文章信息
- 曹俊杰, 李爱芳, 卫亚琳, 廉静, 唐敏.
- CAO Jun-jie, LI Ai-fang, WEI Ya-lin, LIAN Jing, TANG Min.
- Notch信号参与BMP4诱导的间充质干细胞成骨分化及其机制的初步探讨
- Role of Notch Signaling Pathway in Bone Morphogenetic Protein 4-induced Osteogenic Differentiation of Marrow-derived Mesenchymal Stem Cells and Its Mechanism
- 中国生物工程杂志, 2017, 37(4): 48-55
- China Biotechnology, 2017, 37(4): 48-55
- http://dx.doi.org/DOI:10.13523/j.cb.20170407
-
文章历史
- 收稿日期: 2016-08-12
- 修回日期: 2016-08-31
骨髓间充质干细胞是一类多能干细胞,在骨髓中含量最丰富。在体内外相应诱导条件下,可分化为软骨、骨肌、肌肉、脂肪等组织[1-2]。而由于其具有来源丰富,易于分离培养,免疫原性低等特点,它成为骨损伤疾病及组织工程中理想的“种子细胞”[3]。小鼠胚胎成纤维细胞 (mouse embryonic fibroblasts, MEFs) 在体内外具备MSCs特征,是研究MSCs的相关实验的重要细胞[4-6]。
BMP4是TGFβ超家族的一员,在体内外具有促进成骨的能力[7]。Notch信号在进化上是一条保守的信号通路,相邻细胞上的Notch配体与受体相互作用传导信号,对细胞的分化,增殖和凋亡发挥重要作用[8]。目前研究发现,Notch信号在MSCs成骨分化过程中发挥着重要作用,已发现其促进BMP2,9诱导的MSCs成骨分化[9-13]。那么Notch对其他成骨BMPs是否有相同的作用,其类似的信号通路呢?研究发现高密度细胞接触激活Notch信号后,其配体DLL1的表达增高,AdDLL1能激活Notch信号[11, 14]。有研究报道DLL1可以与Notch1结合激活Notch信号,而dnNotch1能抑制Notch信号[13, 15]。所以课题研究应用γ-secretase的抑制剂DAPT和dnNotch1下调Notch信号,用AdDLL1上调Notch信号。故本研究拟采用Notch化学抑制剂DAPT、AddnNotch1下调Notch,AdDLL1上调Notch探讨Notch信号对另一重要的成骨BMPs中的BMP4诱导MSCs成骨分化、增殖的影响及可能的机制,以了解Notch在成骨BMPs诱导成骨中作用的广泛性,为其在临床BMPs的应用提供理论依据。
1 材料与方法 1.1 材料 1.1.1 细胞株及腺病毒MEFs细胞、AdRFP,AdGFP,AdDLL1,AddnNotch1,AdBMP4均由芝加哥大学何通川教授惠赠并由本实验室保存,人结肠癌细胞株HCT116,人胚肾293细胞株 (HEK293) 由重庆医科大学分子实验室保存。
1.1.2 试剂DMEM/HIGH GLUCOSE培养基 (Hyclone公司),胎牛血清 (Gibco公司),免疫细胞化学染色试剂盒 (中杉金桥公司),ALP染色试剂 (Sigma公司),RNA提取试剂Trizol (Invitrogen公司),引物 (Invitrogen公司合成),p-Smad1/5/8抗体 (Cell Signaling Technology公司),DAPT_565784(Calbiochem公司),茜素红S、维生素C和β-磷酸甘油 (Sigma公司)。
1.2 方法 1.2.1 细胞培养与处理复苏HCT116,MEFs细胞,培养于含10 %胎牛血清、1 %青霉素/链霉素 (P/S)、DMEM高糖培养基中。37 ℃、5 % CO2条件下培养。待细胞贴壁生长并融合至80 %左右时,用胰蛋白酶消化传代。
1.2.2 条件培养基的制备HCT116细胞培养于直径100 mm的培养皿中,待细胞融合至70 % ~ 80 %时,加入AdGFP或AdBMP4,控制感染率为80 %,在4 h后换成无血清无抗生素的培养基,分别在24 h和48 h后收集培养基,离心取上清4 ℃保存备用,上清内含有一定浓度的BMP4蛋白,AdGFP的条件培养基作为空白对照。
1.2.3 ALP活性测定与染色将MEFs细胞以2×104/孔接种至24孔板,细胞融合至30 %左右时,加入AdRFP,AdDLL1或AddnNotch1后换液,待红色荧光出现后 (约36 h),加入适当量的BMP4-CM,培养5~7天后进行ALP活性定量与ALP染色分析。ALP染色:弃去培养基,每孔200 μl ALP染色液,避光,约10~30 min后观察染色情况。ALP活性定量分析:弃去培养基,加入细胞裂解液100 μl/孔,静置10 min。刮下细胞裂解物并收集至EP管,13 000 r/min离心5 min。另一EP管中加入ALP底物与Lupo Buffer混合液 (1:3)20μl,加入5μl裂解上清,混匀,避光45~60 min后检测。
1.2.4 茜素红S染色测定细胞钙盐沉积MEFs细胞以2×104/孔接种于24孔板,细胞融合度达到30 %时加入不同的处理因素,6 h后换液,同时加工作浓度50μg /ml维生素C、10mmol /L的β-磷酸甘油,继续培养14d进行茜素红S染色:弃去孔板中的培养基,PBS洗3次后用0.05 %戊二醛固定10 min,去离子水洗3次后加入0.4 %茜素红S,染色5~10 min,弃染液,去离子水终止反应并洗涤,显微镜下观察、成像。
1.2.5 RT-PCR培养MEFs 3天后收集细胞,用Trizol试剂提取细胞总RNA,通过琼脂电泳验证mRNA的质量并进行定量,取1 μg RNA逆转录为cDNA,稀释5倍。PCR: 10 μl体系,反应条件:95 ℃ 5 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 30 s,20~30个循环;72 ℃ 10 min。2 %琼脂糖凝胶跑电泳,凝胶成像,引物见表 1。
| Gene | Forward primer (5 ′~ 3′) | Reverse primer (5′~3′) |
| ALP | CCCCATGTGATGGCGTAT | CGGTAGGGAGAGCACAGC |
| Runx2 | GGTGAAACTCTTGCCTCGTC | AGTCCCAACTTCCTGTGCT |
| Col1a1 | CGGCTCCTGCTCCTCTTA | TTCATTGCATTGCACGTCAT |
| GAPDH | GGCTGCCCAGAACATCAT | ATGATGTTCTGGGCAGCC |
1.2.6 结晶紫染色
细胞培养待汇合至30 %左右时,加入处理因素,分组同前,培养36 h后行结晶紫染色,弃去培养基,PBS洗2~3遍,加入4 %多聚甲醛 (500μl/孔),固定15 min,加入0.05 %结晶紫溶液 (400μl/孔),染色15 min后观察染色情况,弃去染色液终止反应,加入去离子水洗3遍,显微镜下观察、成像。
1.2.7 免疫细胞化学将MEFs细胞接种至24孔板,细胞融合至30 %左右时,加入AdRFP,AdDLL1,6 h换液后加入DAPT,处理3天后,加入适当量的BMP4的条件培养基处理1 h后进行免疫细胞化学染色,操作方法按照试剂盒说明书。
1.2.8 流式细胞术检测细胞周期弃去培养基,胰酶消化,用预冷的PBS洗涤细胞3次,离心去上清,70 %预冷的乙醇重悬细胞,4 ℃过夜,上机检测。
1.3 统计学分析采用SPSS17.0进行统计分析,实验独立重复3次,数据以平均值和标准差 (±S) 表示,组间比较采用单因素方差分析,组间两两比较采用t检验,检验水准P=0.05。
2 结果 2.1 DAPT阻断Notch信号抑制BMP4诱导的MSCs早期成骨分化呈剂量依赖BMP4-CM处理MEFs细胞后,再加入浓度1、5和10μmol/L抑制剂DAPT处理细胞7天后,DAPT能够呈浓度依赖性地抑制BMP4诱导的MEFs细胞的ALP活性 (图 1)。

|
| 图 1 DAPT阻断Notch信号抑制BMP4诱导的MSCs早期成骨分化呈剂量依赖 (×100) Figure 1 BMP4-induced early osteogenic marker ALP stain was significantly inhibited by DAPT in a dose-dependent manner MEFs cells were treated with BMP4-conditioned medium and different concentrations of DAPT; after 36 h treatment, ALP activities were detected by staining assay at day 7 |
用10μmol/L的抑制剂DAPT处理MEFs细胞或用AdDLL1,AddnNotch1或AdRFP感染MEFs细胞,感染效率大约30 %,待红色荧光表达后加入BMP4-CM,诱导成骨分化5天和7天后检测ALP活性和染色的变化,结果显示,DAPT和dnNotch1抑制BMP4诱导的早期成骨分化指标ALP的活性和染色 (P<0.05)(图 2),而DLL1能促进BMP4诱导的ALP的活性和染色 (P<0.05)(图 2)。

|
| 图 2 Notch信号对BMP4诱导的MEFs早期成骨分化的影响 Figure 2 The effect of Notch signaling on BMP4-induced early osteogenic differentiation of MSCs (a) ALP activity determined by ALP stain and ALP quantitative assay at 5 days post-treatment (b) ALP activity determined by ALP stain and ALP quantitative assay at 7 days post-treatment (c) ALP activity determined by ALP stain and ALP quantitative assay at 7 days post-treatment×100, * P < 0.05, ** P < 0.01 |
用抑制剂DAPT或AdDLL1处理BMP4诱导的MEFs细胞3天后,RT-PCR检测成骨相关基因ALP, Col1a1,Runx2的表达,结果显示,DAPT抑制BMP4诱导的成骨相关基因的表达,而DLL1促进BMP4诱导的成骨相关基因的表达 (P<0.05)(图 3)。
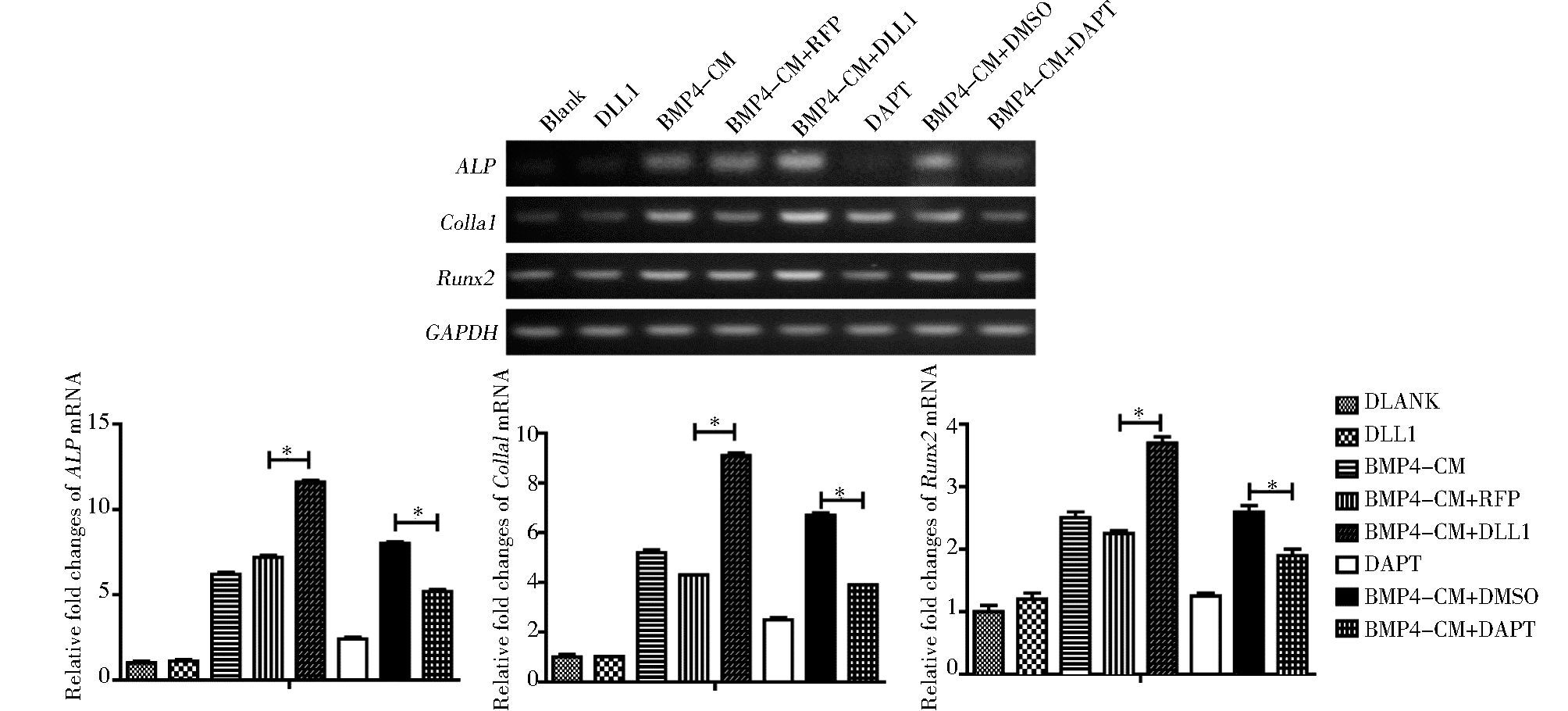
|
| 图 3 Notch信号对BMP4诱导的成骨相关基因的影响 Figure 3 The effect of Notch signaling on the expression of essential osteogenic factors induced by BMP4 in MSCs The gene expression of ALP, Colla1 and Runx2 were detected by semi-quantitative RT-PCR at 3days post-treatment* P < 0.05, compared with BMP4+RFP group, compared with BMP4+DMSO group |
DAPT或AdDLL1处理BMP4诱导的MEFs细胞14天后,茜素红S染色检测成骨晚期指标钙盐沉积,结果显示,DAPT或DLL1能分别抑制或促进BMP4诱导晚期成骨分化 (图 4)。
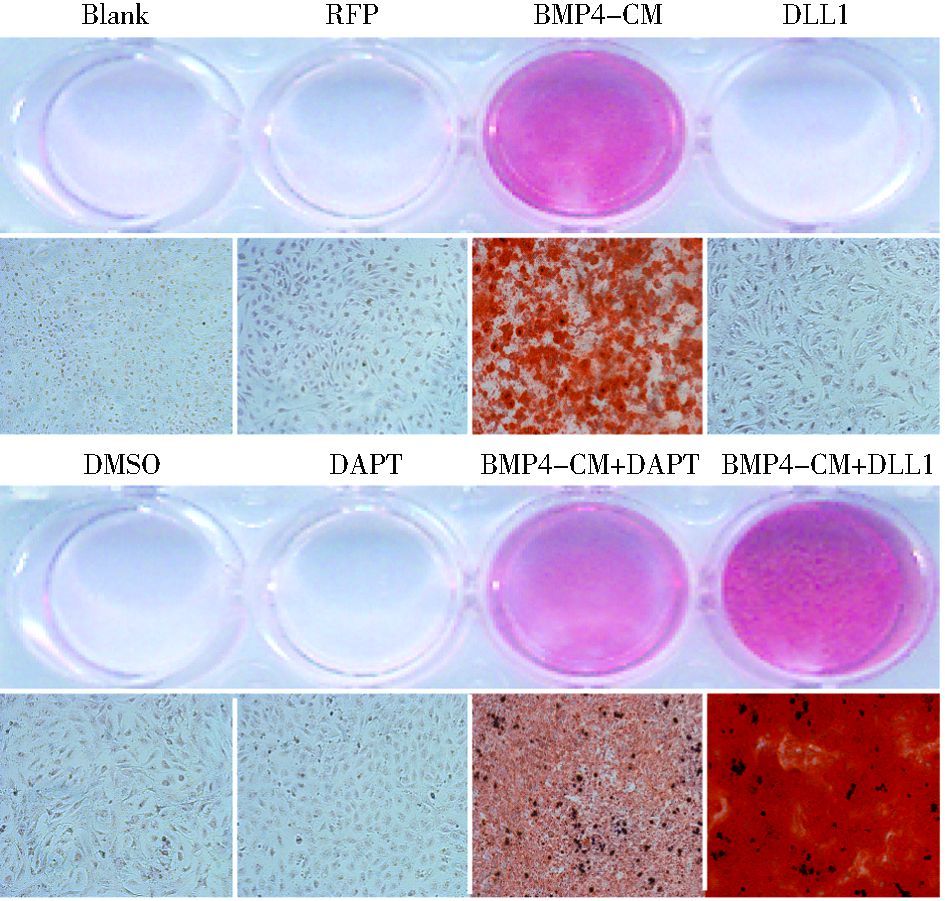
|
| 图 4 Notch信号对BMP4诱导MSCs的晚期成骨分化的影响 Figure 4 The effects of Notch signaling on BMP4-induced late osteogenic differentiation of MSCs (×100) Calcium precipitation was detected by Alizarin Red S staining (×100) |
免疫细胞化学检测细胞处理3天p-Smad1/5/8的蛋白表达 (图 5)。与对照组相比,BMP4和BMP4+DLL1组细胞核内的蛋白表达明显增高,而BMP4+DLL1组的蛋白表达要明显高于BMP4单独处理组。BMP4+DAPT组细胞核内的蛋白表达明显低于BMP4+DMSO组。
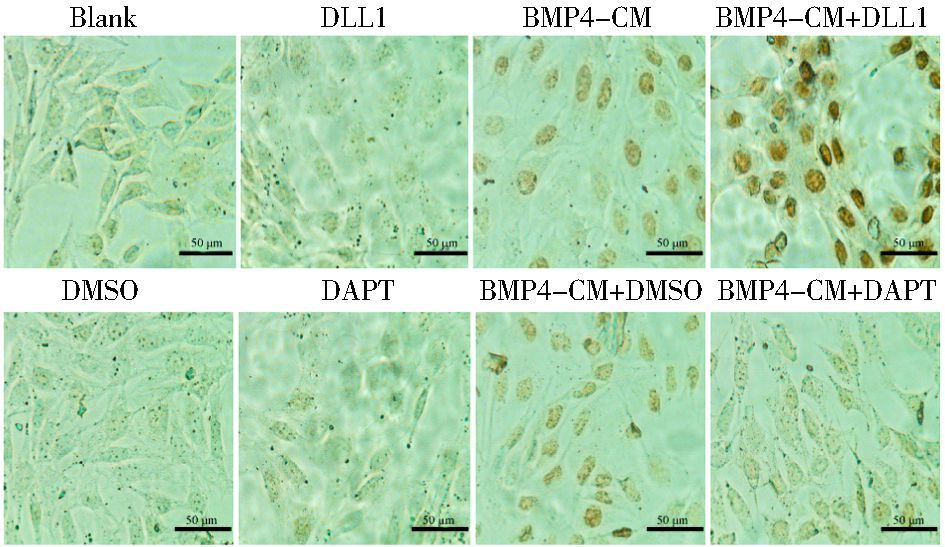
|
| 图 5 Notch信号对BMP4诱导成骨分化过程中的经典信号通路的影响 Figure 5 The effects of Notch signaling on BMP4-induced BMPs/Smads pathway p-Smad1/5/8 was stained by ICC at day 3(×400) |
DAPT或AdDLL1处理BMP4诱导的MEFs细胞,分别于36h后结晶紫检测细胞增殖和3天后流式细胞术检测细胞的增殖情况。结晶紫染色结果 (图 6a) 显示DLL1具有一定的促MEFs增殖的作用而DAPT具有一定的抑制作用;同样,FCM检测细胞周期 (图 6b),发现BMP4+RFP, BMP4+DLL1, BMP4+DMSO, BMP4+DAPT的增殖指数G2+S期分别是30.03%,40.47% 29.80%,20.61%。结果显示,DLL1早期具有一定的促BMP4诱导的MEFs细胞增殖的作用而DAPT具有一定的抑制作用。
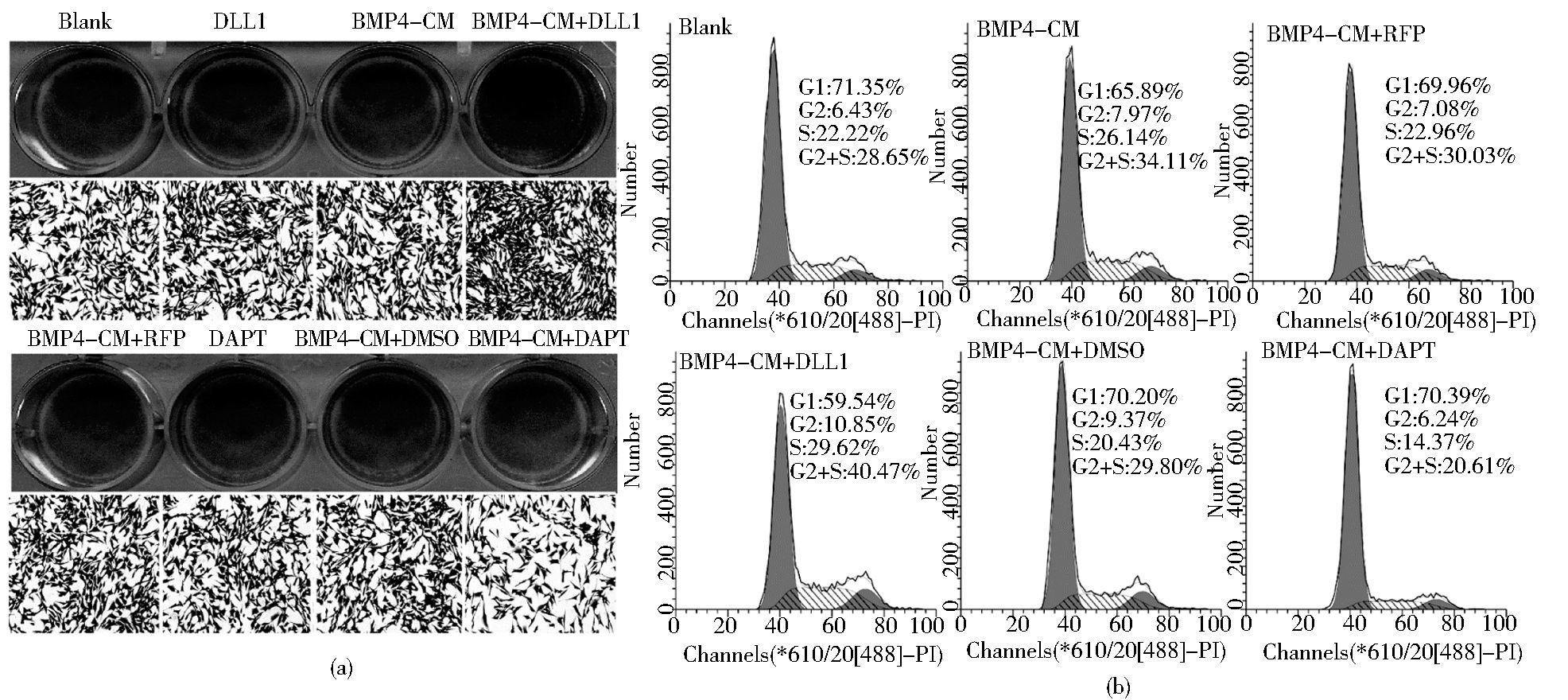
|
| 图 6 Notch信号对BMP4诱导成骨分化中细胞增殖的影响 Figure 6 The effects of Notch signaling on cell proliferation in MSCs during BMP4-induced osteogenic differentiation (a) The cell proliferation was determined with crystal violet staining at 36 h post-treatment (b) The cell cycle was determined with flow cytometry at day 3 |
间充质干细胞是一类多能干细胞,在体内外相应的诱导环境下可以分化为骨,软骨,脂肪,肌肉等组织。因此在再生医学研究中具有重要的作用和地位。研究显示MSCs在成骨BMPs的作用下,可以向成骨细胞分化。其中BMP2和7已经应用于临床,然而由于成骨BMPs诱导MSCs成骨的机制尚不清楚,临床应用效果亦并不如预期,因此进一步明确成骨BMPs诱导MSCs成骨分化的机制,对于改善BMPs在临床中的应用具有重要的意义。MEFs是胚胎成纤维干细胞,由于其具有干细胞的特征,是研究MSCs的重要细胞[2, 6]。本研究用于MSCs成骨分化的研究。
Notch信号是胚胎发育过程中高度保守的信号通路,Notch信号通过调节胚胎干细胞的分化,增殖与凋亡过程,调控哺乳动物的体节发生[8]。在哺乳动物中,Notch的配体有Jagged1, 2和DLL1,3,4,受体有Notch1-4。Notch与相邻细胞上的配体结合,在γ-内分泌酶 (γ-secretase) 的作用下剪切Notch的胞内段 (Notch intracellular domain,NICD),NICD进入细胞核与CSL结合进而调控下游靶基因Hey1,Hes1的表达[16-17]。研究报道Notch信号在骨重建过程中发挥重要作用。我们的研究及一些国外学者的研究都显示Notch单独没有诱导MSCs成骨分化;然而我们发现Notch能够明显促进BMP 9诱导的MSCs成骨,是BMP9诱导MSCs成骨分化中重要的影响因素[11]。这一现象也在BMP2诱导的MSCs成骨分化中发现[10],是否Notch信号在所有的成骨BMPs诱导分化中都具有不可取代的作用呢?Notch信号对其他成骨BMPs,包括BMP4诱导成骨分化的影响目前还不清楚。
BMP4是TGFβ超家族的一员,能诱导间充质干细胞成骨分化,也是成骨BMPs中的一员[18]。在成骨分化过程中,BMP4在原始间质细胞和前成骨细胞中高表达,而在成熟的成骨细胞中不表达。BMP4可以诱导原始间质细胞分化为脂肪,软骨,骨细胞[19]。有研究显示,BMP4依赖于Notch信号表达靶基因Hes1,Hey1,而另一方面,SMADs可以增强NICD介导的转录活性进而促进Notch的靶基因的表达[20]。也有文献报道,BMP4诱导的抑制成肌分化过程中Notch信号发挥着重要作用[21]。这些研究都提示BMP4和Notch之间有密切的关系,那么在BMP4诱导的MSCs成骨分化中,Notch是通过什么样的机制发挥怎样的作用呢?
我们的研究结果显示 (1) DAPT抑制BMP4诱导的早期成骨分化,且呈浓度依赖性;(2) DLL1促进BMP4诱导的成骨分化,DAPT和dnNotch1抑制BMP4诱导的成骨分化;(3) DLL1促进BMP4诱导的成骨相关基因ALP, Runx2,Col1a1的表达,DAPT抑制这些基因的表达;(4) DLL1促进BMP4诱导的细胞核内p-Smad1/5/8的表达,而DAPT抑制其表达;(5) DLL1促进BMP4诱导的细胞增殖,而DAPT抑制BMP4诱导的细胞增殖。研究提示Notch信号通过BMP/Smads信号通路促进BMP4诱导的MSCs成骨分化,在此过程中也有促细胞增殖的作用。其与Notch信号在BMP9和2诱导成骨分化的结果相似,提示Notch信号在促进成骨BMPs诱导成骨中可能存在相同的作用和机制。
本研究作为Notch在成骨BMPs诱导成骨中的作用及机制研究的一部分,但仍需进一步完善Notch在BMP6, 7中的作用研究,才能最终证实Notch在成骨BMPs诱导成骨中的作用广泛性。从而为临床上采用BMPs进行骨组织工程的方法和策略,提供理论和实验依据。
| [1] | Pittenger M F, Mackay A M, Beck S C, et al. Multilineage potential of adult human mesenchymal stem cells. Science, 1999, 284(5411) : 143–147. DOI:10.1126/science.284.5411.143 |
| [2] | Rastegar F, Shenaq D, Huang J, et al. Mesenchymal stem cells:Molecular characteristics and clinical applications. World Journal of Stem Cells, 2010, 2(4) : 67–80. DOI:10.4252/wjsc.v2.i4.67 |
| [3] | Caplan A I, Bruder S P. Mesenchymal stem cells:building blocks for molecular medicine in the 21st century. Trends in Molecular Medicine, 2001, 7(6) : 259–264. DOI:10.1016/S1471-4914(01)02016-0 |
| [4] | Garreta E, Genove E, Borros S, et al. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Engineering, 2006, 12(8) : 2215–2227. DOI:10.1089/ten.2006.12.2215 |
| [5] | Lengner C J, Lepper C, van Wijnen A J, et al. Primary mouse embryonic fibroblasts:a model of mesenchymal cartilage formation. Journal of Cellular Physiology, 2004, 200(3) : 327–333. DOI:10.1002/(ISSN)1097-4652 |
| [6] | Saeed H, Taipaleenmaki H, Aldahmash A M, et al. Mouse embryonic fibroblasts (MEF) exhibit a similar but not identical phenotype to bone marrow stromal stem cells (BMSC). Stem Cell Reviews, 2012, 8(2) : 318–328. DOI:10.1007/s12015-011-9315-x |
| [7] | Peng Y, Kang Q, Cheng H, et al. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. Journal of Cellular Biochemistry, 2003, 90(6) : 1149–1165. DOI:10.1002/(ISSN)1097-4644 |
| [8] | Penton A L, Leonard L D, Spinner N B. Notch signaling in human development and disease. Seminars in Cell & Developmental Biology, 2012, 23(4) : 450–457. |
| [9] | Tezuka K, Yasuda M, Watanabe N, et al. Stimulation of osteoblastic cell differentiation by Notch. Journal of Bone and Mineral Research:The Official Journal of The American Society for Bone and Mineral Research, 2002, 17(2) : 231–239. DOI:10.1359/jbmr.2002.17.2.231 |
| [10] | Canalis E. Notch signaling in osteoblasts. Science Signaling, 2008, 1(17) : e17. |
| [11] | 廉静, 杨伦韵, 曹俊杰, 等. DLL1在骨形态形成蛋白9诱导骨髓间充质干细胞成骨分化中的作用. 第三军医大学学报, 2016, 38(1) : 38–43. Lian J, Yang L Y, Cao J J, et al. Delta-like 1 promotes osteogenic differentiation of mesenchymal stem cells induced by BMP9. Journal of Third Military Medical University, 2016, 38(1) : 38–43. |
| [12] | Viale-Bouroncle S, Gosau M, Morsczeck C. NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells. Biochemical and Biophysical Research Communications, 2014, 443(2) : 500–504. DOI:10.1016/j.bbrc.2013.11.120 |
| [13] | 杨伦韵, 张晓艳, 廉静, 等. Notch1在BMP9诱导iMEF细胞成骨分化中的作用. 第三军医大学学报, 2015, 37(1) : 39–45. Yang L Y, Zhang X Y, Lian J, et al. Role of Notch1 signaling in the process of BMP9-induced osteogenetic differentiation of immortalized mouse embryonic fibroblasts. Journal of Third Military Medical University, 2015, 37(1) : 39–45. |
| [14] | Varnum-Finney B, Wu L, Yu M, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. Journal of Cell Science, 2000, 113(23) : 4313–4318. |
| [15] | Cordle J, Redfieldz C, Stacey M, et al. Localization of the delta-like-1-binding site in human Notch-1 and its modulation by calcium affinity. The Journal of Biological Chemistry, 2008, 283(17) : 11785–11793. DOI:10.1074/jbc.M708424200 |
| [16] | Zanotti S, Canalis E. Notch Signaling and the skeleton. Endocrine Reviews, 2016, 37(3) : 223–253. DOI:10.1210/er.2016-1002 |
| [17] | Kopan R, Ilagan M X. The canonical Notch signaling pathway:unfolding the activation mechanism. Cell, 2009, 137(2) : 216–233. DOI:10.1016/j.cell.2009.03.045 |
| [18] | Luu H H, Song W X, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. Journal of Orthopaedic Research:Official Publication of the Orthopaedic Research Society, 2007, 25(5) : 665–677. DOI:10.1002/(ISSN)1554-527X |
| [19] | Jane J. Ectopic osteogenesis using adenoviral bone morphogenetic protein (BMP)-4 and BMP-6 gene transfer. Molecular Therapy, 2002, 6(4) : 464–470. DOI:10.1006/mthe.2002.0691 |
| [20] | Buas M F, Kadesch T. Regulation of skeletal myogenesis by Notch. Experimental Cell Research, 2010, 316(18) : 3028–3033. DOI:10.1016/j.yexcr.2010.05.002 |
| [21] | Dahlqvist C, Blokzijl A, Chapman G, et al. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development, 2003, 130(24) : 6089–6099. DOI:10.1242/dev.00834 |
 2017, Vol. 37
2017, Vol. 37




