文章信息
- 夏惠, 刘磊, 王秀, 沈妍秋, 郭雨伦, 梁东.
- XIA Hui, LIU Lei, WANG Xiu, SHEN Yan-qiu, GUO Yu-lun, LIANG Dong.
- 苹果6-磷酸山梨醇脱氢酶基因启动子逆境诱导表达特性研究
- Research on Stress-inducible Expression Characteristics of Sorbitol-6-phosphate Dehydrogenase Promoter from Apple
- 中国生物工程杂志, 2017, 37(6): 50-55
- China Biotechnology, 2017, 37(6): 50-55
- http://dx.doi.org/DOI:10.13523/j.cb.20170608
-
文章历史
- 收稿日期: 2017-03-21
- 修回日期: 2017-04-14
2. 四川农业大学果蔬研究所 成都 611130;
3. 成都市农林科学院 成都 611130
2. Institute of Pomology and Olericulture, Sichuan Agricultural University, Chengdu 611130, China;
3. Chengdu Academy of Agriculture and Forestry Sciences, Chengdu 611130, China
山梨醇(Sorbitol)是大多数蔷薇科植物糖代谢的主要形式,如同其他植物中的蔗糖[1-2]。除此之外,山梨醇还可以作为一种小分子渗透调节物质提高植物对干旱[3-4]、火疫病[5]、低温和盐[6]等各种逆境的抗性。6-磷酸山梨醇脱氢酶(Sorbitol-6-phosphate dehydrogenase,S6PDH;EC 1.1.1.200) 在植物体内催化葡萄糖-6-磷酸生成山梨醇-6-磷酸,再经6-磷酸山梨醇磷酸酶(sorbitol-6-phosphatase,SorPP;EC 3.1.3.50) 脱磷酸后生成山梨醇[2]。S6PDH参与的反应被认为是山梨醇合成的关键调控步骤[7-8]。编码S6PDH的基因序列现已获得[9-10]。研究表明,在干旱、低温和盐等逆境胁迫下,S6PDH的表达量和山梨醇含量均会升高[3-4, 6, 11]。
启动子对于一个基因的表达具有非常重要的调控作用。Qi等[12]认为大豆PvSR2基因启动子的(-222/-188) 区和(-187/-147) 区是重金属胁迫响应的关键区域。Xu等[13]发现葡萄芪合成酶基因启动子的(-162/0) 区对胁迫诱导最为关键。Li等[14]的结果也表明GPP基因启动子受不同逆境的诱导。在以前的研究中我们发现,S6PDH基因的启动子(S6PDHp)长2 396bp。通过在线软件预测,发现启动子序列中具有与胁迫响应、光照响应和激素响应等相关的顺式作用元件。烟草瞬时转化试验还发现该启动子具有低温、脱落酸(abscisic acid,ABA)和光照诱导表达的特性[15]。
因此,为了进一步验证和探索S6PDHp的逆境诱导表达特性,在前期获得启动子序列的基础上,通过Gateway技术构建了系列S6PDHp5′端序列缺失体与报告基因GUS的融合表达载体,进一步通过农杆菌介导法转化拟南芥,研究不同诱导条件下转基因拟南芥中GUS蛋白的活性变化。研究结果将为深入了解S6PDHp的功能特性和S6PDH的表达调控等奠定基础。
1 材料与方法 1.1 材料本研究所用的拟南芥(Arabidopsis thaliana)为Columbia野生型。4-甲基伞形酮酰-β-D-葡萄糖酸苷(MUG)、SDS、Tris酚、X-gal、4-甲基伞形酮(4-MU)购自Sigma公司,BP Clonase Enzyme Mix和LR Clonase Enzyme Mix均购自Invitrogen公司,其它试剂均为国产分析纯。载体pDONR221(Kan抗性)、pGWB433、农杆菌EHA105和含有苹果S6PDHp基因的载体(pMD-S6PDHp)为本实验室保存。大肠杆菌TOP10感受态购自天根公司。
1.2 利用Gateway技术构建S6PDHp-GUS系列融合表达载体根据启动子序列测序结果[15]并结合考虑各类作用元件的分布,从启动子5′端碱基开始,依次设计了4个引物attB-PN(N=1、2、3、4)(1F)S(表 1)。使用此4个引物分别与attB-P(1F)A(表 1)配对,以pMD-S6PDHp为模板进行第一轮PCR扩增,产物经1%琼脂糖凝胶电泳后切胶回收纯化。然后以第1轮PCR纯化产物为模板,attB-PN(N=1、2、3、4)(2F)S和attB-P(2F)A(表 1)为引物进行第2轮PCR扩增,产物经1%琼脂糖凝胶电泳后切胶回收纯化。最后再以第二轮PCR纯化产物为模板,attB A/S为引物(表 1)进行第3轮PCR扩增,最终获得携带attB全位点的attB-S6PDHp N(N=1、2、3、4) 产物。以上产物进行回收纯化后用于下一步BP反应构建Entry载体。
利用得到的attB-S6PDHp N(N=1、2、3、4) 产物和pDONR221进行BP反应,构建含卡那霉素抗性的Entry载体。将BP反应产物转化大肠杆菌感受态细胞后,用卡那霉素进行筛选,并对菌液进行PCR鉴定。将获得的Entry载体质粒通过LR反应连接到pGWB433表达载体上,将LR反应产物转化到大肠杆菌感受态细胞中,用壮观霉素进行筛选,并对菌液进行PCR鉴定,引物为attBA/S。鉴定为阳性的菌液进行测序验证。测序正确的菌液提取质粒后转化农杆菌感受态EH105待用。载体结构和缺失体长度如图 1所示。
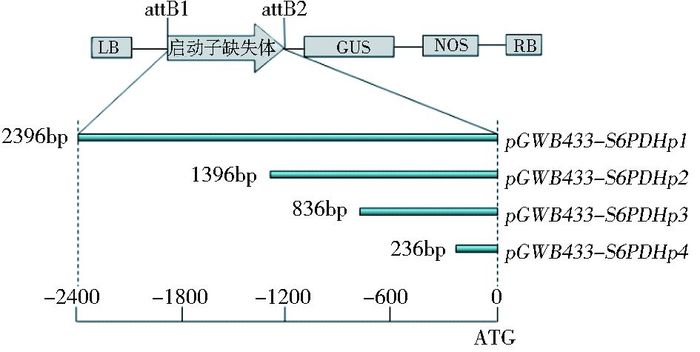
|
| 图 1 S6PDHp系列缺失体GUS融合表达载体构建示意图 Figure 1 Schematic diagram of vector constructions for S6PDHp deletions |
首先用70%的酒精(1min)和NaClO溶液(10%的NaClO水溶液,10min)依次浸泡拟南芥种子;再用无菌水冲洗至少3次;然后把种子均匀悬浮于0.05%的琼脂糖溶液中,继之倾倒于不含抗生素的固体B5培养基上,风干后封口。
将以上培养皿在4℃冰箱中放置4~7天,然后把培养皿放到光照培养箱中进行培养(16h光照25℃,8h黑暗20℃)。待拟南芥长出4~6片真叶时进行移苗土培。移苗培养后3~4周后即可形成大量花簇,可用于下一步转基因试验。移苗土培期间约10d施用一次Hogland培养液。
1.4 拟南芥转基因及转基因植株筛选阳性农杆菌培养至OD600值为1.5~2.0,离心后用适量5%蔗糖溶液重悬至OD600值为0.4~0.7即可,再加入0.02%的Sweet试剂。立即浸染已经提前一天浇好水的拟南芥花絮10s。黑暗培养24h后转至正常条件培养至结果,记为T0代。
将收获的T0代种子进行无菌消毒后,在含有50mg/L的卡那霉素的MS培养基上进行筛选。培养两周后获得T1代拟南芥抗性幼苗,对T1代拟南芥的叶片提取总DNA后进行PCR检测(引物为attB),检测出现目的条带的拟南芥视为转基因成功。以上植株经过连续3代筛选得到T3代纯合体,收取种子后进行后续试验。
1.5 转基因植株的低温和ABA处理将纯化的各缺失体转基因拟南芥种子培养至6~8片真叶后,分别进行4℃低温和ABA(100μmol/L)处理,4℃低温进行12h,ABA处理进行24h。样品采后立即投入液氮并保存于-80℃冰箱备用。
1.6 转基因植株GUS酶活性分析GUS活性测定参照Jefferson等[16]的方法。
2 结果与分析 2.1 基于Gateway技术构建S6PDHp 5′端系列缺失体和GUS融合表达载体首先根据Gateway技术原理,利用Premier(5.0) 软件设计系列引物(表 1),通过3轮PCR扩增,获得带有attB全位点的5′端系列缺失体片段[attB-S6PDHp N(N=1、2、3、4)]。然后将以上缺失体片段回收纯化后和pDONR221进行BP反应,反应后attB-S6PDHpN(N=1、2、3、4) 基因编码序列将pDONR221载体上的ccdB位点置换,形成Entry载体。最后将获得的系列Entry载体质粒通过LR反应连接到pGWB433载体(Destination vector)上形成表达载体(Expression clone),依次命名为pGWB433-S6PDHpN(N=1,2,3,4)(图 1)。通过引物attB A/S对以上4个载体进行PCR鉴定(图 2)和测序分析。经鉴定的阳性质粒转化农杆菌感受态EHA105待用。
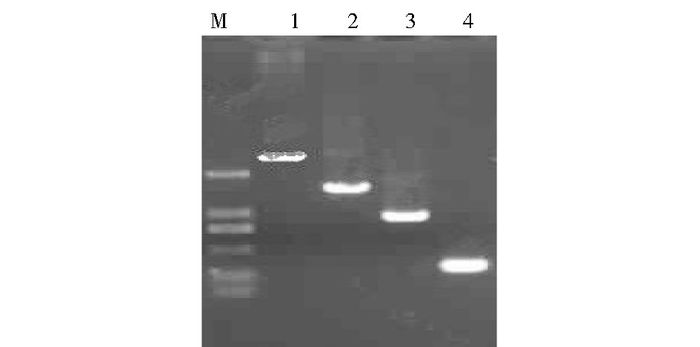
|
| 图 2 S6PDHp系列缺失体和GUS基因融合表达载体PCR检测 Figure 2 PCR identification of S6PDHp∷GUS fusion vectors construction M:DNA Marker DL2000; 1:pGWB433-S6PDHp1; 2:pGWB433-S6PDHp2; 3:pGWB433-S6PDHp3; 4:pGWB433-S6PDHp4 |
农杆菌浸染拟南芥花絮后,获得的T0代种子进行抗性筛选。将经抗性筛选的幼苗移栽并培养至成熟植株后,采集其叶片,提取叶片总DNA,用PCR法对其进行检测。结果表明:除3号缺失体有1株没有检测到目的条带外,其他转基因植株均检测到大小合适的目的条带(图 3)。将检测为阳性的转基因植株继续继代培养,依次获得纯化了的T3代种子,T3代种子经卡那霉素抗性筛选后能全部长出真叶成活。以上结果表明我们已获得了可以用于下一步试验的转基因拟南芥。
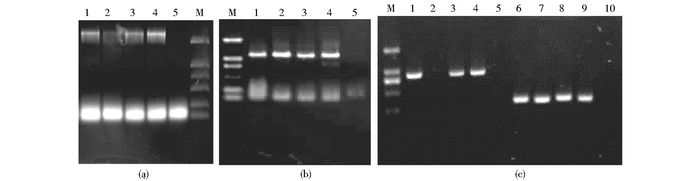
|
| 图 3 S6PDHp系列缺失体转化拟南芥的PCR检测 Figure 3 PCR detection of S6PDHp deletion constructs in transformed Arabidopsis (a) pGWB433-S6PDHp1 M:DNA Marker DL2000, 1~4:Transgenic; 5 Wild type (b) pGWB433-S6PDHp2 M:DNA Marker DL2000, 1~4: Transgenic lines; 5: Wild type (c) pGWB433-S6PDHp3 and pGWB433-S6PDHp4, M:DNA Marker DL2000, 1~4 and 6~9: Transgenic lines, 5, 10: Wild type |
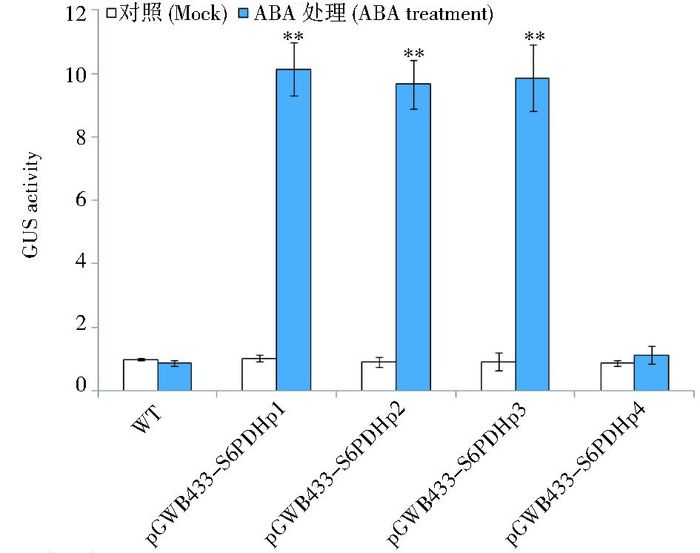
将T3代种子无菌消毒后培养到4~6片真叶时分别进行低温和ABA处理,在分别处理12h和24h后进行GUS酶活检测(图 4和图 5)。结果表明:在低温处理12h后,与常温对照相比,各缺失体转基因株系中GUS活性都有所提高,其中缺失体pGWB433-S6PDHp3转基因植株中的GUS活性增幅最大,达到1.89倍。而其他转基因株系中GUS活性在处理前后变化不显著(图 4),表明启动子序列中-836至-236部分可能具有低温响应的顺式作用原件。与无菌水处理的对照相比,经过24h的ABA处理后,除pGWB433-S6PDHp4外,其余启动子缺失体转基因拟南芥中GUS活性显著升高,分别提高了9.84倍、10.73倍和10.71倍(图 5),表明以上缺失体能被ABA诱导。
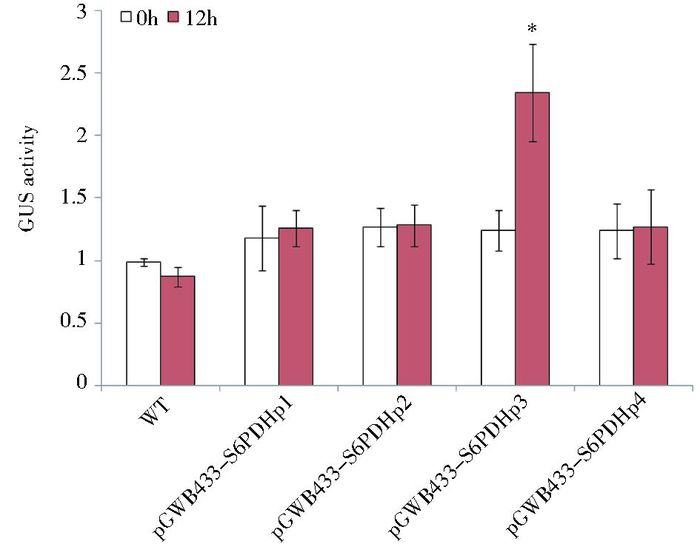
|
| 图 4 低温处理下S6PDHp缺失体转基因拟南芥中GUS活性分析 Figure 4 Analysis of GUS activity against low temperature stress in transformed Arabidopsis by S6PDHp deletions Mean activity was averaged from 3 independent experiments; SD is indicated on each bar; Significant differences between treated (12h) and untreated (0h) were assessed by one-sided paired t-tests (* P < 0.05); WT: Wild type |
|
| 图 5 ABA处理下S6PDHp缺失体转基因拟南芥中GUS活性分析 Figure 5 Analysis of GUS activity for ABA treatment in transformed Arabidopsis by S6PDHp deletions Mean activity was averaged from 3 independent experiments; SD is indicated on each bar. Significant differences between treated and untreated (Mock) were assessed by one-sided paired t-tests (** P < 0.01); WT: Wild type |
低温是一种植物经常遇到的环境胁迫,ABA是公认的植物逆境响应信号,为了探索苹果6-磷酸山梨醇脱氢酶基因启动子是否具有对低温和ABA诱导响应的活性及关键启动子区段,我们在分析了该基因启动子序列的基础上[15],通过Gataway技术构建了含有该基因启动子序列5′端系列缺失体与GUS报告基因融合的表达载体,应用农杆菌介导转化了拟南芥。获得转基因拟南芥后研究了该启动子对信号物质ABA与低温处理的响应。
低温处理的结果表明,pGWB433-S6PDHp3转基因植株中的GUS活性增幅最大,达到显著水平,而其他转基因植株中的GUS活性基本保持不变。这表明在该启动子序列-2396~-836bp区域可能存在低温响应的负调控元件,而-836~-236bp可能存在正调控元件,但目前在该启动子序列上未发现相应的顺式作用元件,以上结果还需要更多的研究来证明。另外,我们注意到,在S6PDHp序列的-1152~-1 147bp存在一个LTR元件(5′-TTTCGG-3′) [15],该元件被认为与植物低温胁迫响应有关[17],但其可能的作用方向与S6PDHp的启动方向相反。由此可推断,LTR的低温胁迫响应功能可能与所在启动子序列的方向有关,LTR在S6PDHp中并未发挥应有的作用。
ABA处理的结果表明,除pGWB433-S6PDHp4外,其余启动子缺失体转基因拟南芥中GUS活性显著升高,这一结果表明,S6PDHp在-2 396~-236bp区域存在很强的正调控元件。前期关于启动子序列分析的结果也证明了这一点,在该启动子序列的-523~-518bp处存在一个ABRE元件[15]。很多研究表明ABRE元件与植物响应ABA有关[18]。一旦缺失了这个元件,植物对ABA的响应能力可能会大幅降低[19]。
综上所述,本研究在前期研究的基础上,进一步证明了低温和外源ABA能够诱导6-磷酸山梨醇脱氢酶基因启动子的表达,不同缺失体的研究结果也初步发现了与逆境胁迫诱导相关的顺式作用元件存在的区段,研究结果对逆境胁迫诱导型启动子的研究和植物抗逆遗传转化具有较好的理论和实践意义。与逆境胁迫诱导相关的新的顺式作用元件的发现和精细定位有待于进一步研究。
| [1] | Loescher W H. Physiology and metabolism of sugar alcohols in higher plant. Physiology Plantarum, 1987, 70(3) : 553–557. DOI:10.1111/ppl.1987.70.issue-3 |
| [2] | Zhou R, Cheng L L, Wayne R. Purification and characterization of sorbitol-6-phosphate phosphatase from apple leaves. Plant Science, 2003, 165(1) : 227–232. DOI:10.1016/S0168-9452(03)00166-3 |
| [3] | Lo Biancoa R, Riegera M, Sung S S. Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiology Plantarum, 2000, 108(1) : 71–78. DOI:10.1034/j.1399-3054.2000.108001071.x |
| [4] | Cui S M, Sadayoshi K, Ogawa Y, et al. Effects of water stress on sorbitol content in leaves and roots, anatomical changes in cell nuclei, and starch accumulation in leaves of young peach trees. Journal of Japanese Society Horticulture Science, 2004, 73(1) : 25–30. DOI:10.2503/jjshs.73.25 |
| [5] | Suleman P, Steiner P W. Relationship between sorbitol and solute potential in apple shoots relative to fire blight symptom development after infection by Erwinia amylovora. Phytopathology, 1994, 84(10) : 1244–1250. DOI:10.1094/Phyto-84-1244 |
| [6] | Kanayama Y, Watanabe M, Moriguchi R, et al. Effects of low temperature and abscisic acid on the expression of the sorbitol-6-phosphate dehydrogenase gene in apple leaves. Journal of Japanese Society Horticulture Science, 2006, 75(1) : 20–25. DOI:10.2503/jjshs.75.20 |
| [7] | Tao G, Suzuki Y, Uratsu S L, et al. Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(49) : 18842–18847. DOI:10.1073/pnas.0605873103 |
| [8] | Cheng L L, Zhou R, Reidel E J, et al. Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta, 2005, 220(5) : 767–776. DOI:10.1007/s00425-004-1384-5 |
| [9] | Kanayama Y, Mori H, Imaseki H, et al. Nucleotide sequence of a cDNA encoding sorbitol-6-phosphate dehydrogenase from apple. Plant Physiology, 1992, 100(3) : 1607–1608. DOI:10.1104/pp.100.3.1607 |
| [10] |
梁东, 马锋旺, 张军科, 等.
苹果6-磷酸山梨醇脱氢酶基因cDNA克隆及其植物表达载体构建. 农业生物技术学报, 2006, 14(4) : 635–636.
Liang D, Ma F W, Zhang J K, et al. Cloning of sorbitol-6-phosphate dehydrogenase S6PDH cDNA from apple and construction of its plant expression vector. Journal of Agricultural Biotechnology, 2006, 14(4) : 635–636. |
| [11] | Meng Y L, Xu X F, Khanizadeh S, et al. The contribution of abscisic acid to sorbitol accumulation in drought-stressed Malus hupehensis. Journal of Food, Agriculture & Environment, 2008, 6(2) : 319–326. |
| [12] | Qi X T, Zhang, Y X, Chai T Y. Characterization of a novel plant promoter specifically induced by heavy metal and identification of the promoter regions conferring heavy metal responsiveness. Plant Physiology, 2007, 143(1) : 50–59. |
| [13] | Xu W R, Yu Y H, Ding J H, et al. Characterization of a novel stilbene synthase promoter involved in pathogen-and stress-inducible expression from Chinese wild Vitis pseudoreticulata. Planta, 2010, 231 : 475–487. DOI:10.1007/s00425-009-1062-8 |
| [14] | Li J, Li M J, Liang D, et al. Expression patterns and promoter characteristics of the gene encoding Actinidia deliciosa L-galactose-1-phosphate phosphatase involved in the response to light and abiotic stresses. Molecular Biology Reporter, 2013, 40(2) : 1473–1485. DOI:10.1007/s11033-012-2190-y |
| [15] | Liang D, Cui M, Wu S, et al. Genomic structure, sub-cellular localization, and promoter analysis of the gene encoding sorbitol-6-phosphate dehydrogenase from apple. Plant Molecular Biology Reporter, 2012, 30(4) : 904–914. DOI:10.1007/s11105-011-0409-z |
| [16] | Jefferson R A, Kavanagh T A, Bevan M W. GUS fusions:beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. European Molecular Biology Organization Jouranl, 1987, 6(13) : 3901–3907. |
| [17] | White A J, Dunn M A, Brown K, et al. Comparative analysis of genomic sequence and expression of a lipid transfer protein gene family in winter barley. Journal of Experimental Botany, 1994, 45(12) : 1885–1892. DOI:10.1093/jxb/45.12.1885 |
| [18] | Yamaguchi S K, Shinozaki K. Arabidopsis DNA encoding two desiccation~responsive rd29 genes. Plant Physiology, 1993, 101(3) : 1119–1120. DOI:10.1104/pp.101.3.1119 |
| [19] | Li J, Liang D, Li M J, et al. Light and abiotic stresses regulate the expression of GDP-l galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light responsive and stress inducible cis-elements in their promoters. Planta, 2013, 238(3) : 535–547. DOI:10.1007/s00425-013-1915-z |
 2017, Vol. 37
2017, Vol. 37





