effect of ANGⅡtypeⅠreceptor antagonist and ACE inhibitor on vitamin D receptor-null mice[J]. Am J Physiol Regul Integr Comp Physiol, 2003, 285(1): R255-R261. DOI:10.1152/ajpregu.00517.2002 |
| [13] |
KONG J, ZHANG ZY, LI DD, et al. Loss of vitamin D receptor produces polyuria by increasing thirst[J]. J Am Soc Nephrol, 2008, 19(12): 2396-2405. DOI:10.1681/asn.2008010011 |
| [14] |
KONG J, KIM GH, WEI MJ, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats[J]. Am J Pathol, 2010, 177(2): 622-31. DOI:10.2353/ajpath.2010.091292 |
| [15] |
KONG J, HAN L, SU H, et al. Riligustilide attenuated renal injury by the blockade of renin[J]. Cell Physiol Biochem, 2018, 50(2): 654-667. DOI:10.1159/000494186 |
| [16] |
KONG J, DING Y, ZHANG C, et al. Severe vitamin D-deficiency and increased bone turnover in patients with hepatitis B from northeastern China[J]. Endocr Res, 2013, 38(4): 215-222. DOI:10.3109/07435800.2013.768266 |
| [17] |
YAZICI G, YILDIZ F, ISKIT A, et al. The effect of vitamin D prophylaxis on radiation induced pulmonary damage[J]. J Radiat Res, 2011, 52(5): 616-621. DOI:10.1269/jrr.11033 |
| [18] |
HEUSER A, EISENHAUER A, SCHOLZ-AHRENS KE, et al. Biological fractionation of stable Ca isotopes in Göttingen minipigs as a physiological model for Ca homeostasis in humans[J]. Isotopes Environ Health Stud, 2016, 52(6): 633-648. DOI:10.1080/10256016.2016.1151017 |
| [19] |
VERONE-BOYLE AR, SHOEMAKER S, ATTWOOD K, et al. Diet-derived 25-hydroxyvitamin D3 activates vitamin D receptor target gene expression and suppresses EGFR mutant non-small cell lung cancer growth in vitro and in vivo[J]. Oncotarget, 2016, 7(1): 995-1013. DOI:10.18632/oncotarget.6493 |
| [20] |
CHEN L, WILSON R, BENNETT E, et al. Identification of vitamin D sensitive pathways during lung development[J]. Respir Res, 2016, 17: 47. DOI:10.1186/s12931-016-0362-3 |
| [21] |
PARK H, BRANNON PM, WEST AA, et al. Maternal vitamin D biomarkers are associated with maternal and fetal bone turnover among pregnant women consuming controlled amounts of vitamin D, calcium, and phosphorus[J]. Bone, 2017, 95: 183-191. DOI:10.1016/j.bone.2016.12.002 |
| [22] |
PARKER VJ, HARJES LM, DEMBEK K, et al. Association of vitamin D metabolites with parathyroid hormone, fibroblast growth factor-23, calcium, and phosphorus in dogs with various stages of chronic kidney disease[J]. J Vet Intern Med, 2017, 31(3): 791-798. DOI:10.1111/jvim.14653 |
| [23] |
CHEN GC, ZHANG ZL, WAN ZX, et al. Circulating 25-hydroxyvitamin D and risk of lung cancer:a dose-response meta-analysis[J]. Cancer Causes Control, 2015, 26(12): 1719-1728. DOI:10.1007/s10552-015-0665-6 |
| [24] |
FENG QQ, ZHANG H, DONG ZQ, et al. Circulating 25-hydroxyvitamin D and lung cancer risk and survival:a dose-response meta-analysis of prospective cohort studies[J]. Medicine (Baltimore), 2017, 96(45): e8613. DOI:10.1097/MD.0000000000008613 |
| [25] |
TAN W, CHEN L, WANG YX, et al. Protectin DX exhibits protective effects in mouse model of lipopolysaccharide-induced acute lung injury[J]. Chin Med J, 2018, 131(10): 1167-1173. DOI:10.4103/0366-6999.227618 |
| [26] |
AN XN, SUN XT, HOU YH, et al. Protective effect of oxytocin on LPS-induced acute lung injury in mice[J]. Sci Rep, 2019, 9(1): 2836. DOI:10.1038/s41598-019-39349-1 |
| [27] |
GALVIN JR, FRAZIER AA, FRANKS TJ. Collaborative radiologic and histopathologic assessment of fibrotic lung disease[J]. Radiology, 2010, 255(3): 692-706. DOI:10.1148/radiol.10090717 |
| [28] |
MYERS JL, KATZENSTEIN AL. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia[J]. Chest, 1988, 94(6): 1309-1311. DOI:10.1378/chest.94.6.1309 |
| [29] |
TODD NW, ATAMAS SP, LUZINA IG, et al. Permanent alveolar collapse is the predominant mechanism in idiopathic pulmonary fibrosis[J]. Expert Rev Respir Med, 2015, 9(4): 411-418. DOI:10.1586/17476348.2015.1067609 |
| [30] |
NIEMAN GF, BREDENBERG CE. High surface tension pulmonary edema induced by detergent aerosol[J]. J Appl Physiol, 1985, 58(1): 129-136. DOI:10.1152/jappl.1985.58.1.129 |
| [31] |
OCHS M. The closer we look the more we see? Quantitative microscopic analysis of the pulmonary surfactant system[J]. Cell Physiol Biochem, 2010, 25(1): 27-40. DOI:10.1159/000272061 |
| [32] |
MARIN L, DUFOUR ME, NGUYEN TM, et al. Maturational changes induced by 1 alpha, 25-dihydroxyvitamin D3 in type Ⅱ cells from fetal rat lung explants[J]. Am J Physiol, 1993, 265(1 Pt 1): L45-L52. DOI:10.1152/ajplung.1993.265.1.L45 |
| [33] |
MARIN L, DUFOUR ME, TORDET C, et al. 1, 25(OH) 2D3 stimulates phospholipid biosynthesis and surfactant release in fetal rat lung explants[J]. Biol Neonate, 1990, 57(3-4): 257-260. DOI:10.1159/000243200 |
| [34] |
NGUYEN TM, GUILLOZO H, MARIN L, et al. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium[J]. Am J Physiol, 1996, 271(3 Pt 1): L392-L399. DOI:10.1152/ajplung.1996.271.3.L392 |
| [35] |
PHOKELA SS, PELEG S, MOYA FR, et al. Regulation of human pulmonary surfactant protein gene expression by 1alpha, 25-dihydroxyvitamin D3[J]. Am J Physiol Lung Cell Mol Physiol, 2005, 289(4): L617 L617-L626. DOI:10.1152/ajplung.00129.2004 |
 2020, Vol. 49
2020, Vol. 49




