文章信息
- 孙元元, 李薇, 叶守东, 刘大海.
- SUN Yuan-yuan, LI Wei, YE Shou-dong, LIU Da-hai.
- Gadd45g诱导小鼠胚胎干细胞向中内胚层分化
- Gadd45g Induces Mesendodermal Differentiation in Mouse Embryonic Stem Cells
- 中国生物工程杂志, 2017, 37(4): 9-17
- China Biotechnology, 2017, 37(4): 9-17
- http://dx.doi.org/DOI:10.13523/j.cb.20170402
-
文章历史
- 收稿日期: 2016-09-22
- 修回日期: 2017-01-02
胚胎干细胞 (embryonic stem cells, ESCs) 分离自着床前的囊胚内细胞团细胞,在体外能够无限地进行自我复制式地增殖 (自我更新),同时还保持着分化为三个胚层来源的各种类型细胞的潜能 (分化),现已成为研究基因功能、筛选药物和制造疾病动物模型的强有力的工具,具有重要的社会和经济价值[1-2]。第一株胚胎干细胞于1981年分离自小鼠 (mouse ESCs, mESCs)[1-2],此后mESCs广泛应用于各类研究中,成为重要的干细胞研究材料。目前人们对于胚胎干细胞如何维持自我更新状态已做了多方面研究,并探索出一些体外维持其处于未分化状态的体系,如含LIF的有血清培养体系[3],以及含GSK3抑制剂CHIR99021和MEK抑制剂PD0325901的无血清培养体系 (即2i)[4]。但目前人们对于mESCs如何退出自我更新状态、向特定发育阶段分化了解得还不够清楚。
生长阻滞和DNA损伤诱导蛋白45γ(Growth arrest and DNA-damage-inducible protein GADD45 gamma,Gadd45g) 是GADD45蛋白家族三成员之一,也叫做细胞因子应答基因6(CR6)[5-7],可影响细胞周期,负调控细胞生长[6, 8]。此前,关于Gadd45g的研究主要集中在肿瘤以及神经前体发育[8-10]等方面,发现Gadd45g可以抑制肿瘤细胞生长,促进其凋亡[8, 10],这种抑制作用部分通过影响LIF/JAK/STAT3信号通路来实现[8],而LIF/JAK/STAT3也是维持ESCs自我更新状态的重要信号通路之一[3, 11];此外,对于非洲爪蟾胚胎发育的研究发现,Gadd45g密切参与囊胚期细胞退出多能性状态、向终末细胞分化的过程[12],但目前关于Gadd45g在ESCs中的作用尚无相关报道。本研究通过在mESCs中过表达Gadd45g,发现其可以抑制细胞的增殖速率,促进mESCs向中内胚层分化,且这种促进作用可能是通过抑制STAT3的磷酸化而实现的。研究结果将扩大人们对ESCs内部调控机制的认识,有利于未来ESCs的基础研究和安全应用。
1 材料与方法 1.1 材料46C小鼠胚胎干细胞系,由剑桥大学Welcome Trust Center的Austin Smith教授馈赠。KOD Hot Start DNA Polymerase PCR试剂盒 (EMD Millipore公司);BglⅡ、XhoⅠ和T4连接酶 (TaKaRa公司);琼脂糖凝胶DNA回收试剂盒、质粒抽提试剂盒 (TIANGEN公司);LTX脂质体 (Invitrogen公司);碱性磷酸酶检测试剂盒 (Sigma公司);Puromycin (Invitrogen公司);α-tubulin (1:200)、Gadd45g (1:200)、Oct4(1:50)、Nanog (1:100)、FLAG (1:1 000)、STAT3(1:1 000) 和Phospho-STAT3(1:2 000) 等抗体购自Santa Cruz、ReproCELL、Sigma和CST等公司。
1.2 mESCs的培养与传代mESCs的有血清培养体系为:DMEM (Gibico)、10%胎牛血清 (Hyclone);1×MEM (Invitrogen)、2 mmol/L L-glutamine (Invitrogen)、0.01 mmol/L β-巯基乙醇 (Sigma) 以及1000 U/ml LIF (Millipore)。mESCs的无血清培养体系为:1×N2, 1×B27(Invitrogen)、DMEM/F12(Invitrogen)、Neurobasal® Medium (Invitrogen)、2mmol/L L-glutamine (Invitrogen)、0.01mmol/L β-巯基乙醇 (Sigma) 以及3μmol/L CHIR99021(Sigma)、1 μmol/L PD0325901(Sigma)。在接种细胞前,培养皿用0.1%的Gelatin (Sigma) 进行包被。当细胞的覆盖密度达到70%~80%时,用胰酶 (Invitrogen) 消化成单个细胞进行传代培养。
1.3 Gadd45g重组质粒的构建、转染和筛选Gadd45g蛋白编码区的克隆引物为Gadd45g-Forward:5′-ATGACTCTGGAAGAAGTCCGTGGC-3′;Gadd45g-Reverse:5′-TCACTCGGGAAGGGTGATGCT-3′。在上下游引物两端分别添加BglⅡ和XhoⅠ限制性内切酶位点,并连入携带Flag标签蛋白的PiggyBac (PB) 系统。Flag标签蛋白为编码8个氨基酸 (DYKDDDDK) 的亲水性多肽,可与目的蛋白Gadd45g融合表达,为后续验证提供方便,通过检测Flag蛋白是否表达即可判断Gadd45g存在与否,而对照组中单独存在的Flag则因分子量过小而不会被检测到。利用LTX脂质体将重组质粒转染进mESCs,两天后加入终浓度为2 μg/ml的Puromycin药物进行筛选。
1.4 细胞计数事先在培养皿中接种一定数量的细胞,在计划的天数利用胰酶进行单细胞消化,离心重悬后,吸取一定量的悬液至血细胞计数板,在显微镜下进行计数 (对于压线细胞,采取计上不计下,计左不计右的原则进行计数)、记录和统计。细胞数目 (个/ ml)=(四角大方格细胞总数/4)×104。
1.5 Western blot检测收集各组细胞,利用添加了蛋白酶抑制剂的RIPA裂解液裂解细胞,利用BCA法测定总蛋白浓度。用4%~12%的SDS-PAGE梯度胶分离蛋白组分。利用湿转系统将蛋白转移至PVDF膜上,经过封闭、一抗孵育、PBST洗脱、HRP标记的二抗 (1:2 000) 孵育、PBST再洗脱等步骤后,ECL化学发光及X光片曝光,经显影和定影处理,最终获得清晰条带。
1.6 荧光实时定量PCR (qRT-PCR)用Trizol法抽提细胞总RNA, 参照TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (TransGen) 试剂盒说明书进行第一链cDNA合成。荧光定量PCR参照TransStart Tip Green qPCR SuperMix (TransGen) 试剂盒执行。GAPDH基因表达水平作为内参。引物序列见表 1。
| Gene | Forward sequence (5′→3′) | Reverse sequence (5′→3′) |
| GAPDH | TGTGAGGGAGATGCTCAGTG | TGTTCCTACCCCCAATGTGTH |
| Gadd45g | GAAGATGAGGGCGACATAGC | GCTCTCCTCGCAGAACAAAC |
| Oct4 | ATCACTCACATCGCCAATCA | TGGGAAAGGTGTCCCTGTAG |
| Nanog | CCAGTGGAGTATCCCAGCAT | GAAGTTATGGAGCGGAGCAG |
| Klf2 | CCAAGAGCTCGCACCTAAAG | GTGGCACTGAAAGGGTCTGT |
| Sox17 | CTCGGGGATGTAAAGGTGAA | GCTTCTCTGCCAAGGTCAAC |
| Gata6 | TCCTCCCCTGCCGAAGTC | AGGGCCAGAGCACACCAA |
| Foxa2 | CCTCAAGGGAGCAGTCTCAC | TTTCTCCTGGTCCGGTACAC |
| T | CCGGTGCTGAAGGTAAATGT | CCTCCATTGAGCTTGTTGGT |
| GSC | CTCGGAGGAGTCAGAAAACG | TCGACTGTCTGTGCAAGTCC |
| Mixl1 | TTGAATTGAACCCTGTTGTCCC | GAAACCCGTTCTCCCATCCACC |
| Nestin | CCAGAGCTGGACTGGAACTC | ACCTGCCTCTTTTGGTTCCT |
| Sox1 | CACAACTCGGAGATCAGCAA | GTCCTTCTTGAGCAGCGTCT |
| Note: GAPDH is the control gene. Gadd45g is the target gene. Oct4、Nanog and Klf2 are self-renewal markers. Sox17, Gata6 and Foxa2 are endoderm markers. T is a mesendoderm marker. GSC and Mixl1 are mesoderm markers. Nestin and Sox1 are ectoderm markers | ||
1.7 碱性磷酸酶染色
弃掉对照组和实验组细胞培养液,加入4%多聚甲醛室温固定2 min。参照说明书将碱性磷酸酶检测试剂盒中的两种染液按照48:2的比例混合。弃掉多聚甲醛,加入混合染液,室温避光30 min。PBS漂洗后在显微镜下观察。
1.8 免疫荧光弃掉培养液,4%多聚甲醛室温固定20min。PBS漂洗后加入封闭液 (含5% BSA,0.2% Triton X-100的PBS),37℃温箱孵育1h。加入含有一抗的稀释液,4℃过夜。次日PBS漂洗3次。添加携带荧光基团的二抗 (1:500,碧云天) 以及Hoechst (1:5 000, Invitrogen) 染料的稀释液,37℃避光孵育1h。PBS漂洗后在荧光显微镜下观察拍照。
1.9 统计学处理实验数据用mean±SD表示,采用SPSS 15.0统计分析软件进行统计检验,组间比较用t检验,P<0.05时认为组间差异显著,P<0.01时认为组间差异极显著,结果具有统计学意义。
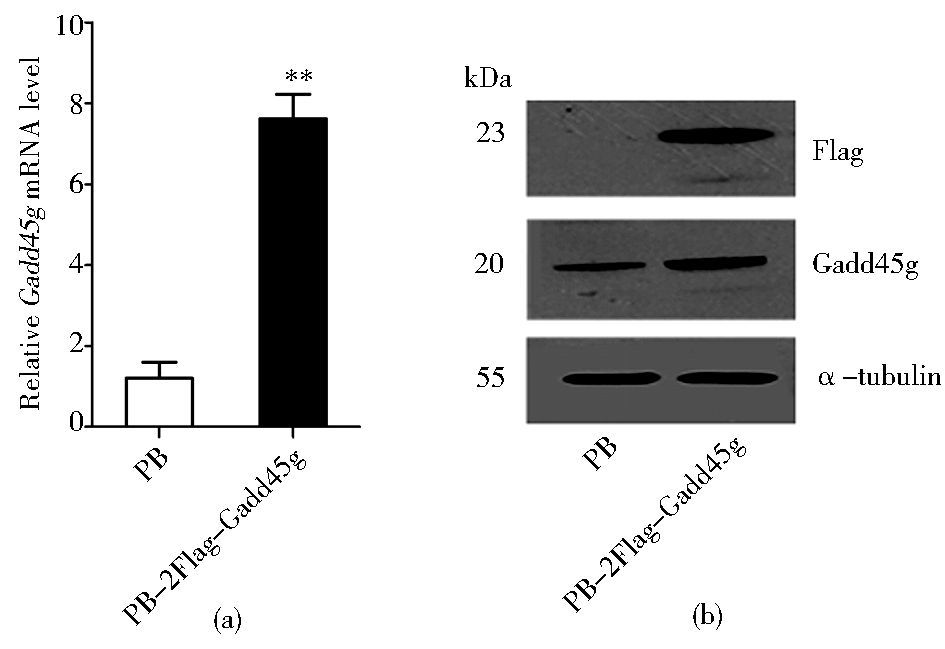
2 结果 2.1 Gadd45g稳定表达细胞株的建立借助LTX,将空白对照质粒PB,以及携带两个Flag标签蛋白的Gadd45g重组质粒 (PB-2Flag-Gadd45g) 分别转染进46C mESCs中。经Puromycin药物筛选一周后,qRT-PCR和Western blot检测结果显示:PB-2Flag-Gadd45g实验组中可检测到Flag蛋白的表达,Gadd45g在转录和蛋白水平的表达量显著增高 (图 1a, b),说明过表达Gadd45g的mESCs系构建成功。
|
| 图 1 Gadd45g在mESCs中过表达的检测 Figure 1 Analysis of Gadd45g overexpression in mESCs (a) qRT-PCR analysis of Gadd45g mRNA level in 46C mESCs transfected with PB or PB-2Flag-Gadd45g, respectively Data represent mean ± SD of three biological replicates. ** P < 0.01vsPB (b) Western blot analysis of 46C mESCs overexpressing FLAG-tagged GADD45G α-tubulin is used as a loading control |
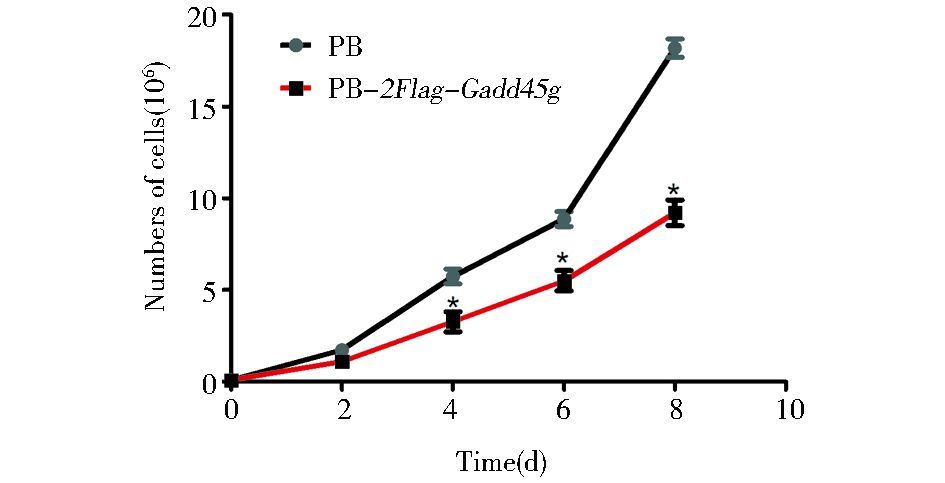
血清和LIF培养体系下,在不同的细胞培养皿中接种起始数目相同的细胞,每2天一次对细胞进行计数,以观察细胞的生长情况。结果显示:PB-2Flag-Gadd45g mESCs的生长速度较PB mESCs缓慢 (图 2),说明Gadd45g减缓了胚胎干细胞的增殖速率,对胚胎干细胞的生长具有抑制作用。
|
| 图 2 Gadd45g对mESCs生长速率的影响 Figure 2 Effects of Gadd45g on the growth rate of mESCs PB mESCs or PB-2Flag-Gadd45g mESCs were counted in the indicated days. Results are shown as mean ± SD of six biological replicates.* P < 0.05 vs PB |
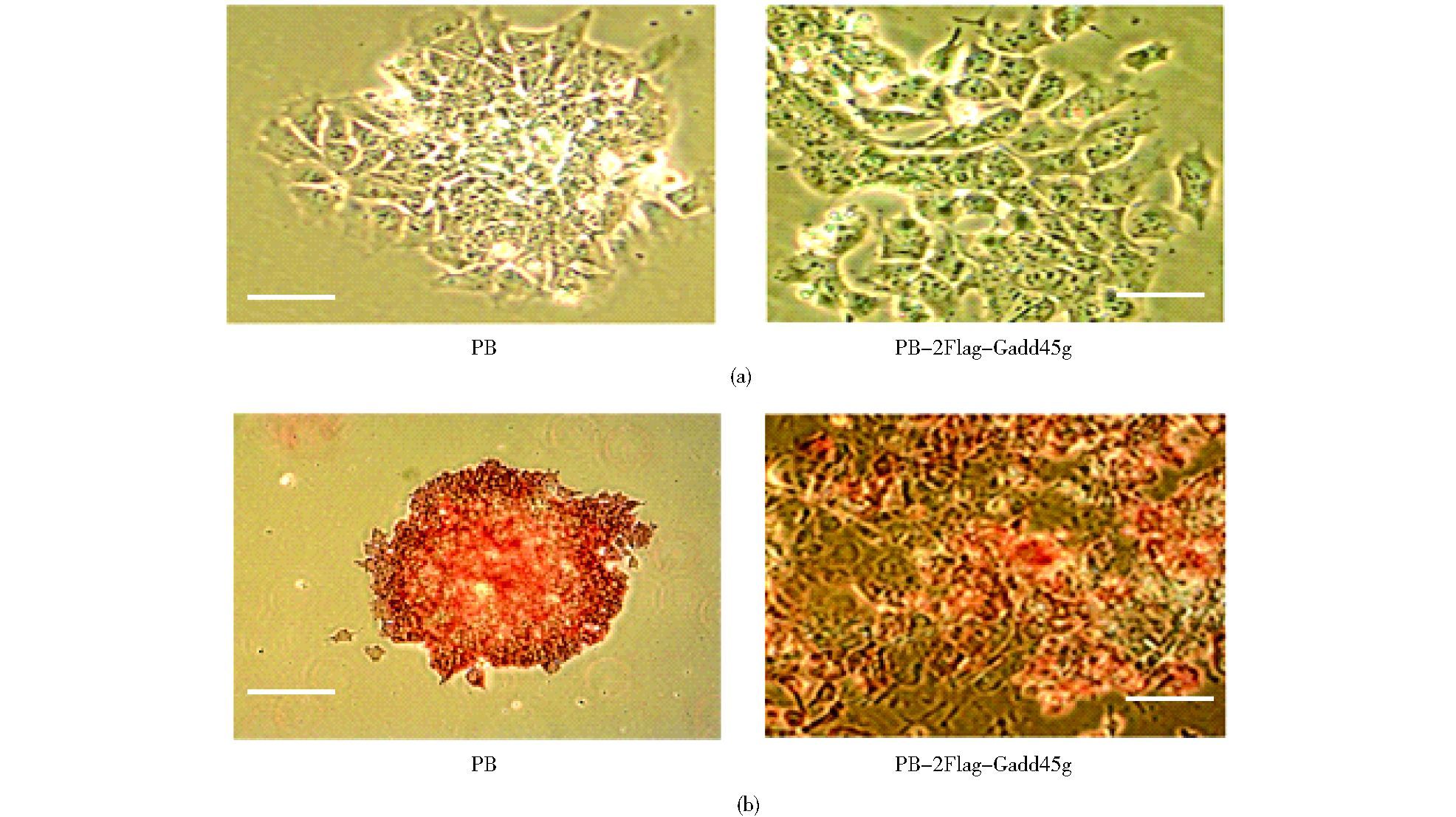
PB-2Flag-Gadd45g mESCs经过两次传代之后,在血清和LIF培养体系下,细胞边缘形态变成纤维状。为了验证这些细胞的自我更新状态,我们进行了碱性磷酸酶染色实验,因为未分化的mESCs具有很高的碱性磷酸酶活性,该特性可作为细胞是否处于自我更新状态的判断依据。结果显示:PB对照组细胞形成的克隆排列密集且边缘较为清晰,具有很高的碱性磷酸酶活性,而PB-2Flag-Gadd45g 细胞染色较浅或丢失 (图 3),因此上调Gadd45g会降低mESCs的碱性磷酸酶活性水平。
|
| 图 3 Gadd45g对mESCs碱性磷酸酶活性的影响 Figure 3 Effects of Gadd45g on mESC alkaline phosphatase activity (a) Representative phase-contrast images of PB and PB-2Flag-Gadd45g mESCs maintained in LIF/serum condition Scale bar, 100 μm (b) Alkaline phosphatase staining of PB and PB-2Flag-Gadd45g mESCs maintained in LIF/serum condition Scale bar, 200 μm (left pane), 100 μm (right panel) |
mESCs在自我更新状态下高表达Oct4、Nanog、Klf2等基因。利用qRT-PCR和免疫荧光技术检测过表达Gadd45g后上述标志基因在细胞中的表达情况。结果显示:与PB对照组细胞相比,PB-2Flag-Gadd45g细胞中Oct4、Nanog、Klf2在转录水平上的相对表达量较低 (图 4a);而在蛋白水平上,过表达Gadd45g的细胞中OCT4和NANOG的表达量亦有所降低 (图 4b),因此上调Gadd45g会抑制mESCs自我更新标志基因的表达。

|
| 图 4 Gadd45g对mESCs自我更新标志基因表达的影响 Figure 4 Effects of Gadd45g on the expression of mESC self-renewal marker genes PB and PB-2Flag-Gadd45g 46C mESCs were cultured in LIF/serum condition for 1 week and were subsequently detected with indicate genes. (a) qRT-PCR analysis of Oct4, Nanog and Klf2 expression in PB and PB-2Flag-Gadd45g mESCs Data represent mean ± SD of triplicate samples from three independent experiments. * P < 0.05 vs PB (b) Immunofluorescence showing stronger OCT4 and NANOG staining in PB mESCs, compared to PB-2Flag-Gadd45g mESCs Hoechst is a nuclear stain. Scale bar, 100 μm |
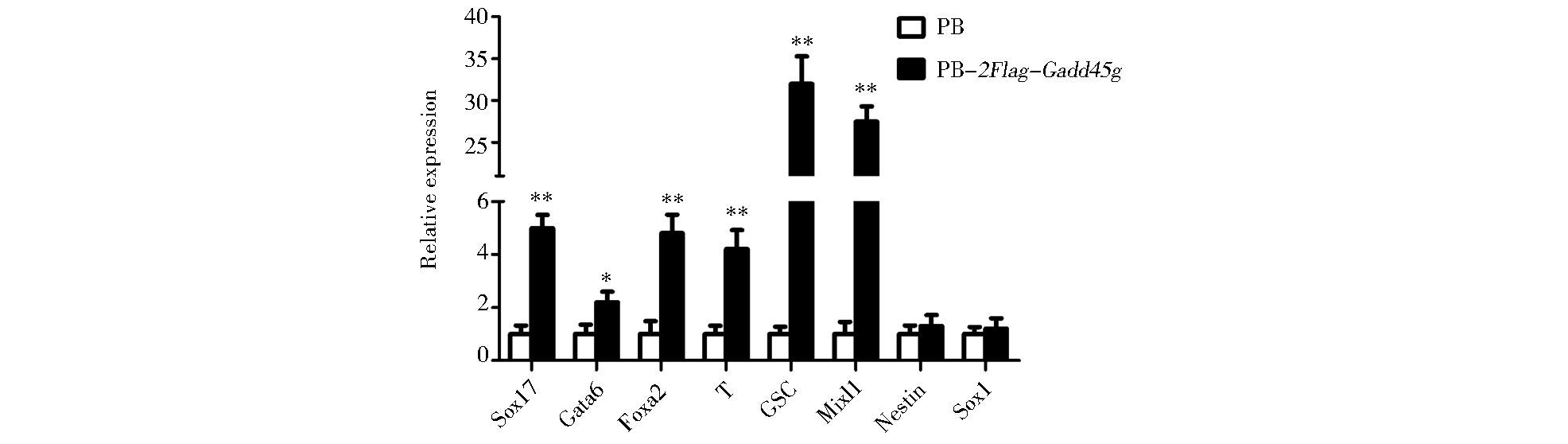
在PB-2Flag-Gadd45g细胞的碱性磷酸酶活性和自我更新标志基因表达都降低的情况下,我们继续应用qRT-PCR来检测细胞中分化基因的表达情况。结果显示:与PB对照组细胞相比,内胚层和中胚层的标志基因在PB-2Flag-Gadd45g细胞中都明显上调,而外胚层标志基因的表达未出现变化 (图 5),说明在自我更新的维持条件下,过表达Gadd45g会促进mESCs向中内胚层分化。
|
| 图 5 Gadd45g对分化基因表达的影响 Figure 5 Effects of Gadd45g on the expression of differentiation genes qRT-PCR analysis of differentiation genes in PB and PB-2Flag-Gadd45g mESCs maintained in LIF/serum. Data represent mean ± SD of triplicate samples from three independent experiments. Sox17, Gata6 and Foxa2 are endoderm markers. T is a mesendoderm marker. GSC and Mixl1 are mesoderm markers. Nestin and Sox1 are ectoderm markers. * P < 0.05 vs PB; ** P < 0.01 vs PB |
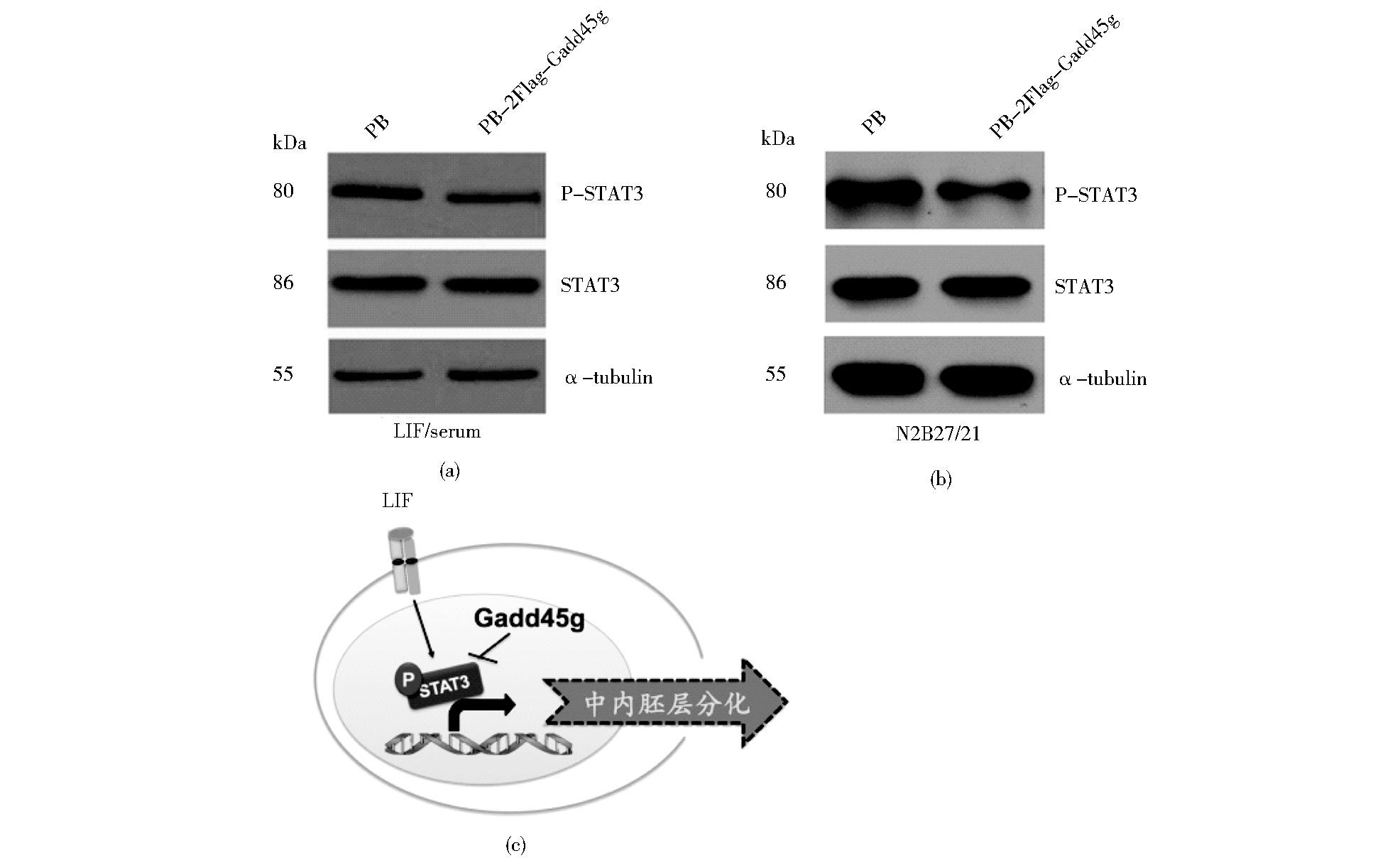
由以上结果可知,在LIF和血清的培养条件下,过表达Gadd45g会诱导mESCs向中内胚层分化,而LIF主要通过激活STAT3来促进mESCs的自我更新,因此我们尝试去检测STAT3的活性,即STAT3第705位酪氨酸磷酸化水平是否出现变化。Western blot结果显示:上调Gadd45g不会改变STAT3总的蛋白表达水平,但是STAT3的磷酸化水平对照组细胞出现了下降 (图 6a),说明Gadd45g通过抑制STAT3的磷酸化,使LIF/STAT3信号通路的激活受阻。为排除由于细胞状态变化而导致STAT3磷酸化水平改变的可能性,我们在N2B27/2i条件下进行了验证,结果显示:在2i的培养条件下,上调Gadd45g依然抑制STAT3的磷酸化水平 (图 6b)。而LIF/STAT3可以通过抑制中内胚层的形成来维持mESCs的未分化状态[12],所以Gadd45g可能通过部分抑制STAT3磷酸化来诱导mESCs退出未分化状态,向中内胚层方向分化。
|
| 图 6 Gadd45g对mESCs中STAT3磷酸化水平的影响 Figure 6 Effects of Gadd45g on STAT3 phosphorylation level in mESCs Western blot analysis of phosphorylated STAT3 and total STAT3 levels in PB and PB-2Flag-Gadd45g 46C mESCs. (a) Western blot analysis of the indicated proteins in mESCs cultured in LIF/serum conditions (b) Western blot analysis of the indicated proteins in mESCs cultured in N2B27/2i (3μmol/L CHIR99021 + 1 μmol/L PD0325901) condition (c) Model of mESC differentiation induced by Gadd45g Overexpression of Gadd45g in mESCs may induce mesendodermal formation partially via inhibiting STAT3 phosphrylation level |
胚胎干细胞能够在体外无限地进行自我更新式的增殖,同时保持着分化成不同类型细胞的潜能,因而在临床医学上具有远大的应用前景。而了解其内部的调控网络与机制是将来安全应用ESCs的基础,现已成为再生医学领域的研究热点。目前已鉴定出一些可以调控mESCs自我更新和分化的转录因子,如Tfcp2l1, Esrrb, Nanog和Gbx2等,这些转录因子可以替代一些外源性的细胞因子来促进mESCs的自我更新[13-15],而Axin2, Otx2和Tfe3等则会诱导mESCs退出自我更新程序[16-18]。本研究结果首次表明Gadd45g可以促进mESCs向中内胚层分化,这种促进作用可能是通过抑制STAT3磷酸化来实现的。
Gadd45蛋白家族参与细胞周期调控,抑制细胞的生长与增殖[5-6],这已被广泛认知,例如,当细胞受到刺激损伤后,p53基因产物被磷酸化,通过激活p21和Gadd45,诱导细胞生长停留[19-21]。在本研究中,Gadd45g对于mESCs同样具有抑制增殖的作用,其内部机制的阐明有待后续深入探究。在发育与分化方面,Gadd45g高表达于脊椎动物的神经前体细胞,通过调控细胞周期蛋白活性,如CDK1和Cyclin B1等,促进神经元的分化和神经系统的发生[22-23];此外,Gadd45g影响小鼠性别的决定和发育,但缺失Gadd45g基因的小鼠不会胚胎致死,说明其对于多能性干细胞的维持来说不是必需的[24],而且有研究表明Gadd45g在mESCs内的表达被多能性基因Oct4抑制,其表达会随着mESCs的分化而升高[25],这与我们的研究结果相一致,说明Gadd45g具有促进mESCs分化的作用。在肿瘤细胞中,Gadd45g会负调控LIF/JAK/STAT3信号通路来抑制肿瘤细胞的生长。而LIF/JAK/STAT3也是维持ESCs未分化状态的重要信号通路之一。LIF与受体结合后招募膜蛋白gp130形成异聚体,随即激活gp130蛋白上的JAK,激活的JAK将磷酸化STAT3第705位酪氨酸,随后磷酸化的STAT3发生二聚化,进入核内激活相应靶基因的表达,从而促进mESCs维持自我更新状态[11]。在mESCs中,LIF通过激活STAT3抑制中内胚层的分化,而血清中的成分,如BMP4,则会阻断外胚层的形成,因而将mESCs维持在未分化状态[11]。本研究中,我们发现在LIF和血清的培养体系下,上调Gadd45g的表达会上调中内胚层标志基因的表达,并且会抑制STAT3的磷酸化,但外胚层标志基因的表达水平不发生改变;为排除细胞状态变化的干扰,我们在2i无血清培养体系中也重复了相关实验,发现Gadd45g同样会抑制STAT3的磷酸化。因此,Gadd45g诱导mESCs向中内胚层分化部分是通过抑制STAT3磷酸化而实现的 (图 6c)。
综上所述,本研究首次探讨了Gadd45g在ESCs中的作用及部分机制,对于更加深入和全面地了解ESCs内部信号调控网络具有重要意义。但目前仍存在一些问题有待进一步探索:Gadd45g以何种方式抑制STAT3磷酸化?该过程是否直接抑制自我更新基因或诱导分化基因表达?下调Gadd45g的表达将对mESCs产生怎样的影响?在细胞周期调控方面,Gadd45g对ESCs的生长抑制作用又与哪些基因或蛋白相关?这一系列问题的答案将在后续实验中逐步阐明,而对于整个干细胞调控网络和细胞命运决定机制的不断揭示与丰富,则能为今后干细胞的安全应用提供有力保障。
| [1] | Martin G R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc NatlAcad Sci U S A, 1981, 78(12) : 7634–7638. DOI:10.1073/pnas.78.12.7634 |
| [2] | Evans M J, Kaufman M H. Establishment in culture of pluripotential cells from mouse embryos. Nature, 1981, 292(5819) : 154–156. DOI:10.1038/292154a0 |
| [3] | Niwa H, Burdon T, Chambers I, et al. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev, 1998, 12(13) : 2048–2060. DOI:10.1101/gad.12.13.2048 |
| [4] | Ying Q L, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature, 2008, 453(7194) : 519–523. DOI:10.1038/nature06968 |
| [5] | Azam N, Vairapandi M, Zhang W, et al. Interaction of CR6(GADD45gamma) with proliferating cell nuclear antigen impedes negative growth control. J Biol Chem, 2001, 276(4) : 2766–2774. DOI:10.1074/jbc.M005626200 |
| [6] | Zhang L, Yang Z, Liu Y. GADD45 proteins:roles in cellular senescence and tumor development. Exp Biol Med, 2014, 239(7) : 773–778. DOI:10.1177/1535370214531879 |
| [7] | Hoffman B, Liebermann D A. Gadd45 in modulation of solid tumors and leukemia. Adv Exp Med Biol, 2013, 793 : 21–33. DOI:10.1007/978-1-4614-8289-5 |
| [8] | Zhang L, Yang Z, Ma A, et al. Growth arrest and DNA damage 45G down-regulation contributes to Janus kinase/signal transducer and activator of transcription 3 activation and cellular senescence evasion in hepatocellular carcinoma. Hepatology, 2014, 59(1) : 178–189. DOI:10.1002/hep.26628 |
| [9] | Huang H S, Kubish G M, Redmond T M, et al. Direct transcriptional induction of Gadd45gamma by Ascl1 during neuronal differentiation. Mol Cell Neurosci, 2010, 44(3) : 282–296. DOI:10.1016/j.mcn.2010.03.014 |
| [10] | Ju S, Zhu Y, Liu L, et al. Gadd45b and Gadd45g are important for anti-tumor immune responses. Eur J Immunol, 2009, 39(11) : 3010–3018. DOI:10.1002/eji.v39:11 |
| [11] | Ying Q L, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell, 2003, 115(3) : 281–292. DOI:10.1016/S0092-8674(03)00847-X |
| [12] | Kaufmann L T, Niehrs C. Gadd45a and Gadd45g regulate neural development and exit from pluripotency in Xenopus. Mech Dev, 2011, 128(7-10) : 401–411. DOI:10.1016/j.mod.2011.08.002 |
| [13] | Tai C I, Ying Q L. Gbx2, a LIF/Stat3 target, promotes reprogramming to and retention of the pluripotent ground state. J Cell Sci, 2013, 126(Pt5) : 1093–1098. |
| [14] | Ye S, Li P, Tong C, et al. Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J, 2013, 32(19) : 2548–2560. DOI:10.1038/emboj.2013.175 |
| [15] | Martello G, Sugimoto T, Diamanti E, et al. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell, 2012, 11(4) : 491–504. DOI:10.1016/j.stem.2012.06.008 |
| [16] | Kim H, Wu J, Ye S, et al. Modulation of beta-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat Commun, 2013, 4 : 2403. |
| [17] | Acampora D, Di Giovannantonio L G, Simeone A. Otx2 is an intrinsic determinant of the embryonic stem cell state and is required for transition to a stable epiblast stem cell condition. Development, 2013, 140(1) : 43–55. DOI:10.1242/dev.085290 |
| [18] | Betschinger J, Nichols J, Dietmann S, et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell, 2013, 153(2) : 335–347. DOI:10.1016/j.cell.2013.03.012 |
| [19] | Carrier F, Georgel P T, Pourquier P, et al. Gadd45, a p53-responsive stress protein, modifies DNA accessibility on damaged chromatin. Mol Cell Biol, 1999, 19(3) : 1673–1685. DOI:10.1128/MCB.19.3.1673 |
| [20] | Kearsey J M, Coates P J, Prescott A R, et al. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene, 1995, 11(9) : 1675–1683. |
| [21] | Macleod K F, Sherry N, Hannon G, et al. P53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev, 1995, 9(8) : 935–944. DOI:10.1101/gad.9.8.935 |
| [22] | Kaufmann L T, Gierl M S, Niehrs C. Gadd45a, Gadd45b and Gadd45g expression during mouse embryonic development. Gene Expr Patterns, 2011, 11(8) : 465–470. DOI:10.1016/j.gep.2011.07.005 |
| [23] | Vairapandi M, Balliet A G, Hoffman B, et al. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol, 2002, 192(3) : 327–338. DOI:10.1002/(ISSN)1097-4652 |
| [24] | Gierl M S, Gruhn W H, von Seggern A, et al. GADD45G functions in male sex determination by promoting p38 signaling and Sry expression. Dev Cell, 2012, 23(5) : 1032–1042. DOI:10.1016/j.devcel.2012.09.014 |
| [25] | Jung M, Peterson H, Chavez L, et al. A data integration approach to mapping OCT4 gene regulatory networks operative in embryonic stem cells and embryonal carcinoma cells. PLoS One, 2010, 5(5) : e10709. DOI:10.1371/journal.pone.0010709 |
| [26] | Huang G, Ye S, Zhou X, et al. Molecular basis of embryonic stem cell self-renewal:from signaling pathways to pluripotency network. Cell Mol Life Sci, 2015, 72(9) : 1741–1757. DOI:10.1007/s00018-015-1833-2 |
 2017, Vol. 37
2017, Vol. 37




