文章信息
- 李雪晴, 袁风娇, 程建青, 董运海, 李剑芳, 邬敏辰.
- LI Xue-qing, YUAN Feng-jiau, CHENG Jian-qing, DONG Yun-hai, LI Jian-fang, WU Min-chen.
- 杂合β-甘露聚糖酶AuMan5Aloop的H321对其酶学性质的影响
- Effect of Amino Acid H321 on the Enzymatic Properties of Hybrid β-Mannanase AuMan5Aloop
- 中国生物工程杂志, 2017, 37(2): 48-53
- China Biotechnology, 2017, 37(2): 48-53
- http://dx.doi.org/DOI:10.13523/j.cb.20170208
-
文章历史
- 收稿日期: 2016-07-15
- 修回日期: 2016-08-30
2. 江南大学无锡医学院 无锡 214122
2. Wuxi Medical School, Jiangnan University, Wuxi 214122, China
内切β-1, 4-D-甘露聚糖酶通常简称为β-甘露聚糖酶 (β-mannanase, EC 3.2.1.78),是甘露聚糖降解酶系中的关键组分[1]。基于酶蛋白一级结构的同源比对及疏水簇分析,绝大多数β-甘露聚糖酶归属糖苷水解酶家族 (GHF) 5、26和113,具有类似的 (β/α)8-TIM桶状结构[2]。β-甘露聚糖酶来源广泛,其中微生物β-甘露聚糖酶较动植物酶拥有更广的作用pH和温度范围以及底物谱,已成为目前研究及应用的热点。
迄今为止,已有多种来源于微生物、不同GHF的β-甘露聚糖酶基因被克隆、改造及表达。Wang等[3]克隆了一种Talaromyces leycettanus β-甘露聚糖酶基因man5A,并在P. pastoris中表达了糖基化程度不同的两种酶Man5A1和Man5A2,它们的最适温度Topt为85~90℃、且在70℃很稳定,比活性分别为2 160和1 800 U/mg。Couturier等[4]采用基因工程手段对两种Podospora anserina β-甘露聚糖酶基因paman5a和paman26a进行了随机突变,经高通量筛选获得了多个单点和双点突变的酶,分别较野生型酶PaMan5A和PaMan26A的催化效率有显著的提高。此外,有研究者发现定向改造临近糖苷水解酶活性中心的loop可提高酶的热稳定性和/或催化效率/或比活性[5-7]。
基于AuMan5A一级和三维结构的分析比对,本实验室前期将其底物结合凹槽侧壁上的loop结构 (316KSPDGGN322) 置换为烟曲霉Aspergillus fumigatus GHF5 β-甘露聚糖酶对应的结构 (PSPNDHF),所获loop置换杂合酶AuMan5Aloop的温度特性和催化效率均有显著的改善[8]。随后证明了D320对AuMan5Aloop酶学性质显著改善所起的重要作用。在上述工作的基础上,进一步分析H321与AuMan5Aloop酶学性质的相关性。基于AuMan5Aloop与AuMan5A一级结构的同源性比对,拟将Auman5Aloop编码H321的密码子突变为Auman5A编码对应的Gly的密码子,将构建的突变酶基因Auman5Aloop/H321G在Pichia pastoris GS115中进行表达,并测定和比较AuMan5Aloop/H321G、AuMan5Aloop和AuMan5A的温度特性、比活性和催化效率。
1 材料与方法 1.1 材料 1.1.1 质粒、菌株和培养基表达质粒pPICZαA、E. coli JM109、DH5α和P. pastoris GS115由作者所在实验室保藏;克隆质粒pUCm-T购自Sangon上海公司;重组质粒pUCm-T-Auman5Aloop及重组毕赤酵母GS115/pPICZαA (空白对照)、GS115/Auman5A和GS115/Auman5Aloop由本实验室构建和保藏[9]。培养基LB、YPDS-Zeocin、BMMY和BMGY的配制参照EasySelectTM Pichia Expression Kit (Invitrogen公司) 操作手册。
1.1.2 主要试剂rTaq DNA聚合酶、限制性内切酶和T4 DNA连接酶购自TaKaRa大连公司;EZ-10柱式DNA胶回收试剂盒、Zeocin、250 bp DNA ladder marker和低分子量protein marker均购自Sangon上海公司;角豆胶、D-甘露糖和考马斯亮蓝G-250为Sigma公司产品;DEAE Sepharose fast flow和Sephadex G-75为Amersham Pharmacia Biotech公司产品;其它试剂均为国产或进口分析纯。
1.2 方法 1.2.1 β-甘露聚糖酶三维结构的分析选择与AuMan5A一级结构 (ADZ99027) 同源性高、分别源自黑曲霉 (Aspergillus niger) 和粗孢篮状菌 (Talaromyces trachyspermus) 的β-甘露聚糖酶晶体结构 (PDB: 3WH9和3WFL) 为模板,运用SALIGN多空间结构比对程序 (http://salilab.org/DBAli/?page=tools_&action=f_salign) 和MODELLER 9.11程序 (http://salilab.org/modeller/) 进行三维结构的多模板同源建模及优化。使用PyMol软件 (http://pymol.org/) 和PIC程序 (http://pic.mbu.iisc.ernet.in/job.html) 对三维模型进行分析。
1.2.2 引物设计基于AuMan5A与AuMan5Aloop一级结构的同源性比对及三维结构的分析,拟将AuMan5Aloop置换loop结构中的H321突变回AuMan5A对应的Gly,即将Auman5Aloop编码H321的密码子CAC突变为Gly的GGT。依据Auman5A (HQ839639) 和Auman5Aloop的核苷酸序列设计PCR引物,由Sangon上海公司合成 (表 1)。
| Primer | Base sequence (5′ 3′) | Size (bp) |
| Man5A-F | GAATTCTCCTTCGCCAGCACCTC | 23 |
| H321G-F | CATCCCCAAATGAC TTCACTATCTACTATG TTCACTATCTACTATG |
33 |
| Man5A-R | GCGGCCGCTTAGGCACTATCAATAGCAG | 28 |
| Note: EcoRⅠ and NotⅠ sites are underlined, and the mutant codon is boxed | ||
1.2.3 Auman5Aloop/H321G重组表达质粒的构建
采用大引物PCR技术[10]扩增突变酶基因Auman5Aloop/H321G。以pUCm-T-Auman5Aloop为模板、H321G-F和Man5A-R为引物,第一轮PCR扩增获基因片段HM;再以同一质粒为模板、Man5A-F和HM为引物,第二轮PCR扩增获得Auman5Aloop/H321G。将纯化的目的PCR产物与pUCm-T连接获重组质粒pUCm-T-Auman5Aloop/H321G,转化E. coli JM109,蓝白斑筛选和DNA测序。将测序正确的重组质粒采用EcoRⅠ和NotⅠ双酶切,回收目的基因,与经同样双酶切的pPICZαA连接,获重组表达质粒pPICZαA-Auman5Aloop/H321G,转化E. coli DH5α,DNA测序。
1.2.4 β-甘露聚糖酶的表达和纯化将pPICZαA-Auman5Aloop/H321G用SacⅠ线性化,电转化至P. pastoris GS115。重组毕赤酵母的鉴定和多拷贝筛选参见EasySelectTM Pichia Expression Kit操作手册。β-甘露聚糖酶的诱导表达和纯化参照文献[11]。采用SDS-PAGE分析重组表达产物。
1.2.5 β-甘露聚糖酶活性的测定在20 ml试管中加入2.4 ml 5 mg/ml角豆胶溶液 (用pH 3.6、50 mmol/L柠檬酸-Na2HPO4缓冲液配制),65℃保温5 min;再加入0.1 ml适当稀释的酶液,准确反应10 min;加入2.5 ml DNS试剂,在沸水浴显色7 min,测定OD540值。在上述反应条件下,每分钟产生1 μmol还原糖 (以D-甘露糖计) 所需的酶量定义为1个酶活性单位 (U)。采用Bradford法测定蛋白质含量[12]。
1.2.6 温度对β-甘露聚糖酶活性的影响在不同温度 (50~75℃) 下,按1.2.5方法测定β-甘露聚糖酶活性;最适温度Topt定义为最高酶活性 (以相对酶活性100%计) 所对应的温度。将酶液置于70℃处理不同时间 (0~600 min),按1.2.5方法测定残留酶活性,未经处理酶液的酶活性以100%计;酶的半衰期t1/270定义为经70℃处理残留酶活性为50%时所对应的时间。
1.2.7 β-甘露聚糖酶催化效率的分析以不同浓度 (1.0~10.0 mg/ml) 的角豆胶溶液 (用pH 3.6、50 mmol/L柠檬酸-Na2HPO4缓冲液配制) 为底物,在β-甘露聚糖酶各自的Topt下按1.2.5方法测定酶活性,采用Origin 8.0软件进行非线性拟合,计算酶的动力学常数kcat/Km及催化效率kcat/Km值。
2 结果与讨论 2.1 AuMan5Aloop拟突变氨基酸的选定将位于AuMan5A一级结构C端、由316KSPDGGN322组成的loop置换为A. fumigatus β-甘露聚糖酶对应的环结构,获杂合酶AuMan5Aloop。酶学性质分析表明,AuMan5Aloop较AuMan5A的温度特性、比活性和催化效率等均有显著的改善[8]。比对AuMan5Aloop与AuMan5A的loop结构发现,两者有5对氨基酸不同,其中性质区别最大的是320和321位的残基:AuMan5A的G320~321被置换为酸性的Asp和碱性的His (图 1)。前期我们证实了D320对AuMan5Aloop酶学性质显著改善所起的作用[9]。因此,本研究选定将AuMan5Aloop置换loop结构中的H321突变回AuMan5A对应的Gly (图 1),构建突变酶AuMan5Aloop/H321G,分析H321对AuMan5Aloop酶学性质的影响。

|
| 图 1 3种β-甘露聚糖酶C末端的多序列同源比对 Figure 1 Multiple sequence alignment at the C-termini among three β-mannanases |
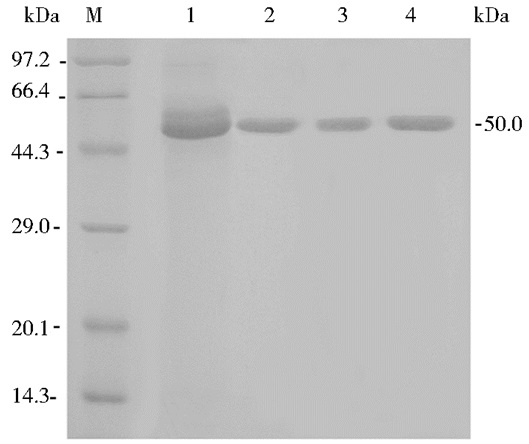
挑选30个在YPDS-Zeocin (400 μg/ml) 平板上生长良好的单菌落,30℃诱导表达96 h,每隔24 h添加终浓度为1.0% (v/v) 的甲醇。按1.2.5方法分别测定表达上清液的酶活性和蛋白质含量,经筛选获得了产AuMan5Aloop/H321G比活性最高的重组毕赤酵母GS115/Auman5Aloop/H321G,粗酶液的比活性为2 209 U/mg。在GS115/pPICZαA (对照) 的上清液中未检测到β-甘露聚糖酶的活性。采用 (NH4)2SO4盐析、DEAE Sepharose fast flow阴离子交换层析和Sephadex G-75凝胶层析对表达上清液进行了纯化。SDS-PAGE分析显示,纯化的AuMan5Aloop/H321G、AuMan5A和AuMan5Aloop均约在表观相对分子质量50.0 kDa处呈现单一蛋白条带 (图 2,泳道2~4)。AuMan5Aloop/H321G纯酶比活性为5 204 U/mg,分别是AuMan5A (406 U/mg) 和AuMan5Aloop (3 631 U/mg) 的12.8和1.43倍。
|
| 图 2 GS115/Auman5Aloop/H321G上清液和各纯化酶的SDS-PAGE分析 Figure 2 SDS-PAGE analysis of the expressed supernatant and purified β-mannanases M:Protein marker; 1: The expressed supernatant of GS115/Auman5Aloop/H321G; 2~4: The purified AuMan5Aloop/H321G, AuMan5A and AuMan5Aloop |
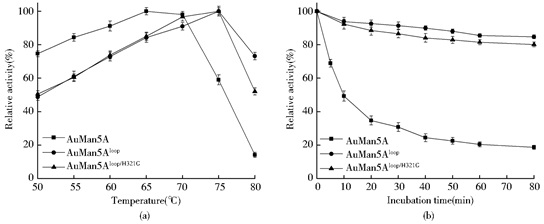
按1.2.6方法分别测定了温度对AuMan5A、AuMan5Aloop和AuMan5Aloop/H321G活性的影响 (图 3)。由图 3a可见,AuMan5Aloop/H321G和AuMan5Aloop的Topt均为75℃,高于AuMan5A的65℃。AuMan5A的t1/270约为10 min,而AuMan5Aloop和AuMan5Aloop/H321G在70℃、80 min内非常稳定 (图 3b);为此继续延长70℃的保温时间至600 min,测得它们的t1/270约为480和300 min。由此可见,将AuMan5Aloop的H321突变回AuMan5A的Gly,突变酶AuMan5Aloop/H321G的Topt与AuMan5Aloop相同、t1/270缩短了180 min,但其温度特性仍明显优于AuMan5A。
|
| 图 3 3种β-甘露聚糖酶的最适温度 (a) 和热稳定性 (b) Figure 3 The temperature optima (a) and thermostabilities (b) of three β-mannanases |
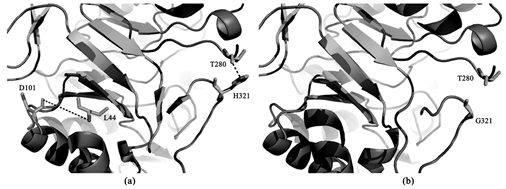
以A. niger (3WH9) 和T. trachyspermus (3WFL) β-甘露聚糖酶晶体结构为模板,按1.2.1方法分别对杂合酶AuMan5Aloop和突变酶AuMan5Aloop/H321G进行了三维结构的多模板同源建模及优化,运用PIC程序分析了两者三维结构中存在的相互作用力的差异。结果表明,AuMan5Aloop置换loop结构中的H321与其临近的T280之间存在氢键相互作用 (图 4a);而AuMan5Aloop/H321G的G321与其周围氨基酸间不形成氢键 (图 4b),从而导致了G321所在loop刚性的减弱[13]。同时,除了共同存在的26对盐桥之外,AuMan5Aloop较AuMan5Aloop/H321G多出1对盐桥L44-D101 (图 4a)。许多研究表明,氢键和盐桥等对蛋白质/酶的热稳定性起到促进作用[14-15],由此可预测AuMan5Aloop的H321突变为Gly将降低酶的热稳定性,与实验结果AuMan5Aloop/H321G的t1/270缩短了180 min相一致。
|
| 图 4 AuMan5Aloop (a) 和AuMan5Aloop/H321G (b) 局部三维结构的比较 Figure 4 The local 3-D structure comparison of AuMan5Aloop (a) with AuMan5Aloop/H321G (b) |
由于Gly侧链仅有一个氢原子,缺乏侧链的约束,从而导致G321所在的loop结构呈现柔性构象 (图 4b)。此外,Gly具有最高的构象熵,在蛋白质解折叠过程中所需能量最少[16]。因此,将特定位点处尤其是loop结构上的Gly置换为其它某种氨基酸残基对提高β-甘露聚糖酶的热稳定性十分有效[8],反之则降低该酶的热稳定性[12]。
2.5 β-甘露聚糖酶催化效率的分析按1.2.7方法测定了3种β-甘露聚糖酶AuMan5A、AuMan5Aloop和AuMan5Aloop/H321G的动力学常数kcat和Km值 (表 2)。分析结果表明,AuMan5Aloop/H321G对角豆胶的催化效率kcat/Km分别是AuMan5A和AuMan5Aloop的14.1和1.12倍。因此,将AuMan5Aloop的H321突变为Gly进一步提高了β-甘露聚糖酶的催化效率。
| β-Mannanase | kcat (1/s) | Km (mg/ml) | kcat/Km (ml/mg·s) | Fold |
| AuMan5A | 409±15 | 1.80±0.06 | 227.2 | 1.0 |
| AuMan5Aloop | 4 050±120 | 1.41±0.04 | 2 872 | 12.6 |
| AuMan5Aloop/H321G | 5 310±150 | 1.66±0.05 | 3 199 | 14.1 |
3 结论
本研究通过对AuMan5Aloop与AuMan5A一级结构的序列比对,将Auman5Aloop编码H321的密码子CAC突变为Gly的GGT,构建了突变酶基因Auman5Aloop/H321G并在P. pastoris GS115中实施了表达。一方面,与AuMan5Aloop相比,突变酶AuMan5Aloop/H321G的Topt不变,但热稳定性有所下降 (t1/270缩短了180 min);结构分析表明,AuMan5Aloop/H321G较AuMan5Aloop仅少了1对氢键T280-H321和1对盐桥L44-D101。由此可见,AuMan5A的loop置换所导致的G321H互换并不是AuMan5Aloop热稳定性提高的主要原因。另一方面,AuMan5Aloop/H321G较AuMan5Aloop的比活性和催化效率提高了1.43和1.12倍,其原因可能是H321突变为Gly后,提高了G321所在loop的柔性,从而有助于D320参与催化底物的水解反应。
本实验室基于GHF5 β-甘露聚糖酶AuMan5A的loop结构置换以及Auman5Aloop的D320和H321分别突变为G320和G321等的研究,深入分析了β-甘露聚糖酶AuMan5A底物结合凹槽侧壁上的loop环结构对其酶学性质的影响,为GHF5 β-甘露聚糖酶及其它酶的酶学性质定向改造提供了新的思路和理论依据。
| [1] | Huang J W, Chen C C, Huang C H, et al. Improving the specific activity of β-mannanase from Aspergillus niger BK01 by structure-based rational design. Biochim Biophys Acta, 2014, 1844(3) : 663–669. DOI:10.1016/j.bbapap.2014.01.011 |
| [2] | Zhang Y, Ju J, Peng H, et al. Biochemical and structural characterization of the intracellular mannanase AaManA of Alicyclobacillus acidocaldarius reveals a novel glycoside hydrolase family belonging to clan GH-A. J Biol Chem, 2008, 283(46) : 31551–31558. DOI:10.1074/jbc.M803409200 |
| [3] | Wang C H, Luo H Y, Niu C F, et al. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl Microbiol Biotechnol, 2015, 99 : 1217–1228. DOI:10.1007/s00253-014-5979-x |
| [4] | Couturier M, Feliu J, Bozonnet S, et al. Molecular engineering of fungal GH5 and GH26 beta-(1, 4)-mannanases toward improvement of enzyme activity. PLoS One, 2013, 8(11) : e79800. DOI:10.1371/journal.pone.0079800 |
| [5] | Chen K, Liu S, Ma J, et al. Deletion combined with saturation mutagenesis of N-terminal residues in transglutaminase from Streptomyces hygroscopicus results in enhanced activity and thermostability. Process Biochem, 2012, 47(12) : 2329–2334. DOI:10.1016/j.procbio.2012.09.013 |
| [6] | Duan X, Chen J, Wu J. Improving the thermostability and catalytic efficiency of Bacillus deramificans pullulanase by site-directed mutagenesis. Appl Environ Microbiol, 2013, 79(13) : 4072–4077. DOI:10.1128/AEM.00457-13 |
| [7] | Voutilainen S P, Murray P G, Tuohy M G, et al. Expression of Talaromyces emersonii cellobiohydrolase Cel7A in Saccharomyces cerevisiae and rational mutagenesis to improve its thermostability and activity. Protein Eng Des Sel, 2010, 23(2) : 69–79. DOI:10.1093/protein/gzp072 |
| [8] | Dong Y H, Li J F, Hu D, et al. Replacing a piece of loop-structure in the substrate-binding groove of Aspergillus usamii beta-mannanase, AuMan5A, to improve its enzymatic properties by rational design. Appl Microbiol Biotechnol, 2016, 100(9) : 3989–3998. DOI:10.1007/s00253-015-7224-7 |
| [9] | 李剑芳, 董运海, 胡蝶, 等. β-甘露聚糖酶AuMan5A/Af酶学性质的改善与其Asp320的相关性分析. 微生物学报, 2016, 56(2) : 301–308. Li J F, Dong Y H, Hu D, et al. Correlation between superior enzymatic properties of β-mannanase AuMan5A/Af and its residue Asp320. Acta Microbiologica Sinica, 2016, 56(2) : 301–308. |
| [10] | Xie Z H, Shi X J. Fast and almost 100% efficiency site-directed mutagenesis by the mega primer PCR method. Prog Biochem Biophys, 2009, 36(11) : 1490–1494. DOI:10.3724/SP.J.1206.2009.00139 |
| [11] | Li J F, Zhao S G, Tang C D, et al. Cloning and functional expression of an acidophilic β-mannanase gene (Anmart5A) from Aspergillus niger LW-1 in Pichia pastoris. J Agric Food Chem, 2012, 60(3) : 765–773. DOI:10.1021/jf2041565 |
| [12] | Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976, 72 : 248–254. DOI:10.1016/0003-2697(76)90527-3 |
| [13] | Fei B J, Xu H, Cao Y, et al. A multi-factors rational design strategy for enhancing the thermostability of Escherichia coli AppA phytase. J Ind Microbiol Biotechnol, 2013, 40(5) : 457–464. DOI:10.1007/s10295-013-1260-z |
| [14] | Missimer J H, Steinmetz M O, Baron R, et al. Configurational entropy elucidates the role of salt-bridge networks in protein thermostability. Protein Sci, 2007, 16 : 1349–1359. DOI:10.1110/ps.062542907 |
| [15] | Ragone R. Hydrogen-bonding classes in proteins and their contribution to the unfolding reaction. Protein Sci, 2001, 10 : 2075–2082. DOI:10.1110/(ISSN)1469-896X |
| [16] | Gray T M, Matthews B W. Structural analysis of the temperature-sensitive mutant of bacteriophage T4 lysozyme, glycine 156 aspartic acid. J Biol Chem, 1987, 262(35) : 16858–16864. |
 2017, Vol. 37
2017, Vol. 37




