文章信息
- 俞达辉, 吴道立, 袁冲, 褚文炎
- YU Dahui, WU Daoli, YUAN Chong, CHU Wenyan
- 癌组织中CD45RO表达水平与肺腺癌患者临床病理特征及生存率的关联分析
- Correlation of Tissue CD45RO with the Clinicopathological Properties and Prognosis in Surgical Patients with Lung Adenocarcinoma
- 中国医科大学学报, 2018, 47(5): 448-453
- Journal of China Medical University, 2018, 47(5): 448-453
-
文章历史
- 收稿日期:2017-04-20
- 网络出版时间:2018-04-26 13:52
肺癌是全球恶性肿瘤相关死亡的主要原因之一,严重威胁着人类的健康和生命。肺腺癌是其最常见的组织学亚型[1]。随着手术治疗、放化疗、靶向治疗以及免疫治疗等多种治疗手段的应用和发展,肺腺癌患者的疗效有所提高,但其5年总生存率仍然为4%~17% [2]。
肿瘤浸润淋巴细胞(tumor-infiltrating lymphocyte,TILs)作为一类由不同细胞构成的异质细胞群,可浸入肿瘤组织直接反映宿主与肿瘤之间的免疫应答状态[3]。CD45分子,即蛋白络氨酸磷酸酶受体C型,可作为T淋巴细胞亚群的分类标志。CD45RO+记忆T细胞是较常见的T细胞亚型,有强大的自我更新能力,可在再次接触抗原后产生强烈的免疫应答,从而在抗肿瘤免疫反应中起着重要作用[4]。然而,关于CD45RO表达水平在肺腺癌患者中的作用目前尚不明确。因此,本研究旨在评估肿瘤组织CD45RO表达水平与肺腺癌手术患者的临床病理参数及预后的相关性。
1 料与方法 1.1 研究对象本研究为前瞻性队列研究设计,连续纳入了121例2009年3月至2012年2月于余姚市人民医院心胸外科接受开胸手术治疗的肺腺癌患者。纳入标准为:(1)所有患者均根据其临床特征、影像学检查和病理证实确诊为肺腺癌;(2)所有患者术前未进行任何新辅助治疗(包括新辅助化疗、放疗等),且均接受了肺部手术治疗;(3)所有患者年龄 > 18岁。排除标准:(1)有严重感染、严重肝功能异常或严重肾功能异常患者,合并其他恶性肿瘤的患者或有其他恶性肿瘤史的患者;(2)预期生命值< 12个月患者;(3)有认知障碍或其他因素导致预估难以执行定期随访和评估的患者。本研究经过余姚市人民医院伦理委员会批准,所有患者均签署了知情同意书。手术中采集肺腺癌组织和癌旁组织标本,然后采用福尔马林浸泡并石蜡封存以备后期使用。
1.2 临床病理特征采集和随访采集所有患者的基线期人口学、临床和病理特征信息(包括年龄、性别、淋巴结转移、病理分期、远端转移、肿瘤大小和TNM分期),按照第6版AJCC肿瘤分期手册进行TNM分期评估。所有患者手术后均接受辅助化疗:长春瑞滨25 mg/m2 d1和d8,顺铂75 mg/m2 d1,q3w治疗3~4个疗程。随访截止日期为2017年2月,中位随访期为44个月。患者术后6个月每月随访1次;6个月到2年每3月随访1次;之后每6月随访1次。无病生存期(disease free survival,DFS)和总体生存期(overall survival,OS)是根据患者疾病复发、死亡和/或最终随访时间计算的。
1.3 免疫组化采用anti-CD45RO抗体(Abcam)对组织样本进行免疫组织化学染色:(1)对石蜡切片进行脱蜡;加入修复液Tris-EDTA(pH = 9.0)进行抗原修复;加入3%的过氧化氢溶液进行内源性过氧化物酶阻断;将其浸入4%牛血清白蛋白进行非特异性内源性抗原的阻断。(2)加入anti-CD45RO抗体,同时将其置于4 ℃冰箱里孵育过夜;洗后,加入辣根过氧化物酶(合)共轭anti-IgG(Abcam)对其再次进行孵育,同时室温静置30 min。(3)清洗和DAB处理;加样器加入苏木精进行复染。(4)脱水,封片。
2位病理科医师采用双盲法分别利用光学显微镜对其阳性细胞进行观察阅片。每张切片随机观察视野肿瘤细胞,计算100个细胞/视野染色阳性的细胞比,在高倍镜视野下(高通滤波器,400倍)。阳性细胞所占比例评分标准为:0分(0%)、1分(> 0%~25%)、2分(> 25%~50%)、3分(> 50%~75%)和4分(> 75%~100%)。CD45RO以胞质出现浅黄色至棕褐色者为阳性,染色强度评分标准:基本不着色为0分、浅黄色为1分、棕黄色为2分、棕褐色为3分。最后,计算染色强度分数和染色面积分数的乘积得到总表达评分,并进行分组:低(0~3分)、中(4~6分)、高(> 6分)。
1.4 统计学分析采用SPSS 22.0软件进行统计分析。数据展示形式主要为x±s和百分比表示。采用χ2检验比较肺腺癌组织和癌旁组织CD45RO表达水平差异;采用Pearson检验分析肺腺癌组织CD45RO水平与患者临床病理特征的关联;采用Kaplan-Meier(K-M)曲线和Log-rank检验分析肺腺癌组织CD45RO表达与DFS及OS的关联;采用单因素和多因素COX风险比回归模型进行基线期因素对于DFS及OS的预测作用分析。P < 0.05为差异有统计学意义。
2 结果 2.1 基线期信息本研究纳入121例肺腺癌手术患者,其中男69例、女52例;平均年龄(62.97±9.87)岁;肿瘤大小≤5 cm 68例(56.2%)、> 5 cm 53例(43.8%);淋巴结转移阴性患者71例(58.7%)、阳性50例(41.3%);远端转移阴性患者119例(98.3%)、阳性2例(1.7%)。见表 1。
| Parameters | LAC patients(n = 121) |
| Age(year) | 62.97±9.87 |
| Gender(male/female) | 69/52 |
| Pathological grade [n(%)] | |
| Poor differentiation | 16(13.2) |
| Moderate differentiation | 77(63.6) |
| Well differentiation | 28(23.1) |
| Tumor size [n(%)] | |
| ≤5 cm | 68(56.2) |
| > 5 cm | 53(43.8) |
| Lymph node metastasis [n(%)] | |
| Negative | 71(58.7) |
| Positive | 50(41.3) |
| Distant metastasis [n(%)] | |
| Negative | 119(98.3) |
| Positive | 2(1.7) |
| TNM stage [n(%)] | |
| Ⅰ | 42(34.7) |
| Ⅱ | 41(33.9) |
| Ⅲ | 36(29.8) |
| Ⅳ | 2(1.7) |
2.2 CD45RO在肺腺癌组织中高表达
免疫组织化学染色显示,CD45RO相对于癌旁组织在肺腺癌组织中高表达,见图 1,表 2。肺腺癌组织和癌旁组织中高、中、低表达CD45RO在肺腺癌组织中的数量分别为82、68、30,然而其表达在癌旁组织中的数量分别为17、59、104;2组差异有统计学意义(P < 0.001),见表 2。

|
| A, high expression of CD45RO was observed in tumor tissue(×100);B, medium and low expression of CD45RO was observed in adjacent lung adenocarcinoma(×100);C, high expression of CD45RO was observed in tumor tissue(×400);D, medium and low expression of CD45RO was observed in adjacent lung adenocarcinoma (×400). 图 1 免疫组化显示CD45RO在肺腺癌组织中高表达 Fig.1 High CD45RO expression in tumor tissue samples |
| Item | Age | Gender(male) | Pathological grade(poor) | Tumor size (> 5 cm) | Lymph node metastasis | Distant metastasis | TNM stage (high) |
| r | -0.008 | 0.016 | 0.064 | -0.198 | -0.184 | 0.135 | -0.098 |
| P | 0.931 | 0.859 | 0.483 | 0.029 | 0.043 | 0.139 | 0.285 |
2.3 肺腺癌组织中CD45RO表达水平与肿瘤大小及淋巴结转移呈负相关
肿瘤组织CD45RO表达水平与肿瘤大小(r = -0.198,P = 0.029)、淋巴结转移(r = -0.184,P = 0.043)均呈负相关。见表 2。
2.4 肺腺癌组织中CD45RO表达水平与DFS及OS的关联采用K-M曲线和Log-rank检验分析肺腺癌组织CD45RO表达与DFS及OS的关联,结果显示肺腺癌组织中的CD45RO表达水平越高,DFS (P < 0.001)和OS (P < 0.001)越长,见图 2。
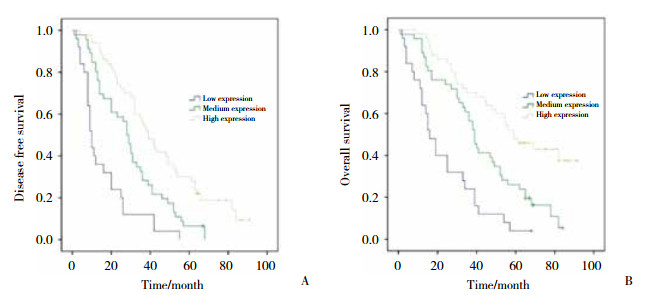
|
| A,DFS;B,OS. 图 2 CD45RO表达水平与DFS以及OS的Kaplan-Meier曲线分析 Fig.2 Kaplan-Meier curve for Correlations of CD45RO expression with DFS and OS |
2.5 肺腺癌患者DFS和OS的预后因素分析
采用单因素COX风险回归模型分析肺腺癌组织CD45RO表达水平以及临床病理特征分别对于其手术患者DFS和OS影响,显示肿瘤组织CD45RO表达量与患者较好的DFS显著相关(P < 0.001)。然而,低分化(P = 0.046)、淋巴转移(P < 0.001)及TNM分级(P = 0.003)与患者较差的DFS显著相关。进一步采用多因素COX回归模型分析肺腺癌手术患者DFS的独立预测因素,发现肺腺癌组织CD45RO表达量(P < 0.001)能独立预测其患者良好的DFS,然而,低分化(P = 0.017)和淋巴结转移(P = 0.004)均能独立预测肺腺癌手术患者不良的DFS。见表 3。
| Item | Univariate Cox proportional hazard regression model (n = 121) | Multivariate Cox proportional hazard regression model (n = 121) | |||||||
| P | HR | 95% CI | P | HR | 95% CI | ||||
| Low | High | Low | High | ||||||
| Tissue CD45RO expression | < 0.001 | 0.474 | 0.363 | 0.617 | < 0.001 | 0.454 | 0.346 | 0.595 | |
| Age(> 60 year) | 0.775 | 0.946 | 0.646 | 1.385 | - | - | - | - | |
| Gender(male) | 0.907 | 0.978 | 0.670 | 1.426 | - | - | - | - | |
| Pathological grade(poor) | 0.046 | 1.363 | 1.005 | 1.848 | 0.017 | 1.553 | 1.083 | 2.228 | |
| Tumor size(> 5 cm) | 0.436 | 1.163 | 0.796 | 1.700 | - | - | - | - | |
| Lymph node metastasis | < 0.001 | 2.245 | 1.524 | 3.307 | 0.004 | 2.104 | 1.259 | 3.514 | |
| Distant metastasis | 0.125 | 3.053 | 0.733 | 12.713 | - | - | - | - | |
| TNM stage(high) | 0.003 | 1.408 | 1.122 | 1.768 | 0.668 | 0.933 | 0.678 | 1.283 | |
单因素COX分析发现,肺腺癌组织CD45RO表达量与患者较好的OS(P < 0.001)有关,而低分化(P = 0.003)、淋巴转移(P < 0.001)、远端转移(P = 0.029)及TNM分级(P = 0.001)均与患者较差的OS有关。多因素COX分析结果显示,肺腺癌组织CD45RO表达量(P < 0.001)能独立预测其手术患者良好的OS,而低分化(P = 0.001)、淋巴转移(P < 0.001)及远端转移(P = 0.001)均能独立预测其手术患者较差的OS。其他临床病理特征对OS的预测作用,见表 4。
| Item | Univariate Cox proportional hazard regression model (n = 121) | Multivariate Cox proportional hazard regression model (n = 121) | |||||||
| P | HR | 95% CI | P | HR | 95% CI | ||||
| Low | High | Low | High | ||||||
| Tissue CD45RO expression | < 0.001 | 0.457 | 0.344 | 0.606 | < 0.001 | 0.396 | 0.296 | 0.532 | |
| Age(> 60 year) | 0.338 | 0.819 | 0.543 | 1.233 | - | - | - | - | |
| Gender(male) | 0.696 | 1.085 | 0.720 | 1.636 | - | - | - | - | |
| Pathological grade(poor) | 0.003 | 1.664 | 1.195 | 2.317 | 0.001 | 1.947 | 1.299 | 2.920 | |
| Tumor size(> 5 cm) | 0.181 | 1.321 | 0.878 | 1.988 | - | - | - | - | |
| Lymph node metastasis | < 0.001 | 2.556 | 1.685 | 3.875 | < 0.001 | 2.878 | 1.631 | 5.081 | |
| Distant metastasis | 0.029 | 4.991 | 1.179 | 21.126 | 0.001 | 17.936 | 3.069 | 104.829 | |
| TNM stage(high) | 0.001 | 1.522 | 1.189 | 1.949 | 0.344 | 0.842 | 0.589 | 1.202 | |
3 讨论
多种TILs,包括T细胞、B细胞以及树突细胞等,可通过特异性识别和清除机体肿瘤细胞,参与肿瘤发生发展的过程[5]。其中CD45RO+TILs被发现在胃癌以及喉癌中呈高表达水平[4, 6]。本研究有相似的发现。这可能是因为肿瘤发生与炎症反应密切相关,肿瘤微环境中存在大量的白细胞,从而在所有白细胞上均有表达,且也称为白细胞共同抗原(leukocyte common antigen,LCA)的CD45被更多的发现[7]。
CD45是一类结构相似的跨膜蛋白组成的分子,由PRPRC基因编码[8]。CD45RO记忆T细胞再次接触到肿瘤细胞时,可快速发生强烈的免疫反应,继而杀伤肿瘤细胞[7]。研究[9]表明,CD45RO通过激活白细胞介素2 (interleukin-2,IL-2)、干扰素γ (interferon-γ,IFN-γ)以及肿瘤坏死因子α (tumor necrosis factor-α,TNF-α)来抵抗宫颈癌细胞的增殖和迁移,继而抑制肿瘤的生长和转移。同时,CD45RO+记忆T细胞减少高迁移率族蛋白B1(high-mobility group box 1,HMGB1)的生成,抑制晚期结肠癌患者肿瘤细胞增殖分化和迁徙[10]。此外,CD45RO+TILs的表达水平与肾细胞癌患者的低分化和TMN分级、乳腺癌患者的淋巴结转移和肿瘤大小、结肠癌患者的淋巴结转移以及血管侵袭均呈负相关[11-13]。本研究有相似的发现,这可能是由于CD45RO可调节多种效应细胞产生大量的细胞因子,发生强烈的免疫应答来杀伤肿瘤细胞从而抑制肿瘤的转移[9-10]。
一些研究[14]评估了CD45RO与恶性肿瘤患者预后的关联。乳腺癌患者中CD45RO高表达组的肿瘤复发率低于低表达组。且CD45RO高表达水平已被证实与肾细胞癌以及胃癌患者良好的DFS和OS均密切相关[12, 15]。本研究有相似的发现。这可能是因为CD45RO能促进大量效应细胞因子的分泌,产生强烈的免疫应答,杀伤肿瘤细胞来抑制肿瘤的生长和转移,继而影响患者的预后情况[9-10]。
本研究仍有有一些不足:(1)主要纳入了可接受肺腺癌手术的Ⅰ~Ⅲ期的患者(Ⅳ期患者仅2例),因此CD45RO表达水平在肺腺癌Ⅳ期患者的预后作用尚不明确;(2)对肺腺癌患者组织样本CD45RO的表达量进行了鉴定,而未对血液中CD45RO表达量进行评估;(3)样本量相对较小。故进一步的研究需要扩大样本容量并且同时纳入肺腺癌Ⅳ期患者且采集患者血液样本。
综上所述,本研究表明,肿瘤组织中CD45RO表达水平可作为新颖且可靠的生物标志物,用于评估肺腺癌手术患者的临床病理特征和预后情况。
| [1] |
CANCER GENOME ATLAS RESEARCH N. Comprehensive molecular profiling of lung adenocarcinoma[J]. Nature, 2014, 511(7511): 543-550. DOI:10.1038/nature13385 |
| [2] |
HERBST RS, HEYMACH JV, LIPPMAN SM. Lung cancer[J]. N Engl J Med, 2008, 359(13): 1367-1380. DOI:10.1056/NEJMra0802714 |
| [3] |
ANDERSEN R, DONIA M, WESTERGAARD MC, et al. Tumor infiltrating lymphocyte therapy for ovarian cancer and renal cell carcinoma[J]. Hum Vaccin Immunother, 2015, 11(12): 2790-2795. DOI:10.1080/21645515.2015.1075106 |
| [4] |
李满意, 刘济生, 周慧, 等. CD45RO在喉鳞状细胞癌中的表达及临床意义[J]. 临床耳鼻咽喉头颈外科杂志, 2014, 28(6): 373-375, 380. DOI:10.13201/j.issn.1001-1781.2014.06.005 |
| [5] |
HALL M, LIU H, MALAFA M, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors[J]. J Immunother Cancer, 2016, 4(1): 61. DOI:10.1186/s40425-016-0164-7 |
| [6] |
OSINSKY S, KOVELSKAYA A, BUBNOVSKAYA L, et al. CD8 and CD45RO T lymphocytes in bone marrow of gastric cancer patients:correlation with disseminated tumor cells and disease outcome[J]. Exp Oncol, 2015, 37(1): 48-52. |
| [7] |
POLLARD JW. Tumour-educated macrophages promote tumour progression and metastasis[J]. Nat Rev Cancer, 2004, 4(1): 71-78. DOI:10.1038/nrc1256 |
| [8] |
SALLUSTO F, GEGINAT J, LANZAVECCHIA A. Central memory and effector memory T cell subsets:function, generation, and maintenance[J]. Annu Rev Immunol, 2004, 22(1): 745-763. DOI:10.1146/annurev.immunol.22.012703.104702 |
| [9] |
RANGEL R, ROCHA L, RAMIREZ JL, et al. Generation of memory CD4+, CD8+, CD45RO+ and CD16-lymphocytes activated with IL-2, INF-gamma, and TNF-alpha with specific cytotoxicity against autologous cervical cancer cells in a mixed leukocyte-tumour cell culture[J]. Eur Cytokine Netw, 1995, 6(3): 195-202. |
| [10] |
PENG RQ, WU XJ, DING Y, et al. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stageⅢB colon cancer[J]. BMC Cancer, 2010, 10: 496. DOI:10.1186/1471-2407-10-496 |
| [11] |
YAJIMA R, YAJIMA T, FUJⅡ T, et al. Tumor-infiltrating CD45RO (+) memory cells are associated with a favorable prognosis breast cancer[J]. Breast Cancer, 2016, 23(4): 668-674. DOI:10.1007/s12282-015-0622-y |
| [12] |
HOTTA K, SHO M, FUJIMOTO K, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma[J]. Br J Cancer, 2011, 105(8): 1191-1196. DOI:10.1038/bjc.2011.368 |
| [13] |
KOELZER VH, LUGLI A, DAWSON H, et al. CD8/CD45RO T-cell infiltration in endoscopic biopsies of colorectal cancer predicts nodal metastasis and survival[J]. J Transl Med, 2014, 12: 81. DOI:10.1186/1479-5876-12-81 |
| [14] |
SALGADO R, DENKERT C, DEMARIA S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer:recommendations by an international TILs working group 2014[J]. Ann Oncol, 2015, 26(2): 259-271. DOI:10.1093/annonc/mdu450 |
| [15] |
WAKATSUKI K, SHO M, YAMATO I, et al. Clinical impact of tumor-infiltrating CD45RO (+) memory T cells on human gastric cancer[J]. Oncol Rep, 2013, 29(5): 1756-1762. DOI:10.3892/or.2013.2302 |
 2018, Vol. 47
2018, Vol. 47