文章信息
- 李丹, 黄鹤.
- LI Dan, HUANG He.
- 纳米抗体异源表达的研究进展
- Heterologous Expression of Nanobodies:a Recent Progress
- 中国生物工程杂志, 2017, 37(8): 84-95
- China Biotechnology, 2017, 37(8): 84-95
- http://dx.doi.org/DOI:10.13523/j.cb.20170813
-
文章历史
- 收稿日期: 2017-03-05
- 修回日期: 2017-04-05
抗体药物已经成为生物药物最有潜力的产业之一,单克隆抗体也已成为治疗和诊断领域的重要工具。2016年美国FDA批准药物中大约30%是单抗药物,治疗范围涉及癌症、呼吸道、免疫、感染等方面[1]。2007年,Ablynx公司靶向vWF的ALX-0081纳米抗体进入临床研究,这是首个进入临床阶段的纳米抗体。但是单克隆抗体在一些应用方面仍存在缺陷:分子质量大(150kDa)限制其组织穿透能力;在体内能够引起免疫反应,进而中和抗体活性;半衰期较长限制其在分子成像中的应用;生产操作复杂、生产成本高。随着分子生物学技术的飞速发展,抗体分子小型化逐渐成为基因工程抗体的主要研究目标。小分子抗体如Fab、Fv、scFv等应运而生,但是它们在稳定性、亲和力等方面有待进一步改进。
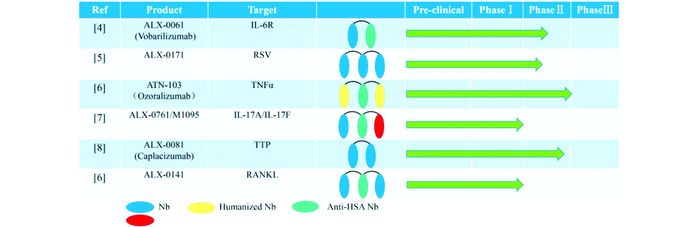
1993年,科学家在骆驼体内发现天然缺失轻链的重链抗体(Heavy chain antibodies,HcAbs)[2],其抗原结合域可变区,也被称为VHH或纳米抗体(nanobody, Nb)(图 1),为抗体小型化研究提供了新的发展方向。随后,软体鱼类体内也发现了骆驼体内类似的抗原受体(new antigen receptor, NAR)[3]。与其他小分子抗体相比,纳米抗体具有如分子质量小(12~15kDa)、亲和力高、免疫源性低等独特的性质,使其在疾病诊断及治疗方面更具优势。近年来,多种纳米抗体药物已进入临床阶段(图 2)。

|
| 图 1 传统抗体与重链抗体结构示意 Figure 1 The structures of conventional antibody and heavy-chain antibody |
|
| 图 2 部分纳米抗体药物的研究阶段 Figure 2 The research stage of some nanobodies |
噬菌体展示等体外筛选技术促进纳米抗体表达系统的发展。由于纳米抗体具有独特的结构和性质[9],使其可在原核及真核细胞中表达。本文综述了近年来纳米抗体及其衍生形式在革兰氏阴性菌(大肠杆菌)、革兰氏阳性菌(芽孢杆菌、乳酸杆菌)、酵母、丝状真菌、昆虫细胞、哺乳动物细胞和植物细胞中表达现状,包括表达系统、宿主、载体特点、载体构建方式及纳米抗体产量等方面。在此基础上,文中从分子水平、表达水平和理性设计三个方面探讨了提高纳米抗体产量的策略,希望为纳米抗体同行研究者提供一些借鉴和思路。
1 纳米抗体的表达 1.1 原核细胞 1.1.1 大肠杆菌大肠杆菌是最早用于外源基因表达的宿主细胞,也是目前应用最广泛的重组蛋白表达系统。利用大肠杆菌表达纳米抗体具有几个优势:大肠杆菌遗传背景清晰、基因操作简单、易培养。另外,大肠杆菌表达系统具有成熟的操作方法以及大量常用的表达载体和宿主(表 1)。在大肠杆菌中表达纳米抗体有两种表达策略:信号肽介导的周质表达和细胞质表达。
大肠杆菌周质空间可提供一个氧化环境,促进二硫键的形成,有利于外源蛋白的正确折叠。介导重组蛋白进入周质常用的信号肽有PelB、OmpA、PhoA等[16-18]。信号肽经信号肽酶切除后,可获得目的蛋白。纳米抗体在周质中的表达量通常较低[19-20],为提高纳米抗体在周质的表达水平,可将纳米抗体与标签蛋白进行融合表达。Behdani等[21]将假单胞菌外毒素A与纳米抗体融合表达,得到融合蛋白产量约为9.2 mg/L。Salema等[22]将纳米抗体与麦芽糖结合蛋白融合表达,将融合蛋白的产量提高到12 mg/L。
重组抗体在细胞质中往往以包涵体形式存在[23],这种方法生产的抗体产量高,但是经过复杂变性、复性手段后只能获得部分有活性的蛋白质[24]。在大肠杆菌谷胱甘肽和硫氧还蛋白缺陷菌株中,共表达周质分子伴侣二硫键异构酶(DsbC)或巯基氧化酶(SO),能够促进二硫键的正确折叠,提高纳米抗体在细胞质的产量[25-26]。Shuffle在细胞质中可过表达DsbC异构酶。Habib等[27]在Shuffle中表达纳米抗体,其产量可达到90~120 mg/L,BL21周质诱导表达产量仅为1~10 mg/L。研究中还发现Shuffle不适用于表达含多对二硫键的蛋白质。Veggiani等[28]将纳米抗体与SO共表达,增加了纳米抗体在细胞质中产量,使其产量达到10~15 mg/L。纳米抗体与DsbC或SO共表达的产量比在周质中的产量高(至少10倍),且表达的纳米抗体均有生物活性。
1.1.2 芽孢杆菌最新研究显示[29],scFv在枯草芽孢杆菌WB800N中达到了目前最高胞外浓度(130mg/L),最高时空收率(8 mg/L·h)。芽孢杆菌高效的蛋白分泌能力,使其有潜力成为生产抗体的重要表达系统。Mizukami等[30]在桥石短芽孢杆菌中表达anti-NDOM-VHH,在100 ml培养基中获得的蛋白分泌量为25~39 mg/L,在3 L的发酵罐中流加培养65h后得到的纳米抗体分泌量高达3 g/L。研究中使用的BIC(Brevibacillus in vivo cloning)质粒构建方法极大地简化了质粒的构建过程。根据目的基因插入的位点不同,pBIC表达载体可在细胞质或分泌表达蛋白,分泌表达时产生不带His-tag、N端带His-tag或C端带His-tag的蛋白。
1.1.3 乳酸杆菌乳酸杆菌是人和动物肠道内重要的益生菌。利用乳酸杆菌生产抗体,通过口服药物治疗胃肠道疾病,将有效地降低生产抗体药物的成本。Pant等[31]首次利用分泌和细胞表面展示两种方式生产抗轮状病毒纳米抗体。在体外实验中,两种方式表达的纳米抗体均显示与病毒的结合能力,在小鼠实验中,仅有展示在乳酸菌表面的纳米抗体能有效缓解小鼠痢疾的病症。Günaydin等[32]将两种不同基因表达盒插入到pAF900表达载体上,使两种蛋白在一个载体上共表达。他们用这种载体表达了识别不同抗原表位的两种纳米抗体,抗体分泌浓度为1~2 mg/L。乳酸杆菌生产的双特异性纳米抗体中和毒素的能力是单体混合物的300倍,双特异纳米抗体能有效延长小鼠的存活时间[33]。利用益生菌生产纳米抗体为人类胃肠道疾病的口服治疗提供了新的方案。
1.2 真核细胞 1.2.1 酵母细胞真核细胞具有高级折叠、翻译后修饰等功能,这些特点能提高抗体(包括全长IgG)的分泌量。酵母是一种低等的单细胞真核生物,相对于其他真核细胞来说,其生长周期短,易培养,基因操作简单。
酿酒酵母遗传背景清楚,长期应用在食品和医药工业,安全可靠,不产生毒素,因此成为生产抗体的重要表达系统之一。Frenken等[34]首次在酿酒酵母中表达纳米抗体,摇瓶产量可达到100 mg/L以上。Thomassen等[35]利用酿酒酵母在10 L的发酵罐中通过流加方式发酵生产纳米抗体,发酵结束后纳米抗体浓度可达到608 mg/L。纳米抗体J区某些氨基酸能参与内质网中分子伴侣的募集,从而缩小酿酒酵母与哺乳动物细胞分子伴侣机制的差异,有利于纳米抗体折叠动力学,进而影响纳米抗体在酿酒酵母中的产量[36]。
毕赤酵母是甲醇营养型酵母,可利用甲醇作为其唯一碳源。外源蛋白在毕赤酵母胞内表达或分泌至胞外。分泌表达时,毕赤酵母只分泌很少的内源蛋白,外源蛋白是培养基中的主要成分。外源蛋白在毕赤酵母中的糖基化程度与哺乳动物细胞糖基化程度相同,降低了外源蛋白的免疫原性。与酿酒酵母相比,毕赤酵母更适合高密度培养,外源蛋白表达量是酿酒酵母10倍以上,有利于工业化生产。有学者[37]发现,毕赤酵母突变体菌株更适合表达蛋白。然而,Schotte[38]发现,突变体菌株在表达纳米抗体时会掺入甲氧基,使得纳米抗体在人体内能够引起免疫反应。Baghban等[39]在大肠杆菌中表达纳米抗体的产量为6~7 mg/L,而在毕赤酵母中的产量(摇瓶培养)可达到16 mg/L。Ji等[40]用毕赤酵母表达anti-TNF-VHH-Fc,与大肠杆菌表达的VHH-Fc相比[41],有更好的生物活性,但产量却仅为5 mg/L。目前,Ablynx公司已经建立成熟的毕赤酵母表达系统及纯化工艺,纳米抗体产量可以达到g/L的水平[5]。
1.2.2 丝状真菌木霉和曲霉属的部分丝状真菌具有高效的蛋白分泌能力,能将大量的蛋白质和代谢物分泌到培养基中。为提高抗体的表达量,抗体基因片段可被插入到表达载体中与葡糖淀粉酶启动子及葡糖淀粉酶的N端进行融合表达,同时引入KexB等酶切位点,以便获得目的蛋白[42]。丝状真菌中蛋白水解酶能够降低蛋白质的产量,可通过基因敲除技术构建蛋白酶缺陷株,减少蛋白损失。Chrysosporium lucknowense C1敲除三个蛋白酶基因(Delta-alp1, Delta-pep4, Delta-alp2)后产生的突变体已经成功地应用于大规模生物反应器[43]。
Okazaki等[44]首次在米曲霉(Aspergillus oryzae)中表达anti-EGFR-VHH,产量为73.8 mg/L,低于大肠杆菌的产量300~800 mg/L[45]。GlaB-VHH融合蛋白在米曲霉中的最终产量可达610 mg/L,培养基中的粗产量为1.35 g/L[46]。GlaB-VHH为纳米抗体高表达提供了可能。但是作者在文章中并没有给出单体纳米抗体的产量,该方法的稳定性也有待验证。
1.2.3 昆虫细胞昆虫细胞是一种应用广泛的真核表达系统,重组杆状病毒表达体系能够专一地转染昆虫细胞。苜蓿银纹夜蛾多核型多角体病毒(Autographica californica MNPV,AcMNPV)是使用最广泛的载体。重组杆状病毒在自然界生存短暂,不能感染脊椎动物,其启动子在哺乳动物中是失活的,因此重组杆状病毒对人类具有安全性[47]。强启动子控制下的外源基因表达蛋白的产量占昆虫总蛋白的0.1%至50%。常用昆虫细胞系包括Sf-9细胞系、Sf-21细胞系、DS2细胞系和High Five细胞系(BTI-TN-5B1-4),High Five细胞系分泌的蛋白产量是Sf-9细胞系的25倍,但Sf-9和Sf-21的转染效率更高,更适于生产高滴度病毒[48]。
纳米抗体在昆虫表达系统中实现高达257 mg/L的产量[49],而IgGs、scFv及Fab在昆虫表达系统中产量约为3~35 mg/L[50],强启动子控制下结构复杂蛋白可能来不及形成正确折叠及翻译后修饰[51],导致目的蛋白发生聚集,降低抗体产量。Vega等[52]在昆虫中表达的anti-VP6-VHH能保护小猪抵制病毒感染和有效减少病毒脱落。
1.2.4 哺乳动物细胞生产重组抗体及抗体片段目前最主要的细胞是CHO、NS0、Sp2/0、HEK293和PER.C6(表 2)。大多数文献中采用CHO生产抗体/抗体片段,迄今为止,50%工业上生产的治疗性蛋白仍然由CHO生产[53]。值得一提的是,被批准用于人类疾病治疗的单克隆抗体仅由CHO、NS0和SP2/0生产[54]。
| 细胞 | 来源 | 特点 | 抗体/抗体片段产量 | 参考文献 |
| CHO | 中国仓鼠卵巢细胞 | 产生最接近天然状态的蛋白质 | 1 g/L (batch)
1~10 g/L (fed-batch) | [55] |
| PER.C6 | 人胚胎视网膜细胞 | 特异性腺病毒载体永生化,高细胞密度 | 0.5 g/L (batch)
8 g/L (fed-batch) 27 g/L (perfusion) | [55]
[56] |
| HEK293 | 人胚肾细胞 | 贴壁生长,瞬时表达 | 0.3g/L (batch)
0.6 g/L fed-batch) | [57]
[58] |
| NS0 | 鼠骨髓瘤细胞 | 胆固醇营养缺陷型,缺乏内源性谷氨酰胺合成酶 | 0.1~0.2 g/L (batch)
0.1~0.8g/L (fed-batches) | [55] |
哺乳动物细胞最适合生产治疗性抗体,其产生抗体的免疫原性最小。Bazl[59]等在CHO细胞表达了anti-GFP-VHH,并且建立了稳定表达的细胞系。VHH-Fc在CHO中分批培养的产量可达到100 mg/L[60]。Fc与纳米抗体融合表达,可延长纳米抗体的半衰期,激活其它免疫反应,增强纳米抗体抗病毒的能力,但是,Fc没有提高纳米抗体穿越血脑屏障的能力[61-62]。
1.2.5 植物纳米抗体已经在高等植物中表达成功。在水稻种子中纯化得到的anti-murine TNF-VHH,能够有效中和抗原、抑制小鼠关节炎[63]。植物表达系统成本低、可规模化生产、无毒素和病毒污染、可控糖基化、产物集中在特定的器官如叶子或种子,但最终抗体浓度通常较低[50]。拟南芥种子中成功表达了VHH和VHH-Fc[64],VHH产量占总可溶蛋白的0.13%,而VHH-Fc占16.25%。Fc在增加蛋白积累量的同时也导致部分蛋白的降解。Anti-α-Cbtx-VHH-Fc在新鲜烟草叶子中产量为129.5 mg/kg[15]。
2 提高纳米抗体产量的策略 2.1 分子水平影响外源基因在不同系统中表达的因素有很多,由于基因片段自身的结构特点,不同基因的表达水平也有很大不同。不同生物或同种生物不同的蛋白编码基因,对简并密码子使用频率具有一定的偏好性。单细胞生物体中,如酿酒酵母和细菌大肠杆菌,基因密码子偏好性与其翻译效率有关[65-66]。利用基因合成设计技术对基因编码序列进行密码子优化,使其翻译效率最大化,可有效增加蛋白质的表达量[67-68]。表达速率控制是与重组抗体产量相关的一个重要因素[69],基于lac启动子及其衍生启动子能够实现抗体及抗体片段的更高产量。大肠杆菌中可通过提高蛋白折叠效率增加重组蛋白可溶性,分子伴侣(GroEL/GroES等)或折叠酶(Dsb家族蛋白)过表达细胞(SHuffle),均可提高重组抗体的产量[70, 27]。另外,目的基因与其他高效表达蛋白序列融合表达也常常被用来提高重组抗体的产量,如假单胞菌外毒素A、硫氧还蛋白、SUMO、麦芽糖结合蛋白等[21, 71, 22]。通过δ位点整合构建酿酒酵母多拷贝表达载体可提高目的产物产量[72]。
2.2 表达水平其他提高重组蛋白可溶表达产量的策略还包括表达系统、表达宿主及表达载体的选择、细胞培养条件的优化等。原核表达系统适合表达不需要糖基化的小分子抗体,如scFv,VHH等[73];真核表达系统能够对重组蛋白进行糖基化等翻译后修饰,适合表达治疗性抗体[74]。优化大肠杆菌宿主细胞可提高生产过程中质粒的稳定性,从而可提高抗体的产量。Zhao等[75]通过优化宿主细胞,使抗人卵巢癌×抗人CD3单链双特异性抗体产量达到了1.2g/L,是优化之前产量的3倍。表达载体的特点及用途如表 3所示。表达载体、宿主菌和培养条件的最佳组合才能实现抗体的高效生产。
| 表达载体 | 特点及用途 | 表达系统 | 参考文献 |
| pET系列 | T7RNA聚合酶诱导蛋白表达 | 大肠杆菌 | [10][12] |
| pMSK | SUMO标签,促溶作用明显,可用于蛋白的可溶性表达 | 大肠杆菌 | [76] |
| pMAL | MBP标签,具有促溶和纯化作用 | 大肠杆菌 | [22] |
| Phen6/pComb3x | 噬菌粒载体,含有琥珀终止密码子,可用于抗体的筛选和可溶性表达 | 大肠杆菌 | [77][13] |
| pBIC | 可构建细胞质表达或分泌表达载体,带his-tag或不带his-tag | 芽孢杆菌 | [30] |
| pLP501 | ldh启动子,氨苄和红霉素抗性,PrtP基因信号肽 | 乳酸杆菌 | [31] |
| pAF900 | Apf启动子,apf基因信号肽,具有prtP锚定区,可用于蛋白的分泌表达或展示细胞表面表达 | 乳酸杆菌 | [32] |
| pUR4548 | 来源于pSY1载体,SUC2信号肽,用于蛋白的分泌表达 | 酿酒酵母 | [34] |
| pPink-HC | AOX1启动子,ADE2筛选基因,可用来进行蛋白的胞内表达 | 毕赤酵母 | [39] |
| pPICZaA | 分泌表达,标记基因博莱霉素,用于蛋白的分泌表达 | 毕赤酵母 | [40] |
| pISI-GFP | 含有根霉(Rhizopus oryzae)脂肪酶的N末端区域28个氨基酸的片段,sodM启动子,glaB终止子 | 米曲霉 | [44] |
| phlACB | hlyA启动子,glaB终止子 | 米曲霉 | [46] |
| pFastMelB2 | 具有来自蜜蜂的信号肽 | 昆虫细胞 | [49] |
| pcDNA3.1(-)-hygro | CMV启动子,可用于蛋白质在哺乳动物细胞内的高表达 | 哺乳动物 | [59] |
| pTT系列 | 具有两个表达盒,强CMV启动子控制目的基因的表达,弱SV40启动子控制表达嘌呤霉素乙酰转移酶基因 | 哺乳动物 | [60] |
| pINFUSE-hIgG1-Fc1 | hEF1-HTLV启动子,带有人源IgG的Fc段,可用于融合表达VHH-Fc | 哺乳动物 | [61] |
| pFUSE-hIgG1e5-Fc2 | 带有人源IgG的Fc段,可用于融合表达VHH-Fc | 哺乳动物 | [62] |
| pZH2Bik45-G1B | 具有两个启动子,prolamin启动子控制目的基因的过表达,mUbi启动子控制RNAi的表达,RNAi能够抑制水稻内源性醇溶蛋白和谷蛋白A储存蛋白 | 植物 | [63] |
| pPphasGW | 具有β-phaseolin基因的启动子Pphas和拟南芥种子储存蛋白基因的信号肽2S2 | 植物 | [64] |
培养条件优化包括温度、pH、培养基、添加剂(甲醇、山梨醇等)及培养方式等方面。在25~37℃时,大肠杆菌中重组蛋白通常以可溶形式存在,在37℃以上诱导表达,则容易形成包涵体[78]。在纳米抗体培养过程中使用营养成分丰富的培养基及维持pH稳定有助于细胞生长,使细胞维持高密度,有利于抗体产量的提高[11]。Rahbarizadeh等[79]研究发现,在毕赤酵母的培养基中加入适量的山梨醇、酸水解酪蛋白或EDTA可以提高纳米抗体的产量。生产系统和生产方式也会影响抗体产量,生物反应器中的细胞密度是摇瓶100倍左右,LEX鼓泡式生物反应器生产anti-MUC1 scFv抗体片段的产量是摇瓶30~40倍[80-81],流加培养的抗体产量高于分批培养[55]。
相关研究结果发现[5],在毕赤酵母中,较高pH可提高ALX-0171的产量。pH6.0时,纳米抗体产量为5.2 g/L,pH6.6时,纳米抗体产量为9.3 g/L。另外,增加甲醇的量会导致ALX-0171产量降低。较高温度可减少不成对半胱氨酸突变体的产生,如果诱导时间增加,可能由于自发氧化,不成对的半胱氨酸突变体也会减少,但是较高温度会增加蛋白降解。
2.3 理性设计理性设计是蛋白质进化的一种方式,通过对蛋白结构、功能等进行模拟分析,设计突变位点并进行定点突变,以期获得有利突变体,具有目的性。利用分子模拟及分子对接技术对目的蛋白可溶性、稳定性以及分子之间相互作用等进行分析,寻找影响纳米抗体产量的关键氨基酸,结合定点突变、框架区或CDRs交换,获得高产量和高质量的功能蛋白分子。Soler等[82]利用分子动力学模拟和分子对接相结合的方法,提出了HADDOCK程序新的应用,即用来预测纳米抗体有效产量和影响蛋白结构域稳定性残基的信息。与不考虑分子间相互作用的评分方法相比,该策略能更精确地预测纳米抗体的有效产量。
3 结论与展望每种表达系统都有其优点和缺点,不存在普适性的抗体表达系统,在选择表达系统时,应基于对实验室条件、抗体结构、功能及用途等的充分认识。
大肠杆菌是抗体噬菌体展示技术中使用的表达宿主,可以在不改变生产系统的情况下进行抗体产生和表达。大肠杆菌中表达的抗体片段主要以分泌到周质中的形式存在,然而从周质中提取蛋白的产量通常较低。芽孢杆菌和乳酸杆菌都是革兰氏阳性菌,没有外膜,可直接在培养上清中分离纳米抗体,但是革兰氏阳性菌的表达系统还处在发展阶段。原核细胞中缺乏糖基化机制,导致了VHH-Fc抗体难以在细菌中表达,从而限制了纳米抗体在临床上的应用。
低等真核细胞酵母,能够对蛋白质进行翻译后修饰,但全长免疫球蛋白在酵母中的表达依然存在困难。丝状真菌已经广泛应用于食品和生物技术产业,但在多数丝状真菌中难以获得高分泌效率的菌株。昆虫表达系统能提供较好的蛋白质折叠及翻译后修饰,成本比哺乳动物低。但是,昆虫表达系统不够成熟,没有稳定表达的细胞系及成熟的操作技术。植物表达系统适合大规模生产抗体,然而其基因操作复杂。此外,从植物中分离纯化纳米抗体也比较困难。哺乳动物表达治疗性抗体成本相对较高,但最高产量的抗体仍是由哺乳动物细胞产生。不同的表达系统中纳米抗体表达情况如表 4所示。
| Host | Nb format | Antigen | Yield | Comments | Ref |
| E.coli | |||||
| BL21(DE3) | VHH(2 different) | urokinase-type plasminogen activator | 3.5 mg /2.1 mg | 5L culture | [19] |
| BL21 | VHH | GPA | 1 ~10 mg/L | 1L shake flask | [27] |
| SHuffle | VHH | GPA | 90~120 mg/L | 1L shake flask | [27] |
| HM140 | MBP-VHH | GFP or human fibrinogen | 12-16 mg/L | shake flask | [22] |
| WK6 | VHH-PE | VEGFR2 | 9.2 mg/L | shake flask | [21] |
| Rosetta | VHH | CD20 | 3~10mg/L | shake flask | [83] |
| BL21 | VHH | Cry1le | 1.125mg/L | shake flask | [20] |
| WK6 | VHH | testostero | 3.9/3.5/2.5mg/L | shake flask | [84] |
| WK6 | VHH | VEGF | 0.3~0.8 g/L | shake flask | [45] |
| WK6 | VHH(6 different) | H7N2 | 5.72/6.89/4.71/
6.77/6.42/6.12mg/L | shake flask | [85] |
| Rosetta | VHH | AFP | 10mg/L | shake flask | [86] |
| Rosetta(DE3) | VHH | mycotoxin deoxynivalenol | 30~40 mg/L | shake flask | [12] |
| WK6 | VHH | Cry1Fa | >10 mg/L | shake flask | [87] |
| WK6 | VHH | Hottentotta saulcyi venom | 2mg/L | _ | [88] |
| WK6 | VHH(11 different) | PCT | 4.5~10.3mg/L | shake flask | [89] |
| XL-1 Blue | VHH | RVA | 9.2mg/L | _ | [77] |
| Non-suppressor top10 | VHH | BoNTs | 5~7mg/L | shake flask | [90] |
| BL21(DE3) | VHH | BoNTs | 55~57mg/L | shake flask | [90] |
| SHuffle | VHH(2 different) | EGFR | 200/130mg/L | fed-batch 1 L shake flask | [11] |
| BL21(DE3) | VHH(2 different) | EGFR | 220/140mg/L | fed-batch 1 L shake flask | [11] |
| Brevibacillus choshinensis | VHH | IZUMO1PFF | 3 g/L | fed-batch 3L scale | [91] |
| Lactobacillus | VHH | HIV | 4mg/L(Secretion concentration) | _ | [92] |
| Lactobacillus | VHH | Rotavirus | 1~2mg/L(Secretion concentration) | _ | [32] |
| Yeast | |||||
| Pichia pastoris | VHH | BoNT | 16mg/L | shake flask | [39] |
| Pichia pastoris | VHH | MUC1 | 10~15 mg/L | 1 L baffled flasks | [79] |
| Pichia pastoris | VHH-Fc | TNFa | 5 mg/L | 1 L flask | [40] |
| Pichia pastoris | VHH | AahI’ | 17mg/L | Small-scale | [93] |
| Saccharomyces cerevisiae | VHH(2 different) | flagellin | 31/153 mg/L | _ | [94] |
| Saccharomyces cerevisiae | VHH | _ | 100mg/L (Secretion concentration) | shake flask | [33] |
| Saccharomyces cerevisiae | VHH | azo-dye RR-6 | production rate 0.213 g.
[kgdryweight]-1h-1 | 15 m3 fed-batch fermentation | [34] |
| Y. lipolytica | Tryp-VHH | African trypanosomes | 30 mg/L | shake flask | [95] |
| Filmentous fungi | |||||
| Aspergillus oryzae | sTAA-VHH (2 different) | EGFR | 21/73.8mg/L | Sakaguchi flask | [44] |
| Aspergillus oryzae | GlaB-VHH | human chorionic gonadotropin | 610mg/L | _ | [46] |
| Insect cells | |||||
| Trichoplusiani | VHH | Rotavirus | 257 mg/L of protein extract | baculovirus | [49] |
| Mammalian cells | |||||
| HEK293T cells | VHH-Fc | human Aβ | 50mg/L | PEI | [61] |
| CHO | VHH-Fc | _ | 100mg/L | shake flask | [60] |
| Plants | |||||
| Nicotiana benthamiana | VHH-Fc | α-Cobratoxin | 129.50 mg/kg | Agrobacterium strains | [15] |
| Arabidopsis seeds | VHH | PSA | 0.13% VHH of total soluble protein | Agrobacterium strain C58C1RifR | [64] |
| Arabidopsis seeds | VHH-Fc | PSA | 16.25% VHH-Fc of total soluble protein | Agrobacterium strain C58C1RifR | [64] |
提高纳米抗体的生产水平将能够显著降低成本。可以从三个方面提高纳米抗体的产量:基因水平方面,对目的基因序列的修饰及载体表达元件的优化等;表达水平方面,对表达系统、宿主、载体的选择及培养条件的优化等;理性设计方面,利用生物信息学,结合体外突变技术,筛选高产菌株。
与传统抗体相比,纳米抗体独特的性质如分子量小、高亲和力、低免疫源性等,拓宽了其在诊断和治疗上的应用。目前,在研的纳米抗体药物涉及炎症、血液、呼吸道、肿瘤等方面,其已经在临床实验中展现了广阔的应用前景。本文对纳米抗体在原核及真核表达系统的生产及产量提高策略进行综述,以期为纳米抗体同行研究者提供一些借鉴,开发出更高效的纳米抗体表达系统,降低生产成本,使纳米抗体得到更广泛的应用。
| [1] |
Mullard A. 2016 FDA drug approvals. Nature Reviews Drug Discovery, 2017, 16: 73-76. DOI:10.1038/nrd.2017.14 |
| [2] |
Hamers C C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains. Nature, 1993, 363(6428): 446-448. DOI:10.1038/363446a0 |
| [3] |
Holliger P, Hudson P J. Engineered antibody fragments and the rise of single domains. Nature Biotechnology, 2005, 23(9): 1126-1136. DOI:10.1038/nbt1142 |
| [4] |
Edwin M, Robert K Z. Topline results from the Phase Ⅱb monotherapy study of vobarilizumab, ALX-0061(anti-IL-6R), in patients with moderate to severe RA. Ablynx webcast presentation, 2016. |
| [5] |
Http://www.ablynx.com
|
| [6] |
Steeland S, Vandenbroucke R E, Libert C. Nanobodies as therapeutics:big opportunities for small antibodies. Drug Discovery Today, 2016, 21(7): 1076-1113. DOI:10.1016/j.drudis.2016.04.003 |
| [7] |
Ablynx's Partner Initiates a Phase I Study and Doses First Healthy Volunteer with ALX0761. Ablynx, 2013.
|
| [8] |
Peyvandi F, Scully M, Kremer Hovinga J A, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. New England Journal of Medicine, 2016, 374(6): 511. DOI:10.1056/NEJMoa1505533 |
| [9] |
Harmsen M M, De Haard H J. Properties, production, and applications of camelid single-domain antibody fragments. Applied Microbiology and Biotechnology, 2007, 77(1): 13-22. DOI:10.1007/s00253-007-1142-2 |
| [10] |
Mei S, Yang X, Dan W, et al. Anti-idiotypicnanobody:A strategy for development of sensitive and green immunoassay for Fumonisin B 1. Talanta, 2015, 143: 388-393. DOI:10.1016/j.talanta.2015.05.010 |
| [11] |
Zarschler K, Witecy S, Kapplusch F, et al. High-yield production of functionalsoluble single-domain antibodies in the cytoplasm of Escherichia coli. Microbial Cell Factories, 2013, 12: 97-110. DOI:10.1186/1475-2859-12-97 |
| [12] |
Qiu Y L, He Q H, Xu Y, et al. Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay. AnalyticaChimicaActa, 2015, 887: 201-208. |
| [13] |
Farajpour Z, Rahbarizadeh F, Kazemi B, et al. A nanobody directed to a functional epitope on VEGF, as a novel strategy for cancer treatment. Biochemical & Biophysical Research Communications, 2014, 446(1): 132-136. |
| [14] |
Fridy P C, Thompson M K, Ketaren N E, et al. Engineered high-affinity nanobodies recognizing staphylococcal Protein A and suitable for native isolation of protein complexes. Analytical Biochemistry, 2015, 477: 92-94. DOI:10.1016/j.ab.2015.02.013 |
| [15] |
Richard G, Meyers A J, Mclean M D, et al. In vivo neutralization of α-cobratoxin with high-affinity Llama single-domain antibodies (VHHs) and a VHH-Fc antibody. Plos One, 2013, 8(7): e69495. DOI:10.1371/journal.pone.0069495 |
| [16] |
Takayama Y, Akutsu H. Expression in periplasmic space of Shewanellaoneidensis. Protein Expression & Purification, 2007, 56(1): 80-84. |
| [17] |
Gophna U, Ideses D, Rosen R, et al. OmpA of a septicemic Escherichia coli O78——secretion and convergent evolution. International Journal of Medical Microbiology Ijmm, 2004, 294(6): 373-381. DOI:10.1016/j.ijmm.2004.08.004 |
| [18] |
Wang Y, Ding H, Du P, et al. Production of phoA, promoter-controlled human epidermal growth factor in fed-batch cultures of Escherichia coli, YK537(pAET-8). Process Biochemistry, 2005, 40(9): 3068-3074. DOI:10.1016/j.procbio.2005.03.010 |
| [19] |
Kaczmarek J Z, Skottrup P D. Selection and characterization of camelid nanobodies towards urokinase-type plasminogen activator. Molecular Immunology, 2015, 65(2): 384-390. DOI:10.1016/j.molimm.2015.02.011 |
| [20] |
Xu C, Liu X, Zhang C, et al. Establishment of a sensitive time-resolved fluoroimmunoassay for detection of Bacillus thuringiensis Cry1Ie toxin based nanobody from a phage display library. Analytical Biochemistry, 2017, 518: 53-59. DOI:10.1016/j.ab.2016.11.006 |
| [21] |
Behdani M, Zeinali S, Karimipour M, et al. Development of VEGFR2-specific nanobody pseudomonas exotoxin A conjugated to provide efficient inhibition of tumor cell growth. New Biotechnology, 2013, 30(2): 205-209. DOI:10.1016/j.nbt.2012.09.002 |
| [22] |
Salema V, Fernández L Á. High yield purification of nanobodies from the periplasm of E. coli, as fusions with the maltose binding protein. Protein Expression & Purification, 2013, 91(1): 42-48. |
| [23] |
Wörn A, Auf M A, Escher D, et al. Correlation between in vitro stability and in vivo performance of anti-GCN4 intrabodies as cytoplasmic inhibitors. Journal of Biological Chemistry, 2000, 275(4): 2795-2803. DOI:10.1074/jbc.275.4.2795 |
| [24] |
Martineau P, Jones P, Winter G. Expression of an antibody fragment at high levels in the bacterial cytoplasm. Journal of Molecular Biology, 1998, 280(1): 117-127. DOI:10.1006/jmbi.1998.1840 |
| [25] |
Djender S, Schneider A, Beugnet A, et al. Bacterial cytoplasm as an effective cell compartment for producing functional VHH-based affinity reagents and Camelidae IgG-like recombinant antibodies. Microbial Cell Factories, 2014, 13(1): 140-149. DOI:10.1186/s12934-014-0140-1 |
| [26] |
Marco A D. Recombinant antibody production evolves into multiple options aimed at yielding reagents suitable for application-specific needs. Microbial Cell Factories, 2015, 14(1): 125-141. DOI:10.1186/s12934-015-0320-7 |
| [27] |
Habib I, Smolarek D, Hattab C, et al. VHH (nanobody) directed against human glycophorin A:A tool for autologous red cell agglutination assays. Analytical Biochemistry, 2013, 438(1): 82. DOI:10.1016/j.ab.2013.03.020 |
| [28] |
Veggiani G, De M A. Improved quantitative and qualitative production of single-domain intrabodies mediated by the co-expression of Erv1p sulfhydryl oxidase. Protein Expression And Purification, 2011, 79(1): 111-114. DOI:10.1016/j.pep.2011.03.005 |
| [29] |
Lakowitz A, Biedendieck R, Krull R. Recombinant production of the antibody fragment D1.3scFv in Bacillus sp. New Biotechnology, 2016, 33: S195-S195. |
| [30] |
Mizukami M, Tokunaga H, Onishi H, et al. Highly efficient production of VHH antibody fragments in Brevibacillus choshinensis expression system. Protein Expression & Purification, 2015, 105: 23-32. |
| [31] |
Pant N, Hultberg A, Zhao Y, et al. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. Journal of Infectious Diseases, 2006, 194(11): 1580-1588. DOI:10.1086/jid.2006.194.issue-11 |
| [32] |
Günaydin G, Álvarez B, Lin Y, et al. Co-expression of anti-rotavirus proteins (Llama VHH Antibody Fragments) in Lactobacillus:Development and Functionality of Vectors Containing Two Expression Cassettes in Tandem. Plos One, 2014, 9(4): e96409. DOI:10.1371/journal.pone.0096409 |
| [33] |
Andersen K K, Strokappe N M, Hultberg A, et al. Neutralization of Clostridium difficile toxin B mediated by engineered lactobacilli producing single domain antibodies. Infection & Immunity, 2016, 84(2): 395-406. |
| [34] |
Frenken L G, Rh V D L, Hermans P W, et al. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. Journal of Biotechnology, 2000, 78(1): 11-21. DOI:10.1016/S0168-1656(99)00228-X |
| [35] |
Thomassen Y E, Meijer W, Sierkstra L, et al. Large-scale production of V HH, antibody fragments by Saccharomyces cerevisiae. Enzyme & Microbial Technology, 2002, 30(3): 273-278. |
| [36] |
Gorlani A, Hulsik D L, Adams H, et al. Antibody engineering reveals the important role of J segments in the production efficiency of llama single-domain antibodies in Saccharomyces cerevisiae. Protein Engineering Design & Selection Peds, 2012, 25(1): 39-46. |
| [37] |
Orman M A, Calik P, Ozdamar T H. The influence of carbon sources on recombinant-human-growth-hormone production by Pichia pastoris is dependent on phenotype:a comparison of Muts and Mut+ strains. Biotechnology & Applied Biochemistry, 2009, 52(3): 245-255. |
| [38] |
Schotte P. Pichia pastoris Mut S strains are prone to misincorporation of O-methyl-l -homoserine at methionine residues when methanol is used as the sole carbon source. Microbial Cell Factories, 2016, 15(1): 1-9. DOI:10.1186/s12934-015-0402-6 |
| [39] |
Baghban R, Gargari S L, Rajabibazl M, et al. Camelid-derived heavy chain nanobody against Clostridium botulinum neurotoxin E in Pichia pastoris. Biotechnology & Applied Biochemistry, 2014, 63(2): 200-205. |
| [40] |
Ji X, Lu W, Zhou H, et al. Covalently dimerized Camelidae antihuman TNFa single-domain antibodies expressed in yeast Pichia pastoris show superior neutralizing activity. Applied Microbiology and Biotechnology, 2013, 97(19): 8547-8558. DOI:10.1007/s00253-012-4639-2 |
| [41] |
Djender S, Schneider A, Beugnet A, et al. Bacterial cytoplasm as an effective cell compartment for producing functional VHH-based affinity reagents and Camelidae IgG-like recombinant antibodies. Microbial Cell Factories, 2014, 13(1): 140. DOI:10.1186/s12934-014-0140-1 |
| [42] |
Ward P P, Piddington C S, Cunningham G A, et al. A system for production of commercial quantities of human lactoferrin:a broad spectrum natural antibiotic. Biotechnology, 1995, 13(5): 498-503. |
| [43] |
Verdoes J C, Punt P J, Burlingame R, et al. Original research:A dedicated vector for efficient library construction and high throughput screening in the hyphal fungus Chrysosporium lucknowense. Industrial Biotechnology, 2007, 3(1): 48-57. DOI:10.1089/ind.2007.3.048 |
| [44] |
Okazaki F, Aoki J, Tabuchi S, et al. Efficient heterologous expression and secretion in Aspergillus oryzae of a llama variable heavy-chain antibody fragment VHH against EGFR. Applied Microbiology and Biotechnology, 2012, 96(1): 81-88. DOI:10.1007/s00253-012-4158-1 |
| [45] |
Kazemilomedasht F, Behdani M, Bagheri K P, et al. Inhibition of angiogenesis in human endothelial cell using VEGF specific nanobody. Molecular Immunology, 2015, 65(1): 58-67. DOI:10.1016/j.molimm.2015.01.010 |
| [46] |
Hisada, Hiromoto, Tsutsumi, et al. High production of llama variable heavy-chain antibody fragment (VHH):fused to various reader proteins by Aspergillus oryzae. Applied Microbiology and Biotechnology, 2013, 97(2): 761-766. DOI:10.1007/s00253-012-4211-0 |
| [47] |
Lackner A, Kreidl E, Peter-Vörösmarty B, et al. Stable protein expression in mammalian cells using baculoviruses. Methods in Molecular Biology, 2012, 801: 75-92. DOI:10.1007/978-1-61779-352-3 |
| [48] |
Davis T R, Wickham T J, Mckenna K A, et al. Comparative recombinant protein production of eight insect cell lines. In Vitro Cellular & Developmental Biology-Animal, 1993, 29(5): 388-390. |
| [49] |
Gómez-Sebastián S. Rotavirus A-specific single-domain antibodies produced in baculovirus-infected insect larvae are protective in vivo. Bmc Biotechnology, 2012, 12(1): 59. DOI:10.1186/1472-6750-12-59 |
| [50] |
Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Frontiers in Immunology, 2013, 4: 217. |
| [51] |
Hasemann C A, Capra J D. High-level production of a functional immunoglobulin heterodimer in a baculovirus expression system. Proceedings of the National Academy of Sciences, 1990, 87(10): 3942-3946. DOI:10.1073/pnas.87.10.3942 |
| [52] |
Vega C G, Bok M, Vlasova A N, et al. Recombinant monovalent llama-derived antibody fragments (VHH) to rotavirus VP6 protect neonatal gnotobiotic piglets against human rotavirus-induced diarrhea. Plos Pathogens, 2013, 9(5): e1003334. DOI:10.1371/journal.ppat.1003334 |
| [53] |
Beck A, Wagner-Rousset E, Bussat M C, et al. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. 2009, 9(6):482-501.
|
| [54] |
Dumont J, Euwart D, Mei B, et al. Human cell lines for biopharmaceutical manufacturing:history, status, and future perspectives. Critical Reviews in Biotechnology, 2015, 36(6): 1-13. |
| [55] |
Renate K, David R. Advances in recombinant antibody manufacturing. Applied Microbiology and Biotechnology, 2016, 100(8): 3451-3461. DOI:10.1007/s00253-016-7388-9 |
| [56] |
Kuczewski M, Schirmer E, Lain B, et al. A single-use purification process for the production of a monoclonal antibody produced in a PER.C6 human cell line. Biotechnology Journal, 2011, 6(1): 56. DOI:10.1002/biot.v6.1 |
| [57] |
Hasegawa H, Woods C E, Kinderman F, et al. Russell body phenotype is preferentially induced by IgG mAb clones with high intrinsic condensation propensity:relations between the biosynthetic events in the ER and solution behaviors in vitro. Mabs, 2014, 6(6): 1518-1532. DOI:10.4161/mabs.36242 |
| [58] |
Jäger V, Büssow K, Wagner A, et al. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. Bmc Biotechnology, 2013, 13(1): 52. DOI:10.1186/1472-6750-13-52 |
| [59] |
Bazl M R, Rasaee M J, Foruzandeh M, et al. Production of chimeric recombinant single domain antibody-green fluorescent fusion protein in Chinese hamster ovary cells. Hybridoma, 2007, 26(1): 1-9. DOI:10.1089/hyb.2006.037 |
| [60] |
Agrawal V, Slivac I, Perret S, et al. Stable expression of chimeric heavy chain antibodies in CHO cells. Methods in Molecular Biology, 2012, 911(911): 287-303. |
| [61] |
Rotman M, Welling M M, Boogaard M L, et al. Fusion of hIgG1-Fc to 111In-anti-amyloid single domain antibody fragment VHH-pa2H prolongs blood residential time in APP/PS1 mice but does not increase brain uptake. Nuclear Medicine & Biology, 2015, 42(8): 695-702. |
| [62] |
Qasemi M, Behdani M, Shokrgozar M A, et al. Construction and expression of an anti-VEGFR2 nanobody-Fc fusionbody in NS0 host cell. Protein Expression & Purification, 2016, 123: 19-25. |
| [63] |
Abe M, Yuki Y, Kurokawa S, et al. A rice-based soluble form of a murine TNF-specific llama variable domain of heavy-chain antibody suppresses collagen-induced arthritis in mice. Journal of Biotechnology, 2014, 175(1): 45-52. |
| [64] |
De B S, Nolf J, De M T, et al. Fusion of an Fc chain to a VHH boosts the accumulation levels in Arabidopsis seeds. Plant Biotechnology Journal, 2013, 11(8): 1006-1016. DOI:10.1111/pbi.12094 |
| [65] |
Angov E. Codon usage:Nature's roadmap to expression and folding of proteins. Biotechnology Journal, 2011, 6(6): 650-659. DOI:10.1002/biot.201000332 |
| [66] |
Lindgreen S. Codon evolution:Mechanisms and models. Trends in Evolutionary Biology, 2012, 4(1): 8. DOI:10.4081/eb.2012.e8 |
| [67] |
Grote A, Hiller K, Scheer M, et al. JCat:a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Research, 2005, 33(Web Server issue): W526. |
| [68] |
Webster G, Teh A Y, Ma J K. Synthetic gene design——The rationale for codon optimization and implications for molecular pharming in plants. Biotechnology & Bioengineering, 2016. |
| [69] |
Schlegel S, Rujas E, Ytterberg A J, et al. Optimizing heterologous protein production in the periplasm of E. coli by regulating gene expression levels. Microbial Cell Factories, 2013, 12(1): 24. DOI:10.1186/1475-2859-12-24 |
| [70] |
Levy R, Weiss R, Chen G, et al. Production of correctly folded Fab antibody fragment in the cytoplasm of Escherichia coli trxB gor mutants via the coexpression of molecular chaperones. Protein Expression Purification, 2001, 23(2): 338-347. DOI:10.1006/prep.2001.1520 |
| [71] |
Sadr V, Saffar B, Emamzadeh R. Functional expression and purification of recombinant Hepcidin25 production in Escherichia coli using SUMO fusion technology. Gene, 2017, 610: 112-117. DOI:10.1016/j.gene.2017.02.010 |
| [72] |
Lian J, Jin R, Zhao H. Construction of plasmids with tunable copy numbers in Saccharomyces cerevisiae and their applications in pathway optimization and multiplex genome integration. Biotechnology & Bioengineering, 2016, 113(11): 2462-2473. |
| [73] |
Gupta S K, Shukla P. Microbial platform technology for recombinant antibody fragment production:A review. Critical Reviews in Microbiology, 2017, 31-42. |
| [74] |
Khan A H, Bayat H, Rajabibazl M, et al. Humanizing glycosylation pathways in eukaryotic expression systems. World Journal of Microbiology & Biotechnology, 2017, 33(1): 4. |
| [75] |
Zhao J B, Wei D Z, Tong W Y. Identification of Escherichia coli host cell for high plasmid stability and improved production of antihuman ovarian carcinoma x antihuman CD3 single-chain bispecific antibody. Applied Microbiology and Biotechnology, 2007, 76(4): 795-800. DOI:10.1007/s00253-007-1050-5 |
| [76] |
Di W, Yang S, Yin S, et al. Characterization of single-domain antibodies against Foot and Mouth Disease Virus (FMDV) serotype O from a camelid and imaging of FMDV in baby hamster kidney-21 cells with single-domain antibody-quantum dots probes. BMC Veterinary Research, 2015, 11(1): 120-130. DOI:10.1186/s12917-015-0437-2 |
| [77] |
Maffey L, Vega C G, Miño S, et al. Anti-VP6 VHH:An experimental treatmentfor rotavirus A-associated disease. Plos One, 2016, 11(9): 1-27. |
| [78] |
甄永苏, 邵荣光. 抗体工程药物. 北京: 化学工业出版社, 2002, 48. Zhen Y S, Shao R G. Antibody Engineering Pharmaceutics. Beijing: Chemical Industry Press, 2002, 48. |
| [79] |
Rahbarizadeh F, Rasaee M J, Forouzandeh M, et al. Over expression of anti-MUC1 single-domain antibody fragments in the yeast Pichia pastoris. Molecular Immunology, 2006, 43(5): 426-435. DOI:10.1016/j.molimm.2005.03.003 |
| [80] |
Kipriyanov S M, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. Journal of Immunological Methods, 1997, 200(1-2): 69. DOI:10.1016/S0022-1759(96)00188-3 |
| [81] |
Miethe S, Meyer T, Wöhlbruhn S, et al. Production of single chain fragment variable (scFv) antibodies in Escherichia coli using the LEXTM bioreactor. Journal of Biotechnology, 2013, 163(2): 105. DOI:10.1016/j.jbiotec.2012.07.011 |
| [82] |
Soler M A, De M A, Fortuna S. Molecular dynamics simulations and docking enable to explore the biophysical factors controlling the yields of engineered nanobodies. Scientific Reports, 2016, 6: 34869. DOI:10.1038/srep34869 |
| [83] |
Liu J L, Zabetakis D, Goldman E R, et al. Selection and characterization of single domain antibodies against human CD20. Molecular Immunology, 2016, 78: 146-154. DOI:10.1016/j.molimm.2016.09.013 |
| [84] |
Li G, Zhu M, Ma L, et al. Generation of small single domain nanobody binders for sensitive detection of testosterone by electrochemical impedance spectroscopy. Acs Applied Materials & Interfaces, 2016, 8(22). |
| [85] |
Xue G, Zhu M, Li G, et al. Specific determination of influenza H7N2 virus based on biotinylated single-domain antibody from a phage-displayed library. Analytical Biochemistry, 2015, 500: 66-72. |
| [86] |
Chen J, He Q H, Xu Y, et al. Nanobody medicated immunoassay for ultrasensitive detection of cancer biomarker alpha-fetoprotein. Talanta, 2016, 147: 523-530. DOI:10.1016/j.talanta.2015.10.027 |
| [87] |
Wang P, Li G, Yan J, et al. Bactrian camel nanobody-based immunoassay for specific and sensitive detection of Cry1Fa toxin. Toxicon, 2014, 92: 186-192. DOI:10.1016/j.toxicon.2014.10.024 |
| [88] |
Darvish M, Behdani M, Shokrgozar M A, et al. Development of protective agent against Hottentotta saulcyi venom using camelid single-domain antibody. Molecular Immunology, 2015, 68(2): 412-420. DOI:10.1016/j.molimm.2015.10.002 |
| [89] |
Yan J, Wang P, Zhu M, et al. Characterization and applications of nanobodies against human procalcitonin selected from a novel naive nanobody phage display library. Journal of Nanobiotechnology, 2015, 13(1): 33. DOI:10.1186/s12951-015-0091-7 |
| [90] |
Bakherad H, Mousavi G S, Rasooli I, et al. In vivo neutralization of botulinum neurotoxins serotype E with heavy-chain camelid antibodies (VHH). Molecular Biotechnology, 2013, 55(2): 159-167. DOI:10.1007/s12033-013-9669-1 |
| [91] |
Mizukami M, Tokunaga H, Onishi H, et al. Highly efficient production of VHH antibody fragments in Brevibacillus choshinensis expression system. Protein Expression & Purification, 2015, 105: 23-32. |
| [92] |
Marcobal A, Liu X, Zhang W, et al. Expression of HIV-1 neutralizing antibody fragments using human vaginal lactobacillus. Aids Research & Human Retroviruses, 2016, 32(10). |
| [93] |
Ezzine A, Sonia M E, Bouhaouala-Zahar B, et al. Efficient expression of the anti-AahI' scorpion toxin nanobody under a new functional form in a Pichia pastoris system. Biotechnology & Applied Biochemistry, 2012, 59(1): 15-21. |
| [94] |
Adams H, Horrevoets W M, Adema S M, et al. Inhibition of biofilm formation by camelid single-domain antibodies against the flagellum of Pseudomonas aeruginosa. Journal of Biotechnology, 2014, 186: 131-138. |
| [95] |
Hofmeyer T, Bulani S I, Grzeschik J, et al. Protein production in Yarrowia lipolytica via fusion to the secreted lipase Lip2p. Molecular Biotechnology, 2014, 56(1): 79-90. DOI:10.1007/s12033-013-9684-2 |
 2017, Vol. 37
2017, Vol. 37




