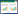文章信息
- 关国欣, 王冰, 黄宝俊, 赵丹懿
- GUAN Guoxin, WANG Bing, HUANG Baojun, ZHAO Danyi
- 沉默牛磺酸上调基因1通过Wnt/CTNNB1信号通路提高胃癌细胞对顺铂和5-氟尿嘧啶的敏感性
- Taurine upregulated gene 1 silencing enhances the sensitivity of gastric cancer cells to cisplatin and 5-fluorouracil through the Wnt/CTNNB1 signaling pathway
- 中国医科大学学报, 2020, 49(12): 1061-1065, 1081
- Journal of China Medical University, 2020, 49(12): 1061-1065, 1081
-
文章历史
- 收稿日期:2020-01-18
- 网络出版时间:2020-12-03 10:23
2. 大连医科大学附属第二医院肿瘤科, 辽宁 大连 116027;
3. 中国医科大学附属第一医院肿瘤外科, 沈阳 110001
2. Department of Oncology, The Second Hospital of Dalian Medical University, Dalian 116027, China;
3. Department of Surgical oncology, The First Hospital, China Medical University, Shenyang 110001, China
胃癌在我国发病率仅次于肺癌,近年来胃癌的发病率和死亡率逐年上升[1-2]。化学治疗(简称化疗)是治疗肿瘤最有效的方法之一。中国临床肿瘤学会对胃癌的一线治疗方案为氟尿嘧啶和铂类药物的联合治疗,如顺铂(cisplatin,CDDP)联合5-氟尿嘧啶(5-fluorouracil,5-FU)。化疗能明显改善胃癌患者的预后,但化疗耐药严重限制了化疗的临床应用。因此,研究并发现提高化疗敏感性的方法尤为重要。
许多非编码RNA被证明参与肿瘤化疗耐药的发生和维持[3-4]。生长抑制特殊转录本5 (growth arrest specific transcript 5,GAS5)降低膀胱癌细胞对阿霉素的耐药性[5],尿路上皮癌相关1 (urothelial carcinoma associated 1,UCA1)敲除抑制胃癌细胞对阿霉素的耐药性[6]。牛磺酸上调基因1 (taurine up-regulated gene 1,TUG1)是在视网膜发育的相关研究中发现并确定的[7],其在膀胱癌和胶质瘤等多种肿瘤中存在异常的表达和功能[8-11]。TUG1在胃癌中高表达,能作为癌基因发挥作用,可以作为胃癌潜在的预后因子[12]。但TUG1在胃癌化疗耐药中的作用尚不清楚。本研究拟探讨TUG1与胃癌细胞对化疗药物CDDP和5-FU的敏感性的关系及其机制。
1 材料与方法 1.1 材料 1.1.1 研究对象76例胃癌及其癌旁正常组织均取自大连医科大学附属第二医院肿瘤科的标本库。本研究获得了大连医科大学附属第二医院伦理委员会的批准,所有患者均签署知情同意书。胃癌SGC7901细胞系以及多药耐药细胞系SGC7901/R由中国医科大学肿瘤外科惠赠。本组患者包括男52例,女24例,年龄43~71岁,中位年龄为61.2岁。纳入标准:所有患者均经胃镜活检及病理检测证实为胃癌,诊断前未接受其他治疗,均选择CDDP和5-FU联合化疗(新辅助化疗或姑息化疗)作为第一阶段的治疗方案。排除标准:患者病理资料不完整,或者患有其他疾病,包括其他肿瘤。所有患者接受2~4个疗程的联合化疗,通过影像学检查(增强CT)和胃镜检查来判断治疗效果。根据瘤体大小变化等评价治疗效果,将患者分为化疗敏感组(47例)和化疗不敏感组(29例)。
1.1.2 主要试剂DMEM高糖培养基和胎牛血清购自北京全式金公司;Trizol和Lipofectamine 3000购自美国赛默飞公司;SYBR RT-PCR试剂盒购自大连宝生物公司;Smart Silencer-TUG1 (ss-TUG1)及阴性对照(ss-NC)购自广州锐博生物公司;增强型CCK8试剂盒购自上海碧云天公司;CDDP和5-FU购自美国Sigma-aldrich公司;pE-CTNNB1质粒及空质粒(pE-NC)购自上海吉玛公司;TOP Flash和FOP Flash荧光素酶报告质粒购自长沙优宝公司;双荧光素酶检测试剂盒购自北京威格拉斯公司;CTNNB1和GAPDH抗体购自云克隆公司。
1.2 方法 1.2.1 细胞培养及转染SGC7901和SGC7901/R细胞均培养于含10%胎牛血清的培养基中,培养条件为37 ℃和5% CO2。将5×104 SGC7901/R细胞接种在6孔板中,培养24 h。应用Lipofectamine 3000按照说明书操作,将Smart Silencer和(或)质粒转染SGC7901/R细胞,培养5~6 h,更换培养基后继续常规培养。
1.2.2 实时定量PCR从组织或细胞标本中提取总RNA,检测RNA的纯度和完整性。以GAPDH为内参基因,应用SYBR RT-PCR试剂盒扩增TUG1基因。引物序列见表 1。反应体系和反应条件参照说明书。应用比较CT值法对TUG1的表达量进行相对定量分析。
| Gene | Primer | Sequence |
| TUG1 | Forward | GCUUGGCUUCUAUUCUGAAUCCUUU |
| Reward | AAAGGAUUCAGAAUAGAAGCCAAGC | |
| GAPDH | Forward | AGGTCGGTGTGAACGGATTTG |
| Reward | GGGGTCGTTGATGGCAACA |
1.2.3 药物敏感性检测
分别用CDDP (0.5 μg/mL、1 μg/mL、5 μg/mL、10 μg/mL和20 μg/mL)或5-FU (1 μg/mL、2.5 μg/mL、5 μg/mL、10 μg/mL和20 μg/mL)处理SGC7901/R细胞。按照说明书使用增强型CCK8试剂盒检测细胞活力。检测450 nm处的吸光度,并计算半数抑制浓度(half maximal inhibitory concentration,IC50)。
1.2.4 荧光素酶实验应用Lipofectamine 3000将荧光素酶报告质粒转染SGC7901/R细胞。采用双荧光素酶检测试剂盒检测荧光素酶活性,以萤火虫荧光素酶为基线,肾素荧光素酶为内控,计算两者比值反映Wnt/CTNNB1信号通路的活性。
1.2.5 Western blotting提取SGC7901/R细胞总蛋白,Bradford法进行蛋白定量。取30 μg进行聚丙烯酰胺凝胶电泳,转移至聚偏二氟乙烯膜,用脱脂牛乳4 ℃封闭过夜,经CTNNB1抗体杂交,二抗孵育,化学发光,凝胶成像仪获取图像。然后,利用Image J软件对图像中的谱带进行检测和分析。
1.3 统计学分析采用SPSS 21.0统计软件对数据进行处理。每组实验重复5次,数据用x±s表示。组间差异采用单因素方差分析,两两比较采用LSD-t检验,2组独立样本均值比较采用t检验。P < 0.05为差异有统计学意义。
2 结果 2.1 TUG1的高表达与化疗敏感性相关胃癌组织表达检测发现,TUG1在不敏感组胃癌组织中的的表达明显高于其在敏感组中的表达(图 1A,P < 0.05)。
|
| A, expression of TUG1; B, IC50 of CDDP; C, IC50 of 5-FU; D, expression of TUG1. *P < 0.05 compared with sensitive group; # P < 0.05 compared with SGC7901 cells. 图 1 TUG1的高表达与胃癌患者的化疗不敏感相关 Fig.1 High TUG1 expression is related to chemotherapy insensitivity in GC |
药物敏感性检测发现,SGC7901和SGC7901/R细胞CDDP的IC50分别为(1.83±0.23) μg/mL和(9.17± 0.89) μg/mL,耐药指数(resistance index,RI)为5.01 (图 1B,P < 0.05);SGC7901和SGC7901/R细胞5-FU的IC50分别为(5.23±0.48) μg/mL和(30.62±2.78) μg/mL,RI为5.85 (图 1C,P < 0.05)。SGC7901/R细胞对CDDP和5-FU的敏感性更低,耐药率更高。与组织表达检测结果相似,TUG1在多药耐药的SGC7901/R细胞中的表达高于其在亲本的SGC7901细胞中的表达(图 1D,P < 0.05)。
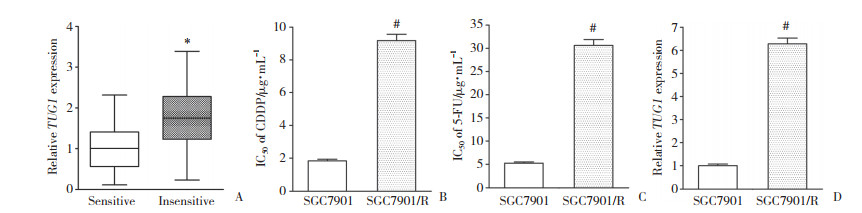
2.2 TUG1沉默增强SGC7901/R细胞对CDDP和5-FU的敏感性将ss-TUG1转染入SGC7901/R细胞可以显著降低TUG1的表达(图 2A,P < 0.05)。药物敏感性检测发现,ss-NC组SGC7901/R细胞中CDDP和5-FU的IC50分别为(8.81±0.62) μg/mL和(31.02±2.64) μg/mL,而TUG1沉默组SGC7901/R细胞中CDDP和5-FU的IC50分别为(3.57±0.41) μg/mL和(8.42±0.93) μg/mL。TUG1的沉默能提高SGC7901/R细胞对CDDP和5-FU的敏感性。
|
| A, transfection of ss-TUG1 silenced the expression of TUG1 in SGC7901/R cells; B, TUG1 silencing decreased the IC50 of CDDP in SGC7901/R cells; C, TUG1 silencing decreased the IC50 of 5-FU in SGC7901/R cells. *P < 0.05 compared with ss-NC group. 图 2 TUG1沉默增强SGC7901/R细胞对CDDP和5-FU的敏感性 Fig.2 TUG1 silencing enhances the sensitivity of SGC7901/R cells to CDDP and 5-FU |
2.3 TUG1沉默失活SGC7901/R细胞中的Wnt/CTNNB1信号通路
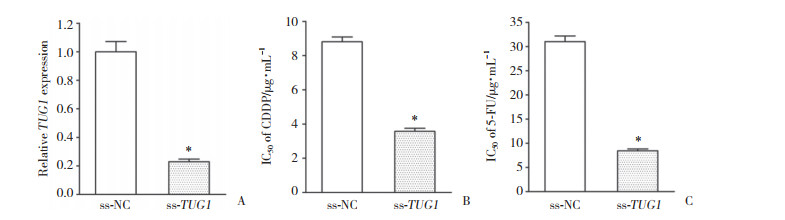
在肿瘤基因组图谱(the cancer genome atlas,TCGA)数据库中对450例胃癌样本进行共表达分析,结果显示,TUG1与CTNNB1呈显著正相关(图 3A,r = 0.324 9,P < 0.05)。其次,TUG1沉默抑制了SGC7901/R细胞中的萤火虫荧光素酶/肾素荧光素酶荧光比值,意味着Wnt/CTNNB1信号通路的活性降低(图 3B,P < 0.05)。此外,TUG1沉默抑制SGC7901/R细胞CTNNB1蛋白的表达(图 3C,P < 0.05)。因此,TUG1沉默能够失活SGC7901/R细胞中的Wnt/CTNNB1信号通路。
|
| A, positive relationship between TUG1 and CTNNB1 was confirmed by co-expression analysis using TCGA database; B, TUG1 silencing restrained the TOP/FOP ratio in SGC7901/R cells; C, TUG1 silencing inhibited CTNNB1 protein expression in SGC7901/R cells. *P < 0.05 compared with ss-NC group. 图 3 TUG1沉默失活SGC7901/R细胞中的Wnt/CTNNB1信号通路 Fig.3 TUG1 silencing inactivates the Wnt/CTNNB1 signaling pathway in SGC7901/R cells |
2.4 Wnt/CTNNB1信号通路介导TUG1对SGC7901/R细胞化疗敏感性的调节
与ss-TUG1+pE-NC组SGC7901/R细胞相比,ss-TUG1+pE-CTNNB1组SGC7901/R细胞的萤火虫荧光素酶/肾素荧光素酶荧光比值和CTNNB1蛋白表达均显著升高(图 4A、4B,P < 0.05)。CTNNB1过表达能够激活由于TUG1沉默而失活的Wnt/CTNNB1信号通路。ss-TUG1+pE-NC组SGC7901/R细胞中CDDP和5-FU的IC50分别为(3.44±0.45) μg/mL和(8.21±0.87) μg/mL,ss-TUG1+pE-CTNNB1组SGC7901/R细胞中CDDP和5-FU的IC50分别为(7.26±0.86) μg/mL和(24.63±2.69) μg/mL (图 4C、4D,P < 0.05)。ss-TUG1+pE-CTNNB1组SGC7901/R细胞中CDDP和5-FU敏感性明显低于ss-TUG1+pE-NC组SGC7901/R细胞。Wnt/CTNNB1信号通路的激活逆转了TUG1沉默对SGC7901/R细胞化疗敏感性的促进作用。
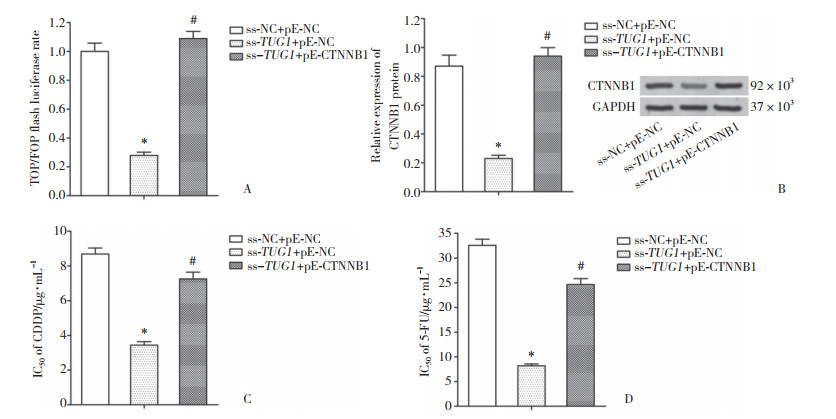
|
| A, TOP/FOP ratio; B, expression of CTNNB1 protein; C, IC50 of CDDP; D, IC50 of 5-FU. *P < 0.05 compared with ss-NC + pE-NC group; # P < 0.05 compared with ss-TUG1 + pE-NC group. 图 4 Wnt/CTNNB1信号通路介导TUG1对SGC7901/R细胞化疗敏感性的调节 Fig.4 Wnt/CTNNB1 signaling pathway mediates the regulation of TUG1 in chemotherapy sensitivity in SGC7901/R cells |
3 讨论
越来越多的研究表明,非编码RNA在肿瘤的发生、发展中起着重要而复杂的作用。其中,lncRNAs因其数量众多、功能和机制复杂等特点,引起了研究者的广泛关注。lncRNAs被证明参与了肿瘤细胞的几乎所有生物学行为,包括化疗敏感性[13-15]。例如,神经母细胞瘤相关转录本1 (neuroblastoma associated transcript 1,NBAT1)可以通过miR21/SOCS6途径抑制膀胱癌细胞的恶性表型[16],核富集丰富转录物1 (nuclear-enriched abundant transcript 1,NEAT1)作为癌基因促进胃癌细胞的恶性生物学行为和阿霉素抗性[17]。
在本研究中,TUG1在化疗耐药的胃癌组织和细胞系中表达上调,TUG1沉默能够提高SGC7901/R细胞对CDDP和5-FU的敏感性。这些发现证实了TUG1参与了胃癌化疗耐药的形成,但其机制尚不清楚。
生物信息学分析为研究TUG1在胃癌化疗耐药中的机制指明了方向。基于TCGA数据库的共表达分析证实TUG1与CTNNB1呈显著正相关,而CTNNB1是Wnt/CTNNB1信号通路的关键分子。此外,TUG1沉默能够失活SGC7901/R细胞中的Wnt/CTNNB1信号通路。Wnt/CTNNB1信号通路是一种经典的、被广泛研究的细胞内信号通路,参与多种细胞生物学功能和行为的调控[18-19]。研究[20-21]表明Wnt/CTNNB1信号通路在多种肿瘤(包括胃癌)化疗耐药的发生和维持中起重要作用。Ma等[22]报道TUG1沉默能够失活肝细胞癌中的Wnt/CTNNB1信号通路。
据此推测TUG1能够通过Wnt/CTNNB1信号通路参与调控胃癌的化疗耐药。为了验证这一假设,进行了一系列的拯救实验。结果发现,Wnt/CTNNB1信号通路的激活逆转了TUG1沉默对胃癌细胞化疗敏感性的促进作用,即Wnt/CTNNB1信号通路介导了TUG1对SGC7901/R细胞化疗敏感性的调节。然而,TUG1调控Wnt/CTNNB1信号通路的机制尚不清楚。
综上所述,TUG1与胃癌患者对CDDP和5-FU的化疗不敏感有关,TUG1沉默通过Wnt/CTNNB1信号通路促进胃癌的化疗敏感性。本研究结论有助于阐明胃癌的化疗耐药机制,同时为胃癌的生物治疗提供了新的靶点。
| [1] |
CHEN WQ, ZHENG RS, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA:A Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [2] |
STRONG VE, WU AW, SELBY LV, et al. Differences in gastric cancer survival between the US and China[J]. J Surg Oncol, 2015, 112(1): 31-37. DOI:10.1002/jso.23940 |
| [3] |
XIE DL, ZHANG H, SHANG C. Long non-coding RNA CDKN2B antisense RNA 1 gene inhibits gemcitabine sensitivity in bladder urothelial carcinoma[J]. J Cancer, 2018, 9(12): 2160-2166. DOI:10.7150/jca.25236 |
| [4] |
LI B, XIE D, ZHANG H. Long non-coding RNA GHET1 contributes to chemotherapeutic resistance to gemcitabine in bladder cancer[J]. Cancer Chemother Pharmacol, 2019, 84(1): 187-194. DOI:10.1007/s00280-019-03873-8 |
| [5] |
ZHANG H, GUO Y, SONG YS, et al. Long noncoding RNA GAS5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma[J]. Cancer Chemother Pharmacol, 2017, 79(1): 49-55. DOI:10.1007/s00280-016-3194-4 |
| [6] |
SHANG C, GUO Y, ZHANG JX, et al. Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer[J]. Cancer Chemother Pharmacol, 2016, 77(5): 1061-1067. DOI:10.1007/s00280-016-3029-3 |
| [7] |
YOUNG TL, MATSUDA T, CEPKO CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina[J]. Curr Biol, 2005, 15(6): 501-512. DOI:10.1016/j.cub.2005.02.027 |
| [8] |
SHAN H, YANG YB, ZHU XH, et al. FAM83H-AS1 is associated with clinical progression and modulates cell proliferation, migration, and invasion in bladder cancer[J]. J Cell Biochem, 2019, 120(3): 4687-4693. DOI:10.1002/jcb.27758 |
| [9] |
BI YY, SHEN G, QUAN Y, et al. Long noncoding RNA FAM83H-AS1 exerts an oncogenic role in glioma through epigenetically silencing CDKN1A (p21)[J]. J Cell Physiol, 2018, 233(11): 8896-8907. DOI:10.1002/jcp.26813 |
| [10] |
BARR JA, HAYES KE, BROWNMILLER T, et al. Long non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16 E6 independently of p53 in cervical cancer cells[J]. Sci Rep, 2019, 9(1): 3662. DOI:10.1038/s41598-019-40094-8 |
| [11] |
GONG YB, ZOU YF. Clinical significance of lncRNA FAM83H-AS1 in ovarian cancer[J]. Eur Rev Med Pharmacol Sci, 2019, 23(11): 4656-4662. DOI:10.26355/eurrev_201906_18045 |
| [12] |
DA J, LIU PP, WANG R, et al. Upregulation of the long non-coding RNA FAM83H-AS1 in gastric cancer and its clinical significance[J]. Pathol Res Pract, 2019, 215(10): 152616. DOI:10.1016/j.prp.2019.152616 |
| [13] |
ZHAO WY, SHAN B, HE D, et al. Recent progress in characterizing long noncoding RNAs in cancer drug resistance[J]. J Cancer, 2019, 10(26): 6693-6702. DOI:10.7150/jca.30877 |
| [14] |
HU XH, HONG Y, SHANG C. Knockdown of long non-coding RNA SNHG5 inhibits malignant cellular phenotypes of glioma via Wnt/CTNNB1 signaling pathway[J]. J Cancer, 2019, 10(5): 1333-1340. DOI:10.7150/jca.29517 |
| [15] |
CHI Y, WANG D, WANG J, et al. Long non-coding RNA in the pathogenesis of cancers[J]. Cells, 2019, 8(9): 1015. DOI:10.3390/cells8091015 |
| [16] |
LIU ZY, XIE DL, ZHANG H. Long noncoding RNA neuroblastoma-associated transcript 1 gene inhibits malignant cellular phenotypes of bladder cancer through miR-21/SOCS6 axis[J]. Cell Death Dis, 9(10): 1042. DOI:10.1038/s41419-018-1090-z |
| [17] |
ZHANG JL, ZHAO BC, CHEN XX, et al. Silence of long noncoding RNA NEAT1 inhibits malignant biological behaviors and chemotherapy resistance in gastric cancer[J]. Pathol Oncol Res, 2018, 24(1): 109-113. DOI:10.1007/s12253-017-0233-3 |
| [18] |
NUSSE R, CLEVERS H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities[J]. Cell, 2017, 169(6): 985-999. DOI:10.1016/j.cell.2017.05.016 |
| [19] |
HU XY, HOU PF, LI TT, et al. The roles of Wnt/β-catenin signaling pathway related lncRNAs in cancer[J]. Int J Biol Sci, 2018, 14(14): 2003-2011. DOI:10.7150/ijbs.27977 |
| [20] |
GARG M, MAURYA N. WNT/β-catenin signaling in urothelial carcinoma of bladder[J]. World J Nephrol, 2019, 8(5): 83-94. DOI:10.5527/wjn.v8.i5.83 |
| [21] |
YANG W, WU B, MA N, et al. BATF2 reverses multidrug resistance of human gastric cancer cells by suppressing Wnt/β-catenin signaling[J]. Vitro Cell Dev Biol-Animal, 2019, 55(6): 445-452. DOI:10.1007/s11626-019-00360-5 |
| [22] |
MA YK, SHEN TH, YANG XY. Upregulation of LncRNA FAM83H-AS1 in hepatocellular carcinoma promotes cell proliferation, migration and invasion by Wnt/β-catenin pathway[J]. Eur Rev Med Pharmacol Sci, 2019, 23(18): 7855-7862. DOI:10.26355/eurrev_201909_18995 |
 2020, Vol. 49
2020, Vol. 49