文章信息
- 窦一涵, 李映, 赵鹏, 范如婷, 田平芳.
- DOU Yi-han, LI Ying, ZHAO Peng, FAN Ru-ting, TIAN Ping-fang.
- 重组肺炎克雷伯氏菌转化甘油为聚3-羟基丙酸
- Metabolic Engineering of Klebsiella pneumoniae for the Production of Poly(3-Hydroxypropionate) from Glycerol
- 中国生物工程杂志, 2017, 37(6): 86-92
- China Biotechnology, 2017, 37(6): 86-92
- http://dx.doi.org/DOI:10.13523/j.cb.20170613
-
文章历史
- 收稿日期: 2016-12-27
- 修回日期: 2017-02-27
2. 北京联合大学生物化学工程学院 北京 100023
2. College of Biochemical Engineering, Beijing Union University, Beijing 100023, China
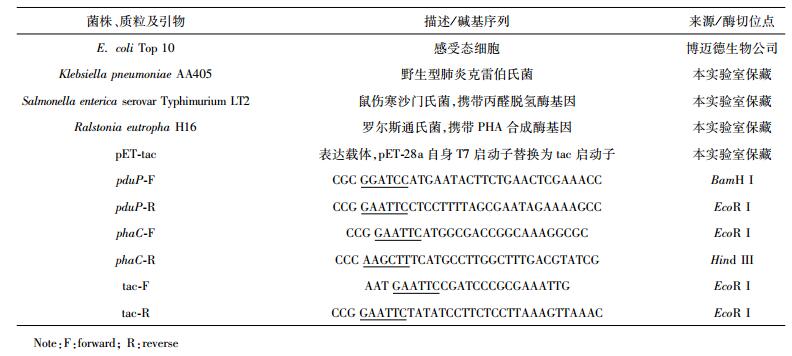
当环境中碳源过剩时,许多微生物在胞内合成聚羟基脂肪酸酯(polyhydroxyalkanoates, PHAs)[1-2]。PHAs是一类由羟基脂肪酸单体聚合而成的线性聚酯。PHAs不仅具有从坚硬质脆到柔软弹性的不同材料学性能[3],而且具备石化基塑料无法比拟的生物可降解性和生物相容性[4]。由于上述优良性能,PHAs已成为医用和食品工业等领域极具前景的新型材料[5-6]。聚3-羟基丙酸[poly (3-hydroxypropionate), P3HP]是PHA家族的新成员[7],其单体3-羟基丙酸(3-HP)是重要的平台化合物[8]。化学合成P3HP包括内酯开环聚合和直接缩聚,因工艺成本高及毒性而限制了研发[9-10]。近年来P3HP的研究日益增多,其工程菌发酵一般采用廉价碳源,且无需添加昂贵前体物[11-12]。
肺炎克雷伯氏菌(Klebsiella pneumoniae)不仅能转化甘油为3-HP,而且生长快[13],因此可作为构建P3HP工程菌的出发菌株。2015年,首次报道了一株经3-HP途径生产P3HP的重组肺炎克雷伯氏菌[14]。本研究分析了K. pneumoniae的生理生化特性,拟按图 1所示构建工程菌,精简了P3HP合成途径。以甘油为底物生产P3HP涉及3步酶催化[15]:(1) 甘油脱水酶(DhaB)催化甘油(Glycerol)生成3-羟基丙醛(3-HPA);(2) 丙醛脱氢酶(PduP)催化3-HPA生成3-羟基丙酰辅酶A(3HP-CoA);(3) 聚酯合成酶(PhaC)将3HP-CoA聚合为P3HP(图 1)。拟以肺炎克雷伯氏菌为宿主,异源表达鼠伤寒沙门氏菌Salmonella enterica serovar Typhimurium LT2的丙醛脱氢酶(PduP)及罗尔斯通氏菌Ralstonia eutropha H16的聚酯合成酶(PhaC),以甘油为碳源发酵生产P3HP。通过优化关键酶表达,提高甘油转化率和P3HP的产量。
|
| 图 1 以甘油为碳源合成P3HP的代谢途径 Figure 1 Biosynthetic pathway of P3HP from glycerol |
菌株K. pneumoniae AA405, S. enterica, R. eutropha及表达载体pET28a为本实验室保存。PCR引物由北京博迈德生物技术有限公司合成(表 1)。
1.2 试剂
DNA聚合酶购自TaKaRa公司;DNA Marker、蛋白Marker、细菌基因组提取试剂盒、质粒提取试剂盒购自博迈德生物技术公司;限制性核酸内切酶、T4 DNA连接酶购自New England Biolabs (北京)有限公司;硫酸卡纳霉素和3-羟基丙酸甲酯(3HP-Methyl)标准品购自宝如亿生物技术有限公司。
1.3 培养基及培养条件LB培养基(g/L):酵母粉,5;胰蛋白胨,10;NaCl,10;固体LB培养基需加入15 g/L琼脂。甘油基本发酵培养基(g/L):K2HPO4·3H2O,3.4;KH2PO4,1.3;(NH4)2SO4,4;MgSO4·7H2O,0.5;CaCO3,0.1;酵母粉,3;甘油,40;微量元素,1.25 ml。微量元素(g/L):MnCl2·4H2O, 0.1;ZnCl2, 0.07;NiCl2·6H2O, 0.025;FeSO4·7H2O, 1;CoCl2·2H2O, 0.2;CuCl2·2H2O, 0.02;Na2MoO4·2H2O, 0.035;硼酸,0.06;饱和盐酸,4 ml。硫酸卡纳霉素:贮存液浓度,10 mg/ml;工作浓度50 μg/ml。
菌种活化:将保存的菌液按1%(V/V)接种于4 ml LB液体培养基,37℃ 200 r/min振荡培养12 h。若质粒携带抗性基因,需添加工作浓度抗生素。摇瓶发酵:活化后的菌液按1%(V/V)接种于含100 ml发酵培养基的锥形瓶(250 ml),封口膜封口,37℃ 200 r/min连续培养24 h(第3 h后加入诱导剂IPTG),每3 h取样检测。
1.4 重组菌构建基因扩增:从GenBank查阅S. enterica serovar Typhimurium LT2的醛脱氢酶基因(pduP)及R. eutropha H16的聚酯合成酶(PHA合成酶)基因(phaC)序列,Primer Premier 5设计引物(表 1);用试剂盒提取细菌基因组,PCR扩增pduP和phaC基因。
重组载体pET-tac-pduP-phaC的构建:回收纯化pduP基因,提取质粒pET-tac,37℃下双酶切(BamH Ⅰ/EcoR Ⅰ)基因和载体2 h,切胶回收后加T4 DNA连接酶16℃连接4 h,热击转化至感受态细胞E. coli TOP10,经菌落PCR及琼脂糖凝胶电泳验证,获得含pET-tac-pduP的重组菌;同样方法获得含pET-tac-phaC的重组菌;双酶切(EcoR Ⅰ/Hind Ⅲ)上述两种质粒,以pET-tac-pduP为骨架,于pduP下游插入phaC,构建含两个关键酶基因的重组载体pET-tac-pduP-phaC。
重组载体pET-tac-pduP-tac-phaC的构建:以pET-tac为模板,PCR扩增tac启动子,用EcoR Ⅰ单酶切pET-tac-pduP-phaC和tac,在pduP和phaC基因之间插入tac启动子序列,转化感受态细胞E. coli TOP 10,筛选阳性克隆。载体构建思路如图 2。

|
| 图 2 重组质粒构建示意图 Figure 2 Diagram of vector construction for P3HP synthesis |
重组菌构建:提取重组载体pET-tac-pduP-phaC和pET-tac-pduP-tac-phaC,电击转化感受态K. pneumoniae,涂布含硫酸卡那霉素的固体平板,菌落PCR及琼脂糖凝胶电泳验证,获得重组菌K. p(pET-tac-pduP-phaC)和K. p(pET-tac-pduP-tac-phaC),保存备用。
1.5 重组菌发酵对以下4株菌进行24 h摇瓶发酵:K. p(pET-tac-pduP-phaC)、K. p(pET-tac-pduP-tac-phaC)、野生型(K. p WT)和空质粒对照K. p(pET-tac)。每3 h各自取样2 ml,测定OD600及甘油剩余量,绘制细菌生长及甘油消耗曲线。另取24 h时的发酵液,通过SDS-PAGE验证蛋白表达情况。
P3HP的检测:用气相色谱测定P3HP [16]。取2 ml 24 h发酵液,离心,洗涤得菌体;加氯仿、甲醇-硫酸溶液(甲醇:硫酸=85:15) 各1 ml;99℃水浴锅回流加热4 h;冷却至室温后加1 ml无菌水,漩涡振荡30 s,静置分层,取下层氯仿层,即为待测样品;以3HP-Methyl标准品为对照,GC检测。检测条件:初始90℃,保持3 min,10℃/min升温至180℃,保持5 min。为进一步验证结果,采用气相色谱-质谱联用(GC-MS)对样品组分进行测定。
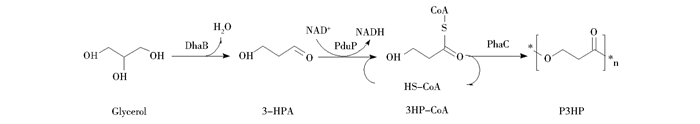
2 结果与讨论 2.1 重组菌的构建PCR克隆鼠伤寒沙门氏菌的pduP基因(1 395 bp),电泳如图 3a所示,1 500 bp附近有一清晰的条带,酶切,连接并转化感受态细胞,经菌落PCR验证,构建得到含有pduP的载体pET-tac-pduP。同样方法克隆罗尔斯通氏菌的PHA合成酶基因phaC(1 770 bp),如图 3b所示,2 000 bp附近有一清晰条带,构建载体pET-tac-phaC。测序结果经DNAMAN软件比对分析,发现与目标序列一致,可用于蛋白表达。
|
| 图 3 基因克隆及重组质粒电泳 Figure 3 Electrophoresis of genes and recombinant plasmids PCR amplification of pduP (a) and phaC (b); Colony PCR analysis of recombinant plasmids pET-tac-pduP-phaC (c) and pET-tac-pduP-tac-phaC (d); M:DNA marker |
双酶切(EcoR Ⅰ/Hind Ⅲ)处理pET-tac-pduP和pET-tac-phaC,再经连接、转化、菌落PCR验证,得到同时携带pduP和phaC的重组载体pET-tac-pduP-phaC,如图 3c所示,在3 000 bp附近有目标条带,表明质粒上同时携带pduP和phaC。
在一个阅读框中串联表达多个基因时,后面基因的表达通常较弱。因此在pduP和phaC之间通过EcoR Ⅰ位点插入包含操纵基因的tac启动子序列,使两个基因的表达受独立操纵基因控制。成功构建表达载体pET-tac-pduP-tac-phaC,如图 3d所示,在3 000 bp附近有目标条带,避免了PHA合成酶基因phaC表达受抑制的可能性。
提取上述两种重组载体,电击转化至K. pneumoniae,构建重组工程菌K. p(pET-tac-pduP-phaC)和K. p(pET-tac-pduP-tac-phaC),保存备用。
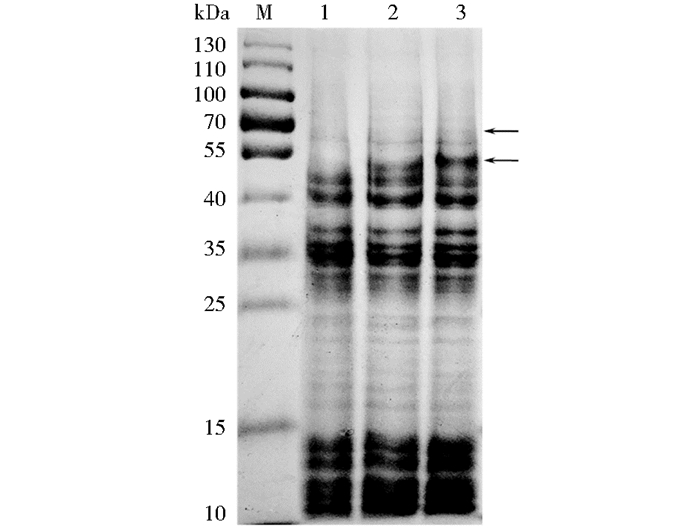
2.2 重组菌蛋白表达摇瓶发酵24 h的菌体进行SDS-聚丙烯凝胶电泳。从SDS-PAGE结果(图 4) 可知,丙醛脱氢酶PduP(49 kDa)在目标位置有明显条带,表达效果较好;PHA合成酶(64.3 kDa)条带浅,表达较弱。
|
| 图 4 SDS-PAGE Figure 4 SDS-PAGE analysis of protein expression M:Marker; 1:K. p WT; 2-K. p(pET-tac-pduP-phaC); 3: K. p(pET-tac-pduP-tac-phaC); arrows indicate PduP (49 kDa) and PhaC(64.3 kDa) |
对重组菌及对照菌进行摇瓶发酵,测吸光度(OD600)和甘油剩余量,绘制生长曲线(图 5a)及甘油剩余量曲线(图 5b)。通过分析和对比可知,4株菌在24 h内的甘油消耗大致相同。从生物量可知,重组菌K. p(pET-tac-pduP-phaC)及空质粒组稍低于野生型,推测是质粒的引入增加了负荷;重组菌K. p(pET-tac-pduP-tac-phaC)在6 h后生物量急剧上升,12 h进入平稳期,最大值为野生型的1.75倍。推测原因如下:其一,表达的丙醛脱氢酶将对细胞有毒的3-HPA转化为3HP-CoA,而强化表达PHA合成酶进一步拉动代谢流,减少了3-HPA累积;其二,P3HP是胞内大分子,可作为碳源促进菌体生长,因此在甘油消耗相同的条件下,细菌表现更高的生物量;其三,生产P3HP颗粒的菌体体积发生明显变化,可能对菌体浓度的测量造成一定干扰。

|
| 图 5 菌体生长及甘油剩余量曲线图 Figure 5 Growth and residual glycerol of recombinant strains |
取发酵24 h的菌液,经甲酯化反应将P3HP转化为3HP-Methyl后用气相色谱检测。如图 6所示,(a)、(b)、(c)分别代表 3HP-Methyl标准品、重组菌K. p(pET-tac-pduP-phaC)和K. p(pET-tac-pduP-tac-phaC)甲酯化样品的GC结果。从图 6可知,样品与标准品的出峰位置吻合,出峰时间(min)分别为:8.413s、8.399s、8.414s,初步认定发酵样品中含有3HP-Methyl;为确证此结果,用气相色谱-质谱联用检测样品,证实样品中对应出峰时间的物质为3HP-Methyl,即菌体产生了P3HP。根据GC结果,重组菌K. p(pET-tac-pduP-tac-phaC)出峰面积大于K. p(pET-tac-pduP-phaC),与预期相符,即独立阅读框的引入,使PHA合成酶表达量增加,P3HP产量也相应增加。

|
| 图 6 气相色谱检测结果 Figure 6 Results of gas chromatography GC results of 3HP-Methyl standard (a), K. p(pET-tac-pduP-phaC) (b) and K. p(pET-tac-pduP-tac-phaC) (c) |
以氯仿为溶剂配制浓度为10、8、5、4、2、1及0.5(g/L)的3HP-Methyl标准品溶液,GC检测,绘制标准曲线(图 7),峰面积计算样品中的3HP-Methyl含量,并换算为重组菌K. p(pET-tac-pduP-phaC)和K. p(pET-tac-pduP-tac-phaC)的P3HP产量,分别为0.054 g/L及0.091 g/L。

|
| 图 7 3HP-Methyl标准曲线 Figure 7 Standard curve of 3HP-Methyl |
(1) PCR克隆了S. enterica的醛脱氢酶基因pduP和R. eutropha的PHA合成酶基因phaC,构建共表达载体后转化K. pneumoniae,SDS-PAGE表明表达成功。
(2) 以甘油为唯一碳源摇瓶发酵,GC及气相色谱-质谱联用证实在K. pneumoniae成功构建了P3HP的合成途径。
(3)pduP和phaC共用tac启动子的工程菌K. p(pET-tac-pduP-phaC)产生0.054 g/L的P3HP,而pduP和phaC各自独用tac启动子的工程菌K. p(pET-tac-pduP-tac-phaC)产生0.091 g/L的P3HP。后者是前者P3HP产量的1.69倍。
| [1] | Wang Y, Yin J, Chen G Q. Polyhydroxyalkanoates, challenges and opportunities. Current Opinion in Biotechnology, 2014, 30(30) : 59–65. |
| [2] | Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnology and Bioengineering, 1996, 49(1) : 1–14. DOI:10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.3.CO;2-1 |
| [3] | Sudesh K, Abe H, Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates:biological polyesters. Progress in Polymer Science, 2000, 25(10) : 1503–1555. DOI:10.1016/S0079-6700(00)00035-6 |
| [4] | Poirier Y, Nawrath C, Somerville C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Nature Biotechnology, 1995, 13(2) : 142–150. DOI:10.1038/nbt0295-142 |
| [5] | Chen G Q, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials, 2005, 26(33) : 6565–6578. DOI:10.1016/j.biomaterials.2005.04.036 |
| [6] | Rai R, Keshavarz T, Roether J A, et al. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Materials Science and Engineering:R:Reports, 2011, 72(3) : 29–47. DOI:10.1016/j.mser.2010.11.002 |
| [7] | Zhu B, Kai W, Pan P, et al. Polymorphic packing and dynamics of biodegradable poly(3-hydroxypropionate). The Journal of Physical Chemistry B, 2008, 112(32) : 9684–9692. DOI:10.1021/jp801538p |
| [8] | Li Y, Wang X, Ge X, et al. High production of 3-hydroxypropionic acid in Klebsiella pneumoniae by systematic optimization of glycerol metabolism. Scientific Reports, 2016, 6 : 26932. DOI:10.1038/srep26932 |
| [9] | Dunn E W, Lamb J R, LaPointe A M, et al. Carbonylation of ethylene oxide to β-propiolactone:a facile route to poly(3-hydroxypropionate) and acrylic acid. ACS Catalysis, 2016, 6(12) : 8219–8223. DOI:10.1021/acscatal.6b02773 |
| [10] | Yamashita M, Takemoto Y, Ihara E, et al. Organolanthanide-initiated living polymerizations of ε-caprolactone, δ-valerolactone, and β-propiolactone. Macromolecules, 1996, 29(5) : 1798–1806. DOI:10.1021/ma951400n |
| [11] | Andreeβen B, Lange A B, Robenek H, et al. Conversion of glycerol to poly(3-hydroxypropionate) in recombinant Escherichia coli. Applied and Environmental Microbiology, 2010, 76(2) : 622–626. DOI:10.1128/AEM.02097-09 |
| [12] | Wang Q, Liu C, Xian M, et al. Biosynthetic pathway for poly(3-hydroxypropionate) in recombinant Escherichia coli. Journal of Microbiology, 2012, 50(4) : 693–697. DOI:10.1007/s12275-012-2234-y |
| [13] | Wang K, Wang X, Ge X, et al. Heterologous expression of aldehyde dehydrogenase from Saccharomyces cerevisiae in Klebsiella pneumoniae for 3-hydroxypropionic acid production from glycerol. Indian Journal of Microbiology, 2012, 52(3) : 478–483. DOI:10.1007/s12088-012-0280-0 |
| [14] | Feng X, Xian M, Liu W, et al. Biosynthesis of poly (3-hydroxypropionate) from glycerol using engineered Klebsiella pneumoniae strain without vitamin B12. Bioengineered, 2015, 6(2) : 77–81. DOI:10.1080/21655979.2015.1011027 |
| [15] | Gao Y, Liu C, Ding Y, et al. Development of genetically stable Escherichia coli strains for poly (3-hydroxypropionate) production. PloS One, 2014, 9(5) : e97845. DOI:10.1371/journal.pone.0097845 |
| [16] | Brandl H, Gross R A, Lenz R W, et al. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Applied and Environmental Microbiology, 1988, 54(8) : 1977–1982. |
 2017, Vol. 37
2017, Vol. 37