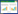文章信息
- 徐健, 赵世杰, 牛一蒙, 齐国先, 田文
- XU Jian, ZHAO Shijie, NIU Yimeng, QI Guoxian, TIAN Wen
- 血小板淋巴细胞比值与老年急性冠状动脉综合征患者冠状动脉介入治疗围术期心肌梗死的相关分析及其预测价值
- Association of Platelet-to-Lymphocyte Ratio with Periprocedural Myocardial Infarction and Its Predictive Value in Elderly Patients Who Underwent Percutaneous Coronary Intervention for Acute Coronary Syndrome
- 中国医科大学学报, 2019, 48(8): 719-725
- Journal of China Medical University, 2019, 48(8): 719-725
-
文章历史
- 收稿日期:2018-08-28
- 网络出版时间:2019-07-15 11:06
经皮冠状动脉介入治疗(percutaneous coronary intervention, PCI)后围术期心肌梗死(periprocedural myocardial infarction, PMI)与冠状动脉粥样硬化性心脏病(以下简称冠心病)患者临床预后不良显著相关[1-5]。LIBBY[6]认为在急性冠状动脉综合征(acute coronary syndromes, ACS)患者中, 炎症在粥样硬化斑块破裂和血栓形成中起着重要作用。炎症标志物, 如C反应蛋白(C-reactive protein, CRP)、高敏C反应蛋白(high-sensitivity C-reactive protein, hs-CRP)、白细胞介素-6 (interleukin-6, IL-6)以及血小板淋巴细胞比值(platelet-to-lymphocyte ratio, PLR)等与心血管不良事件发生风险增加相关[7-10]。其中, PLR同时参与炎症和止血2条通路, 而这2条通路在动脉粥样硬化血栓形成中起着重要作用。然而, 目前关于PLR与PMI的研究尚不多见。本研究拟探讨老年非ST段抬高型急性冠状动脉综合征(non-ST segment elevation acute coronary syndromes, NSTE-ACS)患者PCI术前炎症标志物水平与PMI的关系。
1 材料与方法 1.1 研究对象连续纳入2014年1月至2017年12月于中国医科大学附属第一医院老年心血管病房接受PCI治疗的206例年龄≥65岁的NSTE-ACS病例, 包括不稳定型心绞痛(unstable angina, UA)和非ST段抬高型心肌梗死(non-ST-segment elevation myocardial infarction, NSTEMI)。排除ST段抬高型心肌梗死(ST-segment elevation myocardial infarction, STEMI)、严重肾功能不全(估计肾小球滤过率 < 30 mL·min-1·1.73 m-2)、严重肝功能不全(丙氨酸氨基转氨酶或天冬氨酸氨基转移酶>3倍正常值上限)、严重心功能不全(NYHA分级Ⅳ级或Killip分级Ⅳ级)、恶性肿瘤、急性感染、血液系统疾病以及自身免疫性疾病患者。
1.2 研究方法所有患者于入院次日清晨空腹状态下采集肘正中静脉血, 以检测血常规、肝肾功能、血脂、空腹血糖以及肌钙蛋白Ⅰ (troponinⅠ, cTnⅠ)等。所有患者根据病情需要决定是否给予冠心病优化药物治疗, 如拜阿司匹林、氯吡格雷、RAAS抑制剂[血管紧张素转化酶抑制剂(angiotensin converting enzyme inhibitor, ACEI)、血管紧张素受体拮抗剂(angiotensin receptor blockers, ARB)]、β受体拮抗剂及硝酸酯类等。记录患者PCI术中植入支架的长度及数量, 术后(24±6) h复查cTnⅠ及肾功能。
依据2013年10月美国心血管造影和介入学会(Society for Cardiovascular Angiography and Interventions, SCAI)对PMI的定义[11], 将患者分为PMI组和对照组, 并通过倾向评分匹配(propensity score matching, PSM)的方法将具有相近临床特征的患者进行1︰4配对分组, 最终共116例老年NSTE-ACS患者被纳入本研究。PMI定义[11]应满足以下条件之一: (1)若术前生物标志物正常, 则术后cTnⅠ应≥70倍其正常上限; (2)若术前cTnⅠ升高且已回落, 则术后cTnⅠ升高应≥70倍其正常上限; (3)若术前cTnⅠ升高未回落, 则术后cTnⅠ升高除>70倍正常上限外, 还应同时伴有心电图新发ST段改变和心肌梗死相关症状(如新发或恶化的心力衰竭或持续性低血压)。
1.3 统计学分析所有数据均使用安装了相应PSM的统计软件SPSS 22.0进行分析处理。计量资料采用x±s或中位数(四分位间距)表示; 服从正态分布的资料2组间比较采用两独立样本t检验; 非正态分布资料2组间比较采用两独立样本秩和检验或配对样本的秩和检验。计数资料2组间比较采用χ2检验; logistic回归模型评估老年NSTE-ACS患者PCI术前PLR与PMI的关系; 采用Cochran-Armitage趋势检验判断PLR与PMI间是否存在线性趋势; 采用采试者工作特征(receiver operating characteristic, ROC)曲线分析方法计算PLR预测老年NSTE-ACS患者PCI术后发生PMI的最佳截断值及该截值的特异度和敏感度。以双侧P < 0.05为差异有统计学意义。
2 结果在进行PSM之前, 共206例行PCI的老年NSTE-ACS患者纳入本研究, PMI组28例, 对照组178例, 基线特点见表 1~2。
| Variable | PMI group (n = 28) | Control group (n = 178) | P |
| Age (year) | 74.5(71.0, 79.5) | 70.0(67.0, 76.0) | 0.007 |
| Male [n (%)] | 17(60.7) | 100(56.2) | 0.663 |
| Smoking [n (%)] | 7(25.0) | 67(37.6) | 0.195 |
| Hypertension [n (%)] | 21(75.0) | 117(65.7) | 0.332 |
| Diabetes [n (%)] | 9(32.1) | 58(32.6) | 0.963 |
| Prior MI [n (%)] | 8(28.6) | 37(20.8) | 0.354 |
| Prior PCI [n (%)] | 11(39.3) | 40(22.5) | 0.055 |
| Stroke [n (%)] | 4(14.3) | 28(15.7) | 1.000 |
| ACS diagnosis | 0.015 | ||
| UA [n (%)] | 18(10.8) | 149(89.2) | |
| NSTEMI [n (%)] | 10(25.6) | 29(74.4) | |
| Prior medications | |||
| Aspirin [n (%)] | 27(96.4) | 171(96.1) | 1.000 |
| Clopidogrel [n (%)] | 28(100.0) | 173(97.2) | 0.812 |
| β-blocker [n (%)] | 17(60.7) | 120(67.4) | 0.485 |
| Statins [n (%)] | 28(100.0) | 176(98.9) | 1.000 |
| ACEI/ARB [n (%)] | 15(53.6) | 117(56.7) | 0.213 |
| Ezetimibe [n (%)] | 5(17.9) | 40(22.5) | 0.583 |
| PMI, periprocedural myocardial infarction; PSM, propensity score matching; MI, myocardial infarction; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome; UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blockers. | |||
| Variable | PMI group (n = 28) | Control group (n = 178) | P |
| Leukocytes (×109/L) | 6.54(5.29, 7.68) | 6.27(5.03, 7.66) | 0.830 |
| Neutrophils (×109/L) | 4.26±1.60 | 4.21±1.50 | 0.871 |
| Lymphocytes (×109/L) | 1.51±0.57 | 1.69±0.59 | 0.137 |
| Plaletes (×109/L) | 222.57±63.59 | 208.16±54.01 | 0.202 |
| Monocytes (×109/L) | 0.50±0.17 | 0.46±0.16 | 0.421 |
| NLR | 2.73(2.07, 4.05) | 2.48(1.90, 3.31) | 0.262 |
| PLR | 159.43(104.60, 194.18) | 126.46(94.78, 155.41) | 0.042 |
| Glucose (mmol/L) | 6.75±3.16 | 6.40±2.16 | 0.574 |
| Total cholesterol (mmol/L) | 3.92±1.13 | 4.12±0.98 | 0.316 |
| HDL-C (mmol/L) | 0.93(0.83, 1.11) | 1.07(0.94, 1.22) | 0.026 |
| LDL-C (mmol/L) | 2.50±0.99 | 2.56±0.87 | 0.734 |
| Triglyceride (mmol/L) | 1.25(0.74, 1.89) | 1.26(0.98, 1.70) | 0.581 |
| Average stent length (mm) | 27.88(23.75, 30.60) | 24.17(19.50, 30.00) | 0.254 |
| Number of stents | 2.14±1.21 | 1.57±0.77 | 0.021 |
| PMI, periprocedural myocardial infarction; PSM, propensity score matching; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol. | |||
基线数据分析发现, 2组间既往PCI史存在差异趋势, 故通过PSM对2组患者按照年龄、性别、既往PCI进行1︰4配比, 最终PMI组与对照组患者分别为26例和90例。与对照组相比, PMI组PCI术前诊断NSTEMI的患者更多(P = 0.028), 而术前口服ACEI/ARB的患者更少(P = 0.045)。2组高血压病、糖尿病、既往心肌梗死病史以及术前其他用药史(阿司匹林、氯吡格雷、β受体拮抗剂、他汀类药物)比较无统计学差异。见表 3。
| Variable | PMI group (n = 26) | Control group (n = 90) | P |
| Age (year) | 74.50±5.52 | 74.03±5.90 | 0.719 |
| Male [n (%)] | 15(57.69) | 57(63.33) | 0.602 |
| Smoking [n (%)] | 7(26.92) | 37(41.11) | 0.189 |
| Hypertension [n (%)] | 19(73.08) | 57(63.33) | 0.357 |
| Diabetes [n (%)] | 9(34.62) | 27(30.00) | 0.654 |
| Prior MI [n (%)] | 6(23.08) | 18(20.00) | 0.733 |
| Prior PCI [n (%)] | 9(34.62) | 20(22.22) | 0.199 |
| Stroke [n (%)] | 2(7.69) | 15(16.67) | 0.409 |
| ACS diagnosis | 0.028 | ||
| UA [n (%)] | 17(17.9) | 78(82.1) | |
| NSTEMI [n (%)] | 9(42.9) | 12(57.1) | |
| Prior medications | |||
| Aspirin [n (%)] | 25(96.15) | 86(95.56) | 1.000 |
| Clopidogrel [n (%)] | 26(100) | 88(97.78) | 1.000 |
| β-blocker [n (%)] | 15(57.69) | 62(68.89) | 0.287 |
| Statins [n (%)] | 26(100) | 88(97.78) | 1.000 |
| ACEI/ARB [n (%)] | 13(50.00) | 64(71.11) | 0.045 |
| Ezetimibe [n (%)] | 5(19.23) | 16(61.54) | 1.000 |
| PMI, periprocedural myocardial infarction; PSM, propensity score matching; MI, myocardial infarction; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome; UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blockers. | |||
与对照组比较, PMI组患者术前PLR更高(P = 0.021), 而术前高密度脂蛋白胆固醇(high-density lipoprotein cholesterol, HDL-C)则更低(P = 0.028)。此外, PMI组支架植入数较对照组多(P = 0.009)。然而, 白细胞计数、中性粒细胞计数、淋巴细胞计数、血小板计数、单核细胞计数、中性粒细胞淋巴细胞比值、空腹血糖、低密度脂蛋白胆固醇(low density lipoprotein cholesterol, LDL-C)和植入支架平均长度在2组间均无统计学差异。见表 4。
| Variable | PMI group (n = 26) | Control group (n = 90) | P |
| Leukocytes (×109/L) | 6.28(5.12, 7.81) | 6.44(5.24, 7.74) | 0.910 |
| Neutrophils (×109/L) | 4.03(3.08, 5.18) | 4.00(2.98, 5.37) | 0.766 |
| Lymphocytes (×109/L) | 1.48(1.10, 1.82) | 1.64(1.34, 2.08) | 0.087 |
| Plaletes (×109/L) | 228.73±61.76 | 206.77±52.62 | 0.074 |
| Monocytes (×109/L) | 0.50±0.18 | 0.47±0.16 | 0.42 |
| NLR | 2.69(2.01, 4.00) | 2.36(1.60, 3.50) | 0.188 |
| PLR | 159.43(105.81, 196.13) | 123.86(92.46, 149.60) | 0.021 |
| Glucose (mmol/L) | 6.86±3.24 | 6.22±2.14 | 0.349 |
| Total cholesterol (mmol/L) | 3.97±1.16 | 4.08±0.96 | 0.668 |
| HDL-C (mmol/L) | 0.93(0.84, 1.09) | 1.06(0.94, 1.27) | 0.028 |
| LDL-C (mmol/L) | 2.54±1.02 | 2.52±0.87 | 0.938 |
| Triglyceride (mmol/L) | 1.26(0.81, 1.92) | 1.22(1.01, 1.68) | 0.963 |
| Average stent length (mm) | 27.88(23.92, 30.20) | 25.08(19.50, 30.16) | 0.296 |
| Number of stents | 2.23±1.21 | 1.53±0.74 | 0.009 |
| PMI, periprocedural myocardial infarction; PSM, propensity score matching; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol. | |||
在PMI影响因素的单因素和多因素logistic回归分析中, 将PLR进行四分位数分组(Q1≤94.02, 94.02 < Q2≤126.93, 126.03 < Q3≤162.23, 162.23 < Q4)。将所有P≤0.05的变量纳入分析, 结果示PLR、HDL-C、NSETMI、植入支架数均是PMI的独立影响因素, 见表 5。对HDL-C、ACEI/ARB、支架植入数、术前诊断进行校正之后发现, PLR越高, 则PMI的发生风险也越高(OR = 13.11;95%CI:2.42, 70.92;P-trend=0.017), 见表 6。
| Variable | Univariate analysis | Multivariate analysis | |||||
| OR | 95%CI | P | OR | 95%CI | P | ||
| PLR | |||||||
| Q1 | 1.00 | 1.00 | |||||
| Q2 | 2.26 | 0.51, 10.08 | 0.285 | 5.34 | 0.97, 29.20 | 0.053 | |
| Q3 | 2.26 | 0.51, 10.08 | 0.285 | 3.82 | 0.66, 22.20 | 0.135 | |
| Q4 | 5.30 | 1.29, 21.72 | 0.021 | 13.11 | 2.42, 70.92 | 0.003 | |
| HDL-C | 0.20 | 0.03, 1.23 | 0.083 | 0.07 | 0.01, 0.66 | 0.020 | |
| ACEI/ARB | 0.40 | 0.16, 0.99 | 0.048 | 0.39 | 0.13, 1.19 | 0.098 | |
| ACS diagonsis | |||||||
| UA | 1.00 | 1.00 | |||||
| NSTEMI | 3.44 | 1.25, 9.46 | 0.017 | 4.56 | 1.24, 16.78 | 0.022 | |
| Number of stents | 2.30 | 1.38, 3.82 | 0.001 | 2.83 | 1.52, 5.25 | 0.001 | |
| PCI, percutaneous coronary intervention; ACS, acute coronary syndromes; PMI, periprocedural myocardial infarction; PLR, platelet-to-lymphocyte ratio; HDL-C, high density lipoprotein cholesterol; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blockers; UA, unstable angina; NSTEMI, non-ST-segment elevation myocardial infarction. | |||||||
| Variable | PLR quartiles | P-trend | |||
| Q1 | Q2 | Q3 | Q4 | ||
| PLR | ≤94.02 | > 94.02/≤126.93 | > 126.03/≤162.23 | > 162.23 | |
| OR | 1.00 | 5.34 | 3.82 | 13.11 | 0.017 |
| 95%CI | - | 0.97, 29.20 | 0.66, 22.20 | 2.42, 70.92 | |
| P | - | 0.053 | 0.135 | 0.003 | |
| PLR, platelet-to-lymphocyte ratio. | |||||
通过ROC曲线分析可确定PLR预测老年NSET-ACS患者PCI术后发生PMI的最佳截断值是149.92, 其敏感度为57.70%, 特异度为75.60%, PLR的曲线下面积为0.65 (95%CI:0.53, 0.77, P = 0.021) (图 1)。

|
| 图 1 血小板淋巴细胞比值与PMI的ROC曲线 Fig.1 Receiver operating characteristic analysis of PLR data for PMI |
3 讨论
随着心血管介入治疗技术的进步, PCI很大程度上改善了冠心病患者的预后, 但仍有一些患者远期预后不良, 而PMI是导致患者PCI术后预后较差的主要原因之一[12]。因此, PMI的临床预测非常重要。然而目前对PMI的早期诊断、高危患者的识别以及预防尚缺乏特异性指标和措施。
本研究通过PSM的方法将具有相近临床特征的患者进行配对分组并分析, 发现PLR升高、HDL-C降低、NSTEMI以及植入多枚支架是老年NSTE-ACS患者PCI术后发生PMI的独立危险因素; 同时, PLR升高有助于预测PMI的发生风险, 在PCI术前识别PMI高危人群则有助于有针对性地制定临床治疗策略, 减少PMI发生, 改善患者预后。
炎症在ACS的发生、发展中发挥着重要作用[13-14], 有多中心、随机、双盲、安慰剂对照的临床试验证明了单纯抗炎而非降脂治疗能够明显减少冠心病患者的心血管事件[15]。目前临床上治疗缺血性心脏病的药物中可能具有抗炎作用的药物只有阿司匹林和他汀类药物[12]。有关研究[16-19]发现他汀类药物可通过其抗炎作用减少心血管事件。PLR作为一种易获取且费用便宜的炎症标志物, 目前在心血管领域越来越受到关注。研究[10, 20-22]发现, PLR升高是ACS患者冠状动脉病变严重程度和复杂性的独立危险因素, 还与行冠状动脉造影的冠状动脉粥样硬化性心脏病高危患者以及急性心肌梗死患者的心血管死亡有关。此外, PLR也是急性ST段抬高型心肌梗死患者直接PCI术后发生慢复流或无复流的强烈独立预测因素[23-24], 而无复流与PMI有关[25]。综上所述, PLR升高与冠状动脉粥样硬化性心脏病患者不良心血管事件的发生风险增加有关。PLR在PMI中可能的潜在作用机制如下:一方面, 在炎症反应过程中, 许多炎症介质刺激巨核细胞增殖并导致血小板数量相对性增多, 较高的血小板计数可间接反映潜在的炎症状态[22], 此外, 血小板增多和血小板活化也会增加血管活性介质释放, 并增加富含血小板血栓的形成和血管收缩倾向[24]; 另一方面, 当炎症反应增强时可致使淋巴细胞凋亡增加, 从而导致外周血中淋巴细胞减少[26]。然而, 作为预后标志物, PLR比单独的血小板计数或淋巴细胞计数更好。首先, 淋巴细胞或血小板较PLR更容易受到不同病理生理条件的影响; 其次, PLR还综合了止血和炎症2条通路的作用, 能够更全面地诠释动脉粥样硬化血栓形成的病理机制。
综上所述, 老年NSTE-ACS患者PCI术前PLR升高与PMI发生风险增加有关。此外, 本研究还发现, 在本组研究人群中, LDL-C在PMI组和对照组之间并没有显著差别, 这或许与患者在入院前已经接受他汀类药物降脂治疗有关。本研究是回顾性研究, 样本量相对较小, 没有对患者进行长期随访, 还需进行大规模前瞻性研究进一步验证本研究结果。
| [1] |
CAVALLINI C, SAVONITTO S, VIOLINI R, et al. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary intervention:results of the CK-MB and PCI study[J]. Eur Heart J, 2005, 26(15): 1494-1498. DOI:10.1093/eurheartj/ehi173 |
| [2] |
PATTI G, PASCERI V, COLONNA G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention:results of the ARMYDA-ACS randomized trial[J]. J Am Coll Cardiol, 2007, 49(12): 1272-1278. DOI:10.1016/j.jacc.2007.02.025 |
| [3] |
DI SCIASCIO G, PATTI G, PASCERI V, et al. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention:results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial[J]. J Am Coll Cardiol, 2009, 54(6): 558-565. DOI:10.1016/j.jacc.2009.05.028 |
| [4] |
HERRMANN J. Peri-procedural myocardial injury:2005 update[J]. Eur Heart J, 2005, 26(23): 2493-2519. DOI:10.1093/eurheartj/ehj455 |
| [5] |
LEE DW, CAVENDER MA. Periprocedural myocardial infarction in contemporary practice[J]. Interv Cardiol Clin, 2019, 8(2): 209-223. DOI:10.1016/j.iccl.2018.12.001 |
| [6] |
LIBBY P. Mechanisms of acute coronary syndromes and their implications for therapy[J]. N Engl J Med, 2013, 368(21): 2004-2013. DOI:10.1056/NEJMra1216063 |
| [7] |
FRACASSI F, NICCOLI G, VETRUGNO V, et al. Optical coherence tomography and C-reactive protein in risk stratification of acute coronary syndromes[J]. Int J Cardiol, 2019, pii:S0167-5273(18): 36900-6. DOI:10.1016/j.jcard.2019.01.058 |
| [8] |
TAJFARD M, TAVAKOLY SANY SB, AVAN A, et al. Relationship between serum high sensitivity C-reactive protein with angiographic severity of coronary artery disease and traditional cardiovascular risk factors[J]. J Cell Physiol, 2019, 234(7): 10289-10299. DOI:10.1002/jcp.27945 |
| [9] |
HELD C, WHITE HD, STEWART RAH, et al. Inflammatory biomarkers interleukin-6 and c-reactive protein and outcomes in stable coronary heart disease:experiences from the stability (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial[J]. J Am Heart Assoc, 2017, 6(10): e005077. DOI:10.1161/jaha.116.005077 |
| [10] |
KURTUL A, MURAT SN, YARLIOGLUES M, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes[J]. Am J Cardiol, 2014, 114(7): 972-978. DOI:10.1016/j.amjcard.2019.01.058 |
| [11] |
MOUSSA ID, KLEIN LW, SHAH B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization:an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI)[J]. J Am Coll Cardiol, 2013, 62(17): 1563-1570. DOI:10.1016/j.jacc.2013.08.720 |
| [12] |
MOREIRA DM, DA SILVA RL, VIEIRA JL, et al. Role of vascular inflammation in coronary artery disease:potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease[J]. Am J Cardiovasc Drugs, 2015, 15(1): 1-11. DOI:10.1007/s40256-014-0094-z |
| [13] |
CREA F, LIUZZO G. Pathogenesis of acute coronary syndromes[J]. J Am Coll Cardiol, 2013, 61(1): 1-11. DOI:10.1016/j.jacc.2012.07.064 |
| [14] |
CREA F, LIBBY P. Acute coronary syndromes:the way forward from mechanisms to precision treatment[J]. Circulation, 2017, 136(12): 1155-1166. DOI:10.1161/circulationaha.117.029870 |
| [15] |
RIDKER PM, EVERETT BM, THUREN T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease[J]. N Engl J Med, 2017, 377(12): 1119-1131. DOI:10.1056/NEJMoa1707914 |
| [16] |
NISSEN SE, TUZCU EM, SCHOENHAGEN P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease[J]. N Engl J Med, 2005, 352(1): 29-38. DOI:10.1056/NEJMoa042000 |
| [17] |
BRIGUORI C, VISCONTI G, FOCACCIO A, et al. Novel approaches for preventing or limiting events (Naples)Ⅱtrial:impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction[J]. J Am Coll Cardiol, 2009, 54(23): 2157-2163. DOI:10.1010/j.jacc.2009.07.005 |
| [18] |
RIDKER PM, DANIELSON E, FONSECA FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein[J]. N Engl J Med, 2008, 359(21): 2195-2207. DOI:10.1056/NEJMoa0807646 |
| [19] |
TUNON J, BADIMON L, BOCHATON-PIALLAT ML, et al. Identifying the anti-inflammatory response to lipid lowering therapy:a position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology[J]. Cardiovasc Res, 2019, 115(1): 10-19. DOI:10.1093/cvr/cvy293 |
| [20] |
AZAB B, SHAH N, AKERMAN M, et al. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction[J]. J Thromb Thrombolysis, 2012, 34(3): 326-334. DOI:10.1007/s11239-012-0718-6 |
| [21] |
TEMIZ A, GAZI E, GUNGOR O, et al. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction[J]. Med Sci Monit, 2014, 20: 660-665. DOI:10.12659/msm.890152 |
| [22] |
LEE Y SG, BARADI A, Peverelle M, et al. Usefulness of platelet-to-lymphocyte ratio to predict long-term all-cause mortality in patients at high risk of coronary artery disease who underwent coronary angiography[J]. Am J Cardiol, 2018, 121(9): 1021-1026. DOI:10.1016/j.amjcard.2018.01.018 |
| [23] |
KURTUL A, YARLIOGLUES M, MURAT S N, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction[J]. Am J Cardiol, 2014, 114(3): 342-347. DOI:10.1016/j.amjcard.2014.04.045 |
| [24] |
YILDIZ A, YUKSEL M, OYLUMLU M, et al. The utility of the platelet-lymphocyte ratio for predicting no reflow in patients with ST-segment elevation myocardial infarction[J]. Clin Appl Thromb Hemost, 2015, 21(3): 223-228. DOI:10.1177/1076029613519851 |
| [25] |
CHAN W, STUB D, CLARK DJ, et al. Usefulness of transient and persistent no reflow to predict adverse clinical outcomes following percutaneous coronary intervention[J]. Am J Cardiol, 2012, 109(4): 478-485. DOI:10.1016/j.amjcard.2011.09.037 |
| [26] |
HOTCHKISS RS, KARL IE. The pathophysiology and treatment of sepsis[J]. N Engl J Med, 2003, 348(2): 138-150. DOI:10.1056/NEJMra021333 |
 2019, Vol. 48
2019, Vol. 48