文章信息
- 郭欣欣, 张博涵, 赵梦楠, 王品莹, 陶学恕
- GUO Xinxin, ZHANG Bohan, ZHAO Mengnan, WANG Pinying, TAO Xueshu
- PPARα对肥胖大鼠神经病理性疼痛的作用及其机制
- Effect of PPARα on neuropathic pain in obese rats and its mechanism
- 中国医科大学学报, 2020, 49(2): 124-128
- Journal of China Medical University, 2020, 49(2): 124-128
-
文章历史
- 收稿日期:2019-04-02
- 网络出版时间:2019-12-23 14:25
2. 中国医科大学附属第一医院疼痛科, 沈阳 110001
2. Department of Pain Medicine, The First Hospital, China Medical University, Shenyang 110001, China
研究[1]显示,肥胖与胰岛素抵抗、血脂异常、2型糖尿病和神经退行性疾病相关。最新研究[2]表明肥胖与疼痛易感性增加有关。饮食诱导的肥胖大鼠或肥胖的Zucker大鼠在皮内注射角叉菜胶时表现出增强的外周炎症和炎症痛觉过敏[3-4]。目前没有预防或治愈肥胖相关疼痛的方法,所以阐明其作用机制非常重要。
过氧化物酶体增殖物激活受体α(peroxidosome proliferators activate receptors α,PPARα)与许多脂代谢性疾病(肥胖症等)有关。研究[5]发现在肥胖人群肝脏中PPARα下调。PPARα已被证实在许多炎症相关的神经病理性疼痛(neuropathic pain,Nep)中发挥止痛作用。Nep与中枢小胶质细胞活化及其随后释放的促炎性细胞因子白细胞介素-6(interleukin-6,IL-6)、白细胞介素-1β(interleukin-1β,IL-1β)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)有关[6],研究[7]表明小胶质细胞最终引起脊髓等部分的神经细胞凋亡。有研究[8]发现炎症痛时脊髓中的PPARα激活,并迅速参与到痛觉传导的过程中。另外还有研究[9]发现给予PPARα激动剂可以有效降低硫酸镁注射引起的疼痛,而敲除PPARα基因后发现大鼠的痛觉敏感性增加,而给予PPARα激动剂后却没有显示出明显的抗疼痛作用。肥胖是全身低度的慢性炎症状态,外周脂肪细胞变性坏死促进炎症介质释放,脂肪细胞凋亡导致促炎性细胞因子增多[10],造成疼痛敏感性增强。人外周血单核细胞中PPARα激活还具有抗氧化、抗炎、抗凋亡等重要作用[11]。
本研究采用高脂肪饮食诱导建立肥胖大鼠模型,进而建立Nep模型,探讨PPARα在肥胖大鼠Nep中的作用及其机制。
1 材料与方法 1.1 实验动物及试剂体质量约70 g的雄性SD大鼠(辽宁长生生物中心),饲养在23~25 ℃,12 h光照/黑暗循环的房间中,随意提供食物和水。所有实验均按照《动物和人类研究指导原则》进行。实验程序经中国医科大学动物保护与使用委员会批准。PPARα、Bax、Bcl-2抗体购自美国Cell Signaling Technology公司;PPARα激动剂(PEA)、PPARα抑制剂(GW6471)购自美国APExBIO公司。
1.2 方法 1.2.1 大鼠饲养及分组取SPF级SD大鼠60只,采用随机数字表法随机分为高脂(high fat,HF)组、HF+坐骨神经分支损伤(spared nerve injury,SNI)组、HF+SNI+PEA组、HF+SNI+GW6471组、低脂(low fat,LF)组、LF+SNI组。连续高脂/低脂饲料喂养12周,HF组大鼠给予高脂饮食(含脂肪45% kcal;美国New Brunswick公司)诱导肥胖,具体方法见文献[12],LF组大鼠给予低脂饮食(含脂肪10% kcal)。采用SNI法制备Nep模型,HF或LF组大鼠喂养12周后处死,每组收集部分大鼠L4-6脊髓(spinal cord,SC)和脊髓背根神经节(dorsal root ganglion,DRG)。在大鼠Nep模型建立后14 d收集大鼠L4-6 SC和DRG。HF+SNI+PEA组、HF+SNI+G6471组术后14 d时,鞘内分别给予PEA(0.03 μmol/kg)、GW6471(0.03 μmol/ kg),连续7 d,期间每天测量机械性异常性疼痛,7 d后收集大鼠SC和DRG。
1.2.2 大鼠机械刺激缩爪潜伏期(paw withlraw threshold,PWT)测定采用Von Frey实验评估机械性异常性疼痛,具体方法见文献[13]。将大鼠置于具有网状底部的笼中,并将校准的Von Frey针垂直施用于动物的后爪,直至观察到阳性反应(缩爪和舔或摇动爪)。
1.2.3 大鼠SNI模型制备模拟制作与神经损伤相关的Nep的SNI模型。通过吸入3%异氟烷使大鼠麻醉,并且暴露主要坐骨神经及其后肢的分支。结扎或切断胫神经和腓总神经,保留细小腓肠神经。24 h在后爪和足外侧产生显著疼痛反应。
1.2.4 脊髓蛛网膜下腔的腰椎导管插入术按照文献[14]方法进行鞘内置管。吸入3%异氟烷麻醉大鼠,制作中线侧面切口,并将引导插管(20 G)插入蛛网膜下腔。动物清醒后通过导管施用2%利多卡因(10 μL),通过尾甩动作和后肢麻痹表明定位正确。
1.2.5 Western blotting实验取出大鼠L4-6 SC和DRG,在含有RIPA裂解物(P0013B)的400 μL匀浆缓冲液中匀浆。然后将样品0 ℃温育30 min,在4 ℃ 15 000 r/min离心10 min。收集上清液并使其变性,在十二烷基硫酸钠聚丙烯酰胺凝胶上进行电泳,然后转移到聚偏二氟乙烯膜上。用含有5%脱脂奶粉的封闭缓冲液封闭膜,然后与PPARα一抗(1: 1 000)和GAPDH(1:1 000)孵育。洗涤膜并与适当二抗(1:10 000)温育。用ECL试剂(Millipore Bio-science)显色印迹,获得图像并用分子成像仪(Gel Doc TMXR,170-8170)和相关的Quantity One 4.6.5软件分析。
1.3 统计学分析数据均以x±s表示。使用Graphpad Prism软件进行统计学分析。组间比较使用单向或双向ANOVA分析,然后进行Bonferroni检验。P < 0.05为差异有统计学意义。
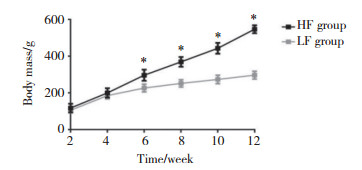
2 结果 2.1 各组大鼠体质量、PWT比较结果显示,喂养6、8、10、12周时HF组大鼠比LF组大鼠体质量增加显著(P < 0.05)。见图 1。
|
| *P < 0.05 vs LF group. 图 1 各组大鼠体质量变化情况 Fig.1 Comparison of body mass of rats in each group |
喂养12周后LF组、HF组、HF+SNI组、LF+SNI组、HF+SNI+PEA组、HF+SNI+GW 6471组50%PWT分别为(13.8±1.10)g、(5.98±1.22)g、(0.11±0.02)g、(0.17±0.40)g、(3.70±1.10)g、(0.07±0.01)g。与LF组比较,HF组大鼠的50%PWT显著降低(P < 0.05);与HF组比较,HF+SNI组大鼠50%PWT进一步降低(P < 0.05);与LF+SNI组比较,HF+SNI组50%PWT也显著降低(P < 0.05);与HF+SNI组比较,HF+SNI+PEA组50%PWT显著提高(P < 0.05),HF+SNI+ GW6471组显著降低(P < 0.05)。
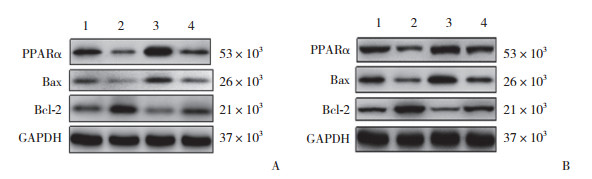
2.2 各组大鼠SC中PPARα、Bax、Bcl-2表达比较结果显示,与LF组比较,HF组SC中凋亡蛋白Bax表达显著提高(P < 0.05),凋亡蛋白Bcl-2表达显著降低(P < 0.05);与HF组比较,HF+SNI组Bax表达进一步上调(P < 0.05),Bcl-2表达进一步下调(P < 0.05)。与HF+SNI组比较,HF+SNI+PEA组SC中凋亡蛋白Bax表达显著降低,Bcl-2表达显著升高(P < 0.05),HF+SNI+ GW6471组凋亡蛋白Bax表达显著升高,Bcl-2表达显著降低(P < 0.05)。见表 1、图 2。
| Group | Bax | Bcl-2 | PPARα |
| HF | 0.60±0.021) | 0.52±0.071) | 0.68±0.121) |
| HF+SNI | 0.86±0.052) | 0.31±0.132) | 0.32±0.042) |
| LF | 0.48±0.03 | 0.89±0.06 | 0.91±0.212) |
| LF+SNI | 0.58±0.04 | 0.49±0.08 | 0.48±0.12 |
| HF+SNI+PEA | 0.58±0.123) | 0.61±0.063) | 0.79±0.213) |
| HF+SNI+ GW 6471 | 1.17±0.093) | 0.13±0.193) | 0.22±0.073) |
| 1)P < 0.05 vs LF group;2)P < 0.05 vs HF group;3)P < 0.05 vs HF+SNI group. | |||
|
| In panel A:1, HF group; 2, HF+SNI group; 3, LF group; 4, LF+SNI group. In panel B:1, HF group; 2, HF+SNI group; 3, HF+SNI+PEA group; 4, HF+SNI+ GW6471 group. 图 2 各组大鼠SC中PPARα,细胞凋亡蛋白Bax、Bcl-2表达情况 Fig.2 Expression of apoptosis proteins Bax and bcl-2, PPARα in the SC of rats in each group |
与LF组比较,HF组PPARα表达显著降低(P < 0.05);与HF组比较,HF+SNI组PPARα表达进一步降低(P < 0.05)。与HF+SNI组比较,HF+SNI+PEA组SC中PPARα表达显著提高(P < 0.05),而HF+SNI+ GW 6471组PPARα表达显著降低(P < 0.05)。见表 1、图 2。
3 讨论研究[15]显示,SC中的PPARα途径在介导Nep中起主要作用,PEA能够逆转SNI野生型小鼠的机械性疼痛和热痛觉过敏,PEA已被证明是通过恢复小鼠SNI中的谷氨酸突触功能来改善疼痛。研究[16-17]显示,PEA通过激活PPARα在慢性疼痛中发挥抗炎、镇痛、免疫调节和神经保护作用。
本研究结果显示,HF诱导的肥胖Nep大鼠机械疼痛敏感性增强,而鞘内注射PPARα激活剂PEA可减轻症状,而鞘内注射PPARα抑制剂(GW6471)则症状加重。HF诱导的肥胖Nep大鼠SC中PPARα表达下降,细胞凋亡因子Bax表达增加,Bcl-2表达降低。而PEA逆转大鼠SC中相关因子的表达。可见鞘内注射PEA可增加HF诱导的肥胖Nep大鼠SC中PPARα活性,导致机械疼痛敏感性降低。相反,鞘内注射GW6471降低SC中PPARα活性,导致机械疼痛敏感度增高。提示HF诱导的肥胖Nep大鼠疼痛增强是SC中PPARα活性降低导致细胞凋亡增加引起的。因此SC中PPARα活性受损是HF诱导的肥胖Nep大鼠疼痛增强的主要原因。
促凋亡蛋白和抑凋亡蛋白共同存在于细胞内,当凋亡信号发出时,2种蛋白平衡模式破坏,促调亡蛋白增多,使细胞发生凋亡[18]。本研究结果显示,HF诱导的肥胖Nep大鼠SC中细胞凋亡过程激活。有研究[19]证实肥胖与氧化应激及慢性炎症反应相关。在肥胖小鼠外周脂肪组织、肝脏、骨骼肌、心肌、血清、关节液等中ROS、SOD、MDA含量显著增加,炎症细胞因子TNF-α、IL-β等明显增加[20-21]。肥胖患者机体激活的免疫细胞产生大量ROS,促进氧化应激、炎症反应等发生,继而引起神经元损伤,引起Nep增强。
综上所述,HF诱导的肥胖大鼠SC中PPARα活性降低。PPARα活性降低导致SC中细胞凋亡蛋白Bax增加,Bcl-2减少,进而促进HF诱导的SNI大鼠疼痛增强。因此推测肥胖诱导的大鼠SC中PPARα活性降低,PPARα可能成为预防和治疗肥胖相关Nep的新靶点。
| [1] |
SCHWARTZ MW, SEELEY RJ, ZELTSER LM, et al. Obesity pathogenesis:an endocrine society scientific statement[J]. Endocr Rev, 2017, 38(4): 267-296. DOI:10.1210/er.2017-00111 |
| [2] |
IANNITTI T, GRAHAM A, DOLAN S. Increased central and peripheral inflammation and inflammatory hyperalgesia in Zucker rat model of leptin receptor deficiency and genetic obesity[J]. Exp Physiol, 2012, 97(11): 1236-1245. DOI:10.1113/expphysiol.2011.064220 |
| [3] |
WANG J, ZHANG Q, ZHAO L, et al. Down-regulation of PPARα in the spinal cord contributes to augmented peripheral inflammation and inflammatory hyperalgesia in diet-induced obese rats[J]. Neuroscience, 2014, 278: 165-178. DOI:10.1016/j.neuroscience.2014.07.071 |
| [4] |
ZHANG YN, SONG CW, LI HO, et al. Ursolic acid prevents augmented peripheral inflammation and inflammatory hyperalgesia in high-fat diet-induced obese rats by restoring downregulated spinal PPARα[J]. Mol Med Rep, 2016, 13(6): 5309-5316. DOI:10.3892/mmr.2016.5172 |
| [5] |
LOVERME J, RUSSO R, LA RANA G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha[J]. J Pharmacol Exp Ther, 2006, 319(3): 1051-1061. DOI:10.1124/jpet.106.111385 |
| [6] |
BARON R, BINDER A, WASNER G. Neuropathic pain:diagnosis, pathophysiological mechanisms, and treatment[J]. Lancet Neurol, 2010, 9(8): 807-819. DOI:10.1016/s1474-4422(10)70143-5 |
| [7] |
KIM D, YOU B, JO EK, et al. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain[J]. Proc Natl Acad Sci USA, 2010, 107(33): 14851-14856. DOI:10.1073/pnas.1009926107 |
| [8] |
KIM M, TIAN R. Targeting AMPK for cardiac protection:opportunities and challenges[J]. J Mol Cell Cardiol, 2011, 51(4): 548-553. DOI:10.1016/j.yjmcc.2010.12.004 |
| [9] |
YANG YJ, HU L, XIA YP, et al. Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK[J]. J Neuroinflammation, 2016, 13(1): 84. DOI:10.1186/s12974-016-0550-6 |
| [10] |
LAUDISIO D, MUSCOGIURI G, BARREA L, et al. Obesity and breast cancer in premenopausal women:Current evidence and future perspectives[J]. Eur J Obstet Gynecol Reprod Biol, 2018, 230: 217-221. DOI:10.1016/j.ejogrb.2018.03.050 |
| [11] |
VARGA T, CZIMMERER Z, NAGY L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation[J]. Biochim Biophys Acta, 2011, 1812(8): 1007-1022. DOI:10.1016/j.bbadis.2011.02.014 |
| [12] |
SONG T, LV LY, XU J, et al. Diet-induced obesity suppresses sevoflurane preconditioning against myocardial ischemia-reperfusion injury:role of AMP-activated protein kinase pathway[J]. Exp Biol Med(Maywood), 2011, 236(12): 1427-1436. DOI:10.1258/ebm.2011.011165 |
| [13] |
FANG XY, XU XR, LIN XW, et al. Downregulated spinal IRF8 and BDNF in NAC are involved in neuropathic pain-induced depression relief via pulsed radiofrequency on dorsal root ganglion in rat SNI model[J]. Brain Res Bull, 2019, 146: 192-200. DOI:10.1016/j.brainresbull.2019.01.008 |
| [14] |
STØRKSON RV, KJØRSVIK A, TJØLSEN A, et al. Lumbar catheterization of the spinal subarachnoid space in the rat[J]. J Neurosci Methods, 1996, 65(2): 167-172. DOI:10.1016/0165-0270(95)00164-6 |
| [15] |
BOCCELLA S, CRISTIANO C, ROMANO R, et al. Ultra-micronized palmitoylethanolamide rescues the cognitive decline-associated loss of neural plasticity in the neuropathic mouse entorhinal cortex-dentate gyrus pathway[J]. Neurobiol Dis, 2019, 121: 106-119. DOI:10.1016/j.nbd.2018.09.023 |
| [16] |
GUIDA F, LUONGO L, MARMO F, et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice[J]. Mol Brain, 2015, 8: 47. DOI:10.1186/s13041-015-0139-5 |
| [17] |
GUIDA F, LUONGO L, BOCCELLA S, et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity:involvement of the CB2 receptor[J]. Sci Rep, 2017, 7(1): 375. DOI:10.1038/s41598-017-00342-1 |
| [18] |
白雪, 宁宏. PP242对人晶状体上皮细胞凋亡的诱导及凋亡相关蛋白Bax表达的影响[J]. 中国医科大学报, 2018, 47(6): 548-551. DOI:10.12007/j.issn.0258-4646.2018.06.015 |
| [19] |
HUANG FF, WANG JJ, YU FM, et al. Protective effect of Meretrix meretrix oligopeptides on high-fat-diet-induced non-alcoholic fatty liver disease in mice[J]. Mar Drugs, 2018, 16(2): E39. DOI:10.3390/md16020039 |
| [20] |
LEE BC, KIM MS, PAE M, et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity[J]. Cell Metab, 2016, 23(4): 685-698. DOI:10.1016/j.cmet.2016.03.002 |
| [21] |
BOULANGÉ CL, NEVES AL, CHILLOUX J, et al. Impact of the gut microbiota on inflammation, obesity, and metabolic disease[J]. Genome Med, 2016, 8(1): 42. DOI:10.1186/s13073-016-0303-2 |
 2020, Vol. 49
2020, Vol. 49