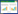文章信息
- 杜国强, 王立银, 王晓凤, 李君, 张明明, 王瑾
- DU Guoqiang, WANG Liyin, WANG Xiaofeng, LI Jun, ZHANG Mingming, WANG Jin
- APE1通过NF-κB通路调节PD-L1在喉鳞状细胞癌中的表达
- APE1 Regulates the Expression of PD-L1 through the NF-κB Pathway in Laryngeal Squamous Cell Carcinoma
- 中国医科大学学报, 2018, 47(10): 865-870
- Journal of China Medical University, 2018, 47(10): 865-870
-
文章历史
- 收稿日期:2018-04-10
- 网络出版时间:2018-09-27 9:35
2. 中国医科大学附属盛京医院急诊科, 沈阳 110004;
3. 中国医科大学附属盛京医院病理科, 沈阳 110004
2. Emergency Department, Shengjing Hospital, China Medical University, Shenyang 110004, China;
3. Pathology Department, Shengjing Hospital, China Medical University, Shenyang 110004, China
喉鳞状细胞癌是头颈部鳞状细胞癌中最常见的肿瘤,占全身恶性肿瘤的2%~3%,其发病率呈上升趋势。目前尽管针对喉癌的治疗手段有所改进,但患者5年生存率并没有显著提高[1]。探究如何有效地评估喉癌治疗效果及其预后一直是研究热点。近年来,程序性死亡蛋白配体1(programmed death ligand 1,PD-L1)在肿瘤免疫治疗中的地位受到广泛关注,其与受体程序性死亡蛋白组成机体重要的免疫检查点,可抑制T细胞的活化,参与肿瘤的免疫逃逸[2]。但其在肿瘤微环境中的调控机制尚不明确。脱嘌呤/脱嘧啶内切核酸酶1(apurinic/apyrimidinic endonuclease 1,APE1)具有DNA损伤修复功能以及氧化还原功能,可以调节多种转录因子,APE1在亚细胞的定位分布变化与肿瘤的增殖、浸润和对放化疗抵抗性有一定关系[3-4]。已有研究[5-8]表明,APE1和PD-L1在多种恶性肿瘤中共同参与肿瘤的浸润、转移和预后,但在喉癌组织中未明确其相关性。本研究拟检测PD-L1和APE1在喉癌中的表达情况,并初步探讨其可能存在的相互作用机制。
1 材料与方法 1.1 材料随机收集2013年2月至2015年12月中国医科大学附属盛京医院耳鼻咽喉科手术治疗的60例喉鳞状细胞癌患者的肿瘤标本,随机选取其中30例的癌旁黏膜组织(距肿瘤边缘 > 0.5 cm)作为对照组。患者喉部肿瘤切除范围根据术前TNM分期和术中所见确定。所有患者术前均未接受放疗或化疗。标本收集在术前征得患者同意,且通过伦理委员会批准(伦理编号:2018PS56K)。人喉鳞状细胞癌Hep-2细胞系由中国医科大学基础医学院医学遗传学教研室馈赠。
1.2 主要试剂1640培养基、胎牛血清(以色列BI公司);Lipofectamine 2000(美国Invitrogen公司);反转录试剂盒、RNA提取试剂Trizol、SYBR(日本TaKaRa公司);蛋白提取试剂盒、PDTC(中国碧云天公司);兔抗人APE1、核因子κB(nuclear factor κB,NF-κB)、pNF-κB多克隆抗体(英国Abcam公司);兔抗人PD-L1多克隆抗体(美国CST公司);鼠抗人Tubulin抗体、兔抗人GAPDH抗体、HRP标记羊抗兔(鼠)二抗(中国中杉金桥公司);ECL超敏发光液(英国GE公司);脂多糖(lipopolysaccharide,LPS;美国Sigma公司);APE1-siRNA序列(5’-GUCUGGUACGACUGGAGUACC-3’,5’-UACUCCAGUCGUACCAGACCU-3’)(上海吉玛公司)。
1.3 实时定量PCR检测喉癌以及癌旁组织APE1、PD-L1基因表达水平Trizol提取组织内总RNA,纯度合格后逆转录成cDNA。设计引物:APE1上游引物5’-CAATACTGGTCAGCTCCTTCG-3’,下游引物5’-TGCCGTAAGAAACTTTGAGTGG-3’;PD-L1上游引物5’-GCTGCACTAATTGTCTATTGGGA-3’,下游引物5’-AATTCGCTTGTAGTCGGCACC-3’;内参GAPDH上游引物5’-GTCTCCTCTGACTTCAACAGCG-3’,下游引物5’-ACCACCCTGTTGCTGTAGCCAA-3’。进行定量分析,反应条件为95 ℃预变性30 s,95 ℃变性5 s,60 ℃退火30 s,40个循环,收集信号。基因相对表达量用2-∆Ct方法计算分析。每个样本3个重复孔。
1.4 细胞培养、APE1-siRNA转染、LPS/PDTC干预Hep-2细胞用含10%胎牛血清的1640完全培养基于37 ℃、5% CO2孵箱培养,48 h更换培养基1次。将状态良好的Hep-2细胞以2×105/孔接种于6孔板,用无双抗培养基培养至细胞融合度约50%,更换Opti-MEM培养基。设置实验组(转染APE1-siRNA)、阴性对照组(转染Negative control-siRNA)、空白对照组(未干预)。
脂质体用量5 μL/孔,siRNA 5 μL/孔。转染6 h后,更换为含10%胎牛血清的1640培养基,继续培养48 h。将细胞接种于6孔板,以10%血清1640培养基培养至细胞融合度约80%后更换培养基。单纯LPS干预组按浓度0、0.5、1、2、4 μg/mL加入LPS后继续培养24 h。单纯PDTC组按浓度0、25、50、100、200 μmol/L加入PDTC后继续培养12 h。转染+LPS组按脂质体转染步骤转染24 h后加入LPS(浓度2 μg/mL)继续培养24 h。
1.5 Western blotting检测APE1、PD-L1、NF-κB、pNF-κB蛋白表达情况按蛋白提取试剂盒说明提取总蛋白,高温变性。样品行SDS-PAGE电泳后电转至PVDF膜,5%脱脂奶常温封闭2 h后孵育一抗,其中anti-APE1(1: 500)、anti-PD-L1(1: 200)、anti-NF-κB(1: 1 000)、anti-pNF-κB(1: 500)、anti-tubulin(1: 10 000)、anti-GAPDH(1: 10 000),4 ℃摇床过夜。二抗(1: 40 000)常温孵育2 h,显影后用Image J软件分析蛋白条带灰度,以目的蛋白/内参蛋白灰度值进行分析。
1.6 统计学分析采用SPSS 19.0统计软件,数据用x±s表示,采用独立样本t检验及单因素方差分析分析差异性,用Spearman等级相关分析分析相关性,P < 0.05为差异有统计学意义。
2 结果 2.1 RT-PCR检测PD-L1、APE1基因在临床样本中的表达水平 2.1.1 PD-L1、APE1 mRNA的表达在喉鳞癌组织中APE1、PD-L1基因表达显著高于癌旁组织,有统计学差异(P < 0.05)。利用Spearman相关分析显示,APE1与PD-L1两者基因表达存在正相关关系(r = 0.37,P < 0.01)。见图 1。
|
| A, APE1 mRNA expression; B, PD-L1 mRNA expression; C, correlation analysis of APE1 and PD-L1 mRNA expressions. N, normal tissues; T, tumor tissues. * P < 0.05. 图 1 RT-PCR检测喉鳞状细胞癌和癌旁黏膜中APE1和PD-L1 mRNA表达 Fig.1 APE1 and PD-L1 mRNA levels in laryngeal squamous cell carcinoma and normal tissues evaluated by RT-PCR |
2.1.2 PD-L1、APE1 mRNA表达与临床病理特征的关系
PD-L1 mRNA的表达与肿瘤分化程度、TNM分期、淋巴结转移无关,与喉癌发生部位有关,声门型喉癌组织中PD-L1 mRNA表达水平显著高于声门上型和声门下型(P < 0.05)。同时,喉癌组织中APE1 mRNA的表达与淋巴结转移与否具有相关性(P < 0.05),与肿瘤生长部位、分化程度、TNM分期无关。见表 1。
| Clinicopathological feature | n | PD-L1 mRNA | APE1 mRNA | |||
| 2-∆Ct | P | 2-∆Ct | P | |||
| Pathogenic site | < 0.01 | 0.78 | ||||
| Subglottic | 19 | 0.005±0.001 | 0.015±0.002 | |||
| Glottic | 30 | 0.020±0.007 | 0.017±0.002 | |||
| Supraglottic | 11 | 0.006±0.001 | 0.016±0.002 | |||
| Differentiation | 0.31 | 0.41 | ||||
| Poor | 8 | 0.022±0.005 | 0.013±0.003 | |||
| Moderate | 21 | 0.006±0.001 | 0.016±0.002 | |||
| Well | 31 | 0.015±0.006 | 0.017±0.002 | |||
| TNM stage | 0.06 | 0.56 | ||||
| Ⅰ-Ⅱ | 26 | 0.020±0.008 | 0.015±0.002 | |||
| Ⅲ-Ⅳ | 34 | 0.007±0.002 | 0.016±0.002 | |||
| Lymphatic metastasis | 0.18 | 0.02 | ||||
| Negative | 37 | 0.017±0.006 | 0.013±0.001 | |||
| Positive | 23 | 0.007±0.002 | 0.019±0.002 | |||
2.2 APE1-siRNA转染Hep-2细胞对APE1、PD-L1表达的影响
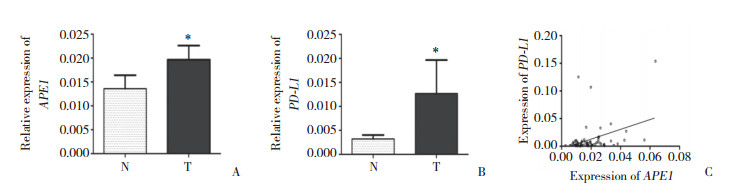
Hep-2细胞转染APE1-siRNA及NC-siRNA 48 h后,采用RT-PCR及Western blotting分别检测APE1、PD-L1 mRNA及蛋白水平的表达,结果显示APE1与PD-L1在Hep-2细胞中有表达,且实验组与阴性对照组及空白对照组相比,APE1、PD-L1相对表达量显著下降,差异有统计学意义(P < 0.05)。见图 2。
|
| A, APE1 mRNA expression, B, PD-L1 mRNA expression; C, APE1 and PD-L1 protein expressions; D, statistical analysis of APE1 and PD-L1 protein expression levels. si-APE1, experimental group; NC, negative control group; control, blank control group. * P < 0.05; ** P < 0.01; *** P < 0.001. 图 2 沉默APE1基因后Hep-2细胞APE1、PD-L1 mRNA及蛋白表达的变化 Fig.2 The changes in the expressions of APE1 and PD-L1 mRNA and protein in Hep-2 cells after siRNA transfection |
2.3 APE1对PD-L1的影响依赖NF-κB信号通路
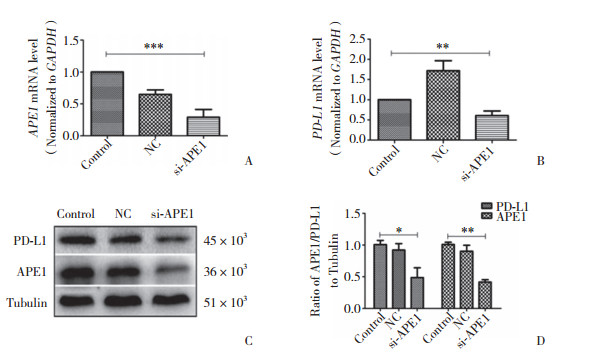
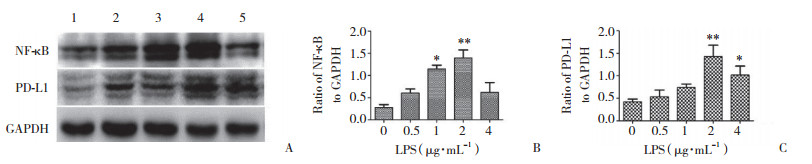
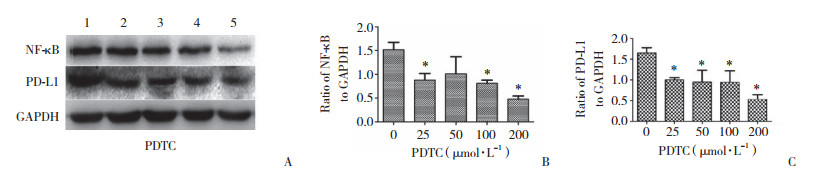
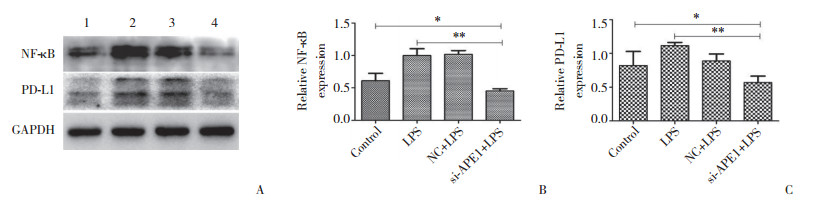
为探究APE1对PD-L1的表达是否存在调控机制,本研究进一步在Hep-2细胞中沉默APE1基因的表达,检测NF-κB、pNF-κB蛋白的表达水平。结果显示,实验组与空白对照组、阴性对照组相比,NF-κB、pNF-κB蛋白表达量显著下降(图 3)。予以不同浓度LPS干预Hep-2细胞,在一定浓度范围内,PD-L1、NF-κB蛋白表达较0浓度组逐渐增高(图 4)。以不同浓度PDTC干预Hep-2细胞,在一定浓度范围内,PD-L1、NF-κb蛋白表达较0浓度组逐渐降低(图 5)。沉默APE1表达后给予LPS干预,NF-κB、PD-L1蛋白表达较未干预组减低,较单纯LPS干预组显著降低(图 6),差异均有统计学意义(P < 0.05)。这些结果表明,APE1可能通过NF-κB信号通路调节PD-L1的表达。

|
| A, results of Western blotting; B, statistical analysis of NF-κB, pNF-κB, and PD-L1 protein expression. si-APE1, experimental group; NC, negative control group; control, blank control group. * P < 0.05. 图 3 沉默APE1基因后Hep-2细胞中NF-κB、pNF-κB及PD-L1蛋白表达的变化 Fig.3 The changes in NF-κB, pNF-κB, and PD-L1 protein expression in Hep-2 cells after siRNA transfection |
|
| A, NF-κB and PD-L1 activity in Hep-2 cells treated with different concentrations of LPS for 24 h; B, statistical analysis of NF-κB protein expression level; C, statistical analysis of PD-L1 protein expression level. 1, 0 μg/mL LPS; 2, 0.5 μg/mL LPS; 3, 1 μg/mL LPS; 4, 2 μg/mL LPS; 5, 4 μg/mL LPS. * P < 0.05; ** P < 0.01. 图 4 激动NF-κB信号通路提高PD-L1和NF-κB蛋白的表达 Fig.4 The activation of the NF-κB signaling pathway is effective in improving PD-L1 and NF-κB expressions |
|
| A, NF-κB and PD-L1 activities in Hep-2 cells treated with different concentrations of PDTC for 12 h; B, statistical analysis of NF-κB protein expression level; C, statistical analysis of PD-L1 protein expression level. 1, 0 μmol/L PDTC; 2, 25 μmol/L PDTC; 3, 50 μmol/L PDTC; 4, 100 μmol/L PDTC; 5, 200 μmol/L PDTC. * P < 0.05. 图 5 抑制NF-κB信号通路降低PD-L1和NF-κB蛋白的表达 Fig.5 The inhibition of the NF-κB signaling pathway is effective in abolishing PD-L1 and NF-κB expression |
|
| A, results of Western blotting; B, statistical analysis of NF-κB protein expression level; C, statistical analysis of PD-L1 protein expression level. 1, control; 2, LPS; 3, NC+LPS; 4, si-APE1+LPS. * P < 0.05; ** P < 0.01. 图 6 NF-κB信号通路参与APE1对PD-L1的调节 Fig.6 The NF-κB signaling pathway is involved in the regulation of PD-L1 by APE1 |
3 讨论
机体的免疫微环境与肿瘤的发生发展有着密切关系[9]。对于免疫治疗来说,寻找能够评估肿瘤对免疫治疗敏感程度的指标及调控肿瘤细胞免疫检查点表达的分子生物学通路,成为选择适合免疫治疗的患者和评估预后的关键问题。
PD-L1是主要表达于恶性肿瘤细胞、抗原呈递细胞、T细胞的B7超家族蛋白。其受体程序性死亡蛋白1主要表达于T淋巴细胞。PD-L1与程序性死亡蛋白1+ T细胞结合后,可通过激活免疫受体酪氨酸抑制基序(ITIM)使下游磷脂酰肌醇3激酶-蛋白激酶(PI3K)发生去磷酸化,阻断PI3K-AKT通路的激活,阻碍活化T细胞的形成[2, 10]。恶性肿瘤因发病部位、组织来源的不同,在其发生发展过程中可能形成相对特异的免疫微环境,因此对免疫相关治疗的敏感程度也可能存在差异性。相关研究[10-11]显示,多种类型的肿瘤细胞表面PD-L1的表达情况反映了肿瘤免疫微环境活化的程度,并且与肿瘤患者对免疫治疗敏感程度和患者的预后、生存率呈正相关。本研究表明,PD-L1在声门型喉癌中的表达显著高于声门上型及声门下型,APE1在淋巴结转移喉癌组织中明显高表达,提示PD-L1与APE1参与了喉癌的进展,且两者可能存在互相调控关系。声门区可能存在相对活跃的肿瘤免疫微环境,对免疫检查点抑制剂类的免疫治疗预后可能相对好于其他型喉癌。
APE1通过改变自身在恶性肿瘤细胞中的亚细胞空间定位,由核表达变为核浆共表达,调节包括NF-κB、AP-1、P53在内的多种转录因子,从而调控多种蛋白的表达,影响了肿瘤细胞的分化、增殖、凋亡以及对治疗的耐药性[12-14]。Qing等[8]发现APE1、PD-L1在胃癌中普遍高表达,并存在相关性,可共同作为评估胃癌发生的危险因素及预测其预后的标志物。本研究显示,在喉癌组织中APE1与PD-L1基因较癌旁组织高表达且呈正相关。进一步沉默人Hep-2细胞APE1基因表达,发现PD-L1蛋白的表达随着APE1蛋白表达的抑制而降低,验证了在喉鳞状细胞癌中APE1可能对PD-L1存在正向调控作用。
核转录因子NF-κB是Rel蛋白家族成员,参与了肿瘤的增殖和转移[15]。既往研究[16-17]显示,NF-κB活性受到抑制时PD-L1的表达相应下降,提示NF-κB转录因子参与PD-L1的表达调控。本研究通过抑制或激动Hep-2细胞NF-κB蛋白表达,正向调节PD-L1的蛋白表达。同时,沉默APE1表达后,Hep-2细胞中NF-κB、pNF-κB的表达显著降低。APE1表达沉默后的细胞给予NF-κB激动剂处理后,发现PD-L1蛋白水平的表达无明显增高。以上结果提示,APE1可能通过NF-κB通路调节PD-L1表达。
综上所述,在喉鳞状细胞癌中PD-L1和APE1表达增高,两者参与了喉癌的发展。本研究显示PD-L1表达与喉癌发生部位相关,声门型喉癌患者可能在免疫相关治疗中更加获益。APE1可能通过NF-κB通路对喉癌细胞PD-L1基因表达实行调控,但这仍需通过大量基础研究在更多的肿瘤类型中进一步证实。
| [1] |
CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [2] |
TOPALIAN SL, HODI FS, BRAHMER JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer[J]. New Eng J Med, 2012, 366(26): 2443-2454. DOI:10.1056/NEJMoa1200690 |
| [3] |
LI M, WILSON DM. Human apurinic/apyrimidinic endonuclease 1[J]. 2014, 20(4): 678-707. DOI: 10.1089/ars.2013.5492.
|
| [4] |
LAEV SS, SALAKHUTDINOV NF, LAVRIK OI. Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1(APE1/Ref-1)[J]. Bioorg Med Chem, 2017, 25(9): 2531-2544. DOI:10.1016/j.bmc.2017.01.028 |
| [5] |
JIANG X, SHAN J, DAI N, et al. Apurinic/apyrimidinic endonuclease 1 regulates angiogenesis in a transforming growth factor beta-dependent manner in human osteosarcoma[J]. Cancer Sci, 2015, 106(10): 1394-1401. DOI:10.1111/cas.12763 |
| [6] |
MANDAI M, HAMANISHI J, ABIKO K, et al. Anti-PD-L1/PD-1 immune therapies in ovarian cancer:basic mechanism and future clinical application[J]. Int J Clin Oncol, 2016, 21(3): 456-461. DOI:10.1007/s10147-016-0968-y |
| [7] |
KAN G, DONG W. The expression of PD-L1 APE1 and P53 in hepatocellular carcinoma and its relationship to clinical pathology[J]. Eur Rev Med Pharmacol Sci, 2015, 19(16): 3063-3071. |
| [8] |
QING Y, LI Q, REN T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer[J]. Drug Des Devel Ther, 2015, 9: 901-909. DOI:10.2147/DDDT.S75152 |
| [9] |
GARNELO M, TAN A, HER Z, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma[J]. Gut, 2017, 66(2): 342-351. DOI:10.1136/gutjnl-2015-310814 |
| [10] |
ROPER E, LUM T, PALME CE, et al. PD-L1 expression predicts longer disease free survival in high risk head and neck cutaneous squamous cell carcinoma[J]. Pathology, 2017, 49(5): 499-505. DOI:10.1016/j.pathol.2017.04.004 |
| [11] |
GANDINI S, MASSI D, MANDALA M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies:a systematic review and meta-analysis[J]. Crit Rev Oncol Hematol, 2016, 100: 88-98. DOI:10.1016/j.critrevonc.2016.02.001 |
| [12] |
NISHI T, SHIMIZU N, HIRAMOTO M, et al. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo[J]. J Biol Chem, 2002, 277(46): 44548-44556. DOI:10.1074/jbc.M202970200 |
| [13] |
KELLEY MR, GEORGIADIS MM, FISHEL ML. APE1/Ref-1 role in redox signaling:translational applications of targeting the redox function of the DNA repair/redox protein APE1/Ref-1[J]. Curr Mol Pharmacol, 2012, 5(1): 36-53. DOI:10.2174/1874467211205010036 |
| [14] |
SAKAI Y, YAMAMORI T, YASUI H, et al. Downregulation of the DNA repair enzyme apurinic/apyrimidinic endonuclease 1 stimulates transforming growth factor-beta1 production and promotes actin rearrangement[J]. Biochem Biophys Res Commun, 2015, 461(1): 35-41. DOI:10.1016/j.bbrc.2015.03.163 |
| [15] |
YANG C, FU ZX. PEG-liposomal oxaliplatin combined with nuclear factor-kappaB inhibitor (PDTC) induces apoptosis in human colorectal cancer cells[J]. Oncol Rep, 2014, 32(4): 1617-1621. DOI:10.3892/or.2014.3336 |
| [16] |
GOWRISHANKAR K, GUNATILAKE D, GALLAGHER SJ, et al. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-kappaB[J]. PLoS One, 2015, 10(4): e0123410. DOI:10.1371/journal.pone.0123410 |
| [17] |
LIN K, CHENG J, YANG T, et al. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-kappaB[J]. Biochem Biophys Res Commun, 2015, 463(1/2): 95-101. DOI:10.1016/j.bbrc.2015.05.030 |
 2018, Vol. 47
2018, Vol. 47