文章信息
- 郑天祥, 钱雨农, 张大羽.
- ZHENG Tian-xiang, QIAN Yu-nong, ZHANG Da-yu.
- 昆虫脂肪酸合成通路关键基因的研究进展
- Key Genes Involved in Fatty Acids Biosynthesis in Insects
- 中国生物工程杂志, 2017, 37(11): 19-27
- China Biotechnology, 2017, 37(11): 19-27
- http://dx.doi.org/DOI:10.13523/j.cb.20171104
-
文章历史
- 收稿日期: 2017-09-18
2. 浙江农林大学农业与食品科学学院 临安 311300;
3. 浙江省2011绿色农药协同创新中心 临安 311300
2. College of Agricultural and Food Science, Zhejiang A & F University, Linan 311300, China;
3. Collaborative Innovation Center of Green Pesticide, Zhejiang Province, Zhejiang A & F University, Linan 311300, China
脂肪酸(fatty acids, FAs)是一类一端含有一个羧基的脂肪族碳氢链。其生物合成是一个复杂的多步反应过程,主要由乙酰辅酶A羧化酶(acetyl-coenzyme A carboxylase, ACC)、脂肪酸合成酶(fatty acid synthase, FAS)、超长链脂肪酸延伸酶(elongase of very long chain fatty acid,ELO)、去饱和酶(desaturase,desat或fatty acid desaturase, FAD)、脂酰辅酶A还原酶(fatty acyl-CoA reductase,FAR)等参与完成[1]。在昆虫中,脂肪酸衍生物蜡酯、脂肪醇、烃类物质是昆虫表皮的重要组成成分[2-4]。同时,超长链脂肪酸是鞘脂类和甘油脂类的前体,这两种物质是细胞膜结构必需的组成成分,并参与多种细胞生物学过程[5-7]。此外,脂肪可储存和提供能量,是昆虫重要的供能物质,昆虫体内不饱和脂肪酸的比例及脂肪酸含量还影响昆虫耐寒能力[8]。
脂肪酸在体内合成步骤如图 1所示。乙酰辅酶A在ACC作用下生成丙二酸单酰辅酶A;在FAS作用下,乙酰辅酶A与丙二酸单酰辅酶A反应,每一个循环增加2个碳链长,最终可以形成C16脂酰辅酶A;之后,≥C12脂酰辅酶A可以在ELO作用下进行延伸,形成更长链的脂肪酸;另外,饱和脂肪酸可以在desat的作用下形成不饱和脂肪酸。饱和脂肪酸(SFAs)、单不饱和脂肪酸(MUFAs)和多不饱和脂肪酸(PUFAs)可以进一步反应,得到脂肪醇(由FAR催化)、鞘脂类、甘油酯类、烃类和蜡酯类衍生物。
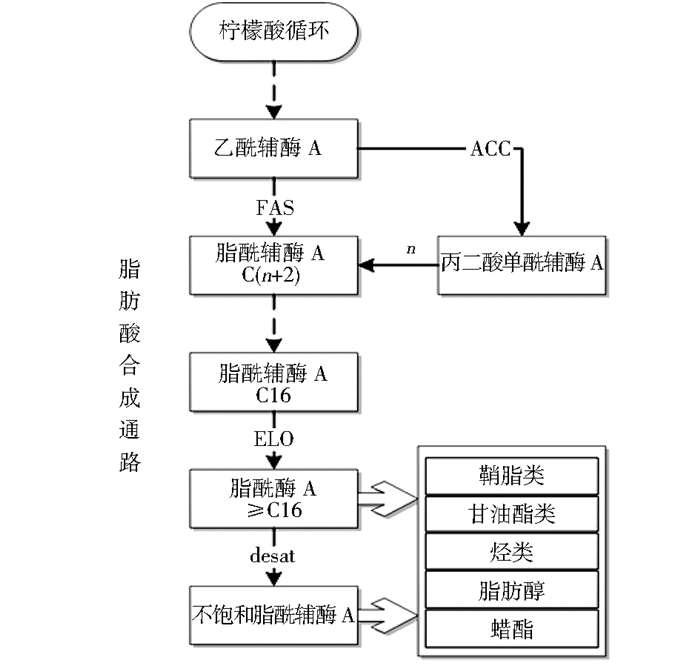
|
| 图 1 昆虫脂肪酸合成通路及参与的关键酶 Figure 1 Fatty acid synthesis pathway and key enzymes in insect |
脂肪酸是膜组分和信号分子的重要前体[9-10],脂肪酸合成通路在维持昆虫体内平衡中起重要作用。以下主要介绍ACC、FAS、ELO、desat和FAR在昆虫中的研究进展。
1.1 ACCACC是脂肪酸合成第一步反应的限速酶。ACC分为两大类,一类称为多亚基型ACC,这类ACC由4个亚基组成,即生物素羧化酶(BC)、生物素羧基载体蛋白(BCCP)及羧基转移酶(CT)的两个亚基α-CT和β-CT,合称为ACC全酶,但该酶很不稳定[11]。另一类为多功能型ACC,其BC、BCCP及CT功能域依次分布在同一条多肽链上,是分子质量在265~280kDa的多结构域酶[12],大部分真核生物ACC属于此类。基因组数据表明,昆虫ACC为单基因编码的多结构域酶[13]。ACC催化乙酰辅酶A羧化形成丙二酸单酰辅酶A通过两个单独的反应进行,分别由BC和CT催化:在BC催化的反应中,生物素辅因子N1原子被碳氢酸盐类羧化,该反应需要ATP参与,且2个Mg2+被磷酸盐螯合;在CT催化的反应中,被羧化的生物素将羧基转移到受体底物乙酰辅酶A中,生成丙二酸单酰辅酶A[14-15];整个反应过程生物素由BCCP运载[16]。
ACC在昆虫中的主要作用包括影响脂质积累、生殖能力以及表皮的正常功能等。此外,有研究报道了以ACC为靶标的杀虫剂螺虫乙酯的作用机制。
1.1.1 ACC影响脂质积累昆虫脂肪体(FB)和绛色细胞被认为具有与哺乳动物脂肪和肝相似的功能[17-18],昆虫吸收的大部分营养物质被运输到FB和绛色细胞中进行代谢,以甘油三酯(TGs)形式储存[19]。研究结果显示,敲除黑腹果蝇Drosophila melanogaster FB中ACCCG1198,会导致TGs显著下降,糖原含量增加,但不会出现致死表型[13],TGs和糖原代谢在维持体内平衡中起重要作用并与多种疾病相关[20-21]。此外,干扰埃及伊蚊Aedes aegypti ACC后也有相似结果,其TGs和磷脂(PLs)水平显著下降[22]。
1.1.2 ACC影响生殖能力ACCCG11198突变会导致黑腹果蝇胚胎死亡[13],与敲除小鼠Mus musculus胚胎ACC1的表型相同[23],说明该基因对胚胎发育十分重要。而RNA干扰埃及伊蚊ACC仅在第一营养周期造成卵壳发育缺陷,使卵死亡率提高,但在后一营养周期中该缺陷会消失,表明卵壳缺陷是滤泡上皮细胞ACC缺失造成的[22]。作者认为,卵壳脂成分是通过卵巢组织从头合成脂质来提供的,而ACC缺失会中断这一过程,从而导致卵壳缺陷[24];也可能是ACC缺乏导致底物乙酰辅酶A增加或产物丙二酸单酰辅酶A减少从而影响了卵壳的形成[24]。在第一和第二营养周期,干扰ACC还会使埃及伊蚊产卵量和繁殖能力下降[22]。此外,井冈霉素处理提高的褐飞虱Nilaparvata lugens繁殖力和种群数量,通过RNA干扰ACC能恢复到正常水平[25]。
1.1.3 ACC影响表皮功能绛色细胞是存在于昆虫外表皮腹部两侧与腹部气孔紧密相连的一类细胞,能控制气体进入气管系统[26]。ACCCG11198除了在黑腹果蝇FB高表达外,在绛色细胞中也高表达。在果蝇绛色细胞中突变ACCCG11198,会使绛色细胞凋亡,并导致绛色细胞中至少一个超长链脂肪酸(VLCFA)合成通路被破坏,使气管系统将气孔腺分泌的脂滴转移到气孔口的过程阻断,水密性丧失,果蝇死亡[13]。前人研究也发现,脂滴转移过程在陆生昆虫中的作用是防止水分从气孔和角质层散失[27]。这说明ACCCG11198对于果蝇的存活至关重要。
1.1.4 ACC作为杀虫剂靶标ACC是杀虫剂螺虫乙酯的作用靶标,研究人员对桃蚜Myzus persicae、草地夜蛾Spodoptera frugiperda以及在昆虫细胞中成功表达的二斑叶螨Tetranychus urticae ACC进行纯化并进行研究,结果表明螺虫乙酯烯醇对ACC的CT结构域起作用[16]。
1.2 FASFAS定位于细胞质中,能催化乙酰辅酶A与丙二酸单酰辅酶A反应,最终可以得到软脂酸[28]。在生物体中,存在两种类型的FAS。Ⅰ型FAS是由多亚基蛋白组成的合酶,主要存在于动物和真菌中;而Ⅱ型FAS酶主要存在于细菌和真核生物叶绿体、线粒体等细胞器中[29]。FAS催化合成脂肪酸的反应在不同亚基的功能域分步进行[29]。
FAS在昆虫中的研究集中于时空表达和功能以及对昆虫滞育的影响两个方面。
1.2.1 FAS时空表达和功能在黑腹果蝇基因组中发现了3个FAS基因,称为FASCG3523、FASCG3524、FASCG17374[13]。在其幼虫中,所有组织中都能检测到FASCG3523表达,而FASCG3524和FASCG17374仅在角质层、表皮、肌肉和绛色细胞中表达[30]。在埃及伊蚊中,检测喂食血液后埃及伊蚊FAS1在48h后的FB和72h后的卵巢中表达量最高,而在饲喂后FAS2无显著差异[22]。
RNA干扰FAS1能显著降低埃及伊蚊TGs和PLs的含量,与干扰ACC相似。此外,抑制FAS1表达还会影响该蚊消化血液的能力。另外,通过RNA干扰FAS可使褐飞虱卵巢和FB中FAs含量降低,繁殖力下降[31]。
1.2.2 FAS对滞育的影响Robich和Denlinger[32]和Zhou和Miesfeld[33]发现,尖音库蚊Culex pipiens FAS在滞育早期表达上调。Sim和Denlinger[34]确定了尖音库蚊滞育期脂肪储存和消耗的调控基因,发现fas-1基因在滞育早期上调。RNA干扰雌蚊fas-1和fas-3后,雌蚊不能储存过冬所需的脂质,因此这两个FAS在滞育早期对脂肪储存具有重要作用。除尖音库蚊外,在大猿叶虫Colaphellus bowringi Baly中,FAS1的表达受到保幼激素(JH)的抑制,而FAS1表达量降低会阻碍滞育发生[35]。FAS2表达量在滞育准备期高于FAS1,并在该时期,FAS2在雌虫FB中的表达量远高于其他组织。干扰FAS2会导致脂质储存降低,影响耐逆性基因表达,并增加虫体含水量,但不影响滞育准备时长及发生率。此外,FAS2与FAS1一同受到JH和保幼激素受体JH-Met的抑制,缺少JH-Met会诱导FAS2表达,促进滞育期雌虫脂质储存[36]。
1.3 ELOELO是脂肪酸延伸反应第一步的限速缩合酶[37]。昆虫脂肪酸延伸循环一般认为可从C14开始,较短链FAs由FAS从头合成或从体外获得[1]。脂肪酸以其活化形式脂酰辅酶A参与延伸循环,经过缩合、还原、脱水、再还原4个步骤,生成较长链FAs。,分别由ELO、3-ketoacyl-CoA reductase(KAR)、3-dehydratase-CoA dehydratase(HACD)和trans-2, 3-enoyl-CoA reductase(TER)催化[1]。
ELO在昆虫中的研究集中于黑腹果蝇,主要包括ELO对生殖能力影响、在信息素合成中的作用以及对表皮功能的影响。
1.3.1 ELO对生殖能力的影响果蝇基因组中的脂肪酸延伸循环通路基因包括20个ELO、2个TER、2个HADC和1个KAR[13]。其中ELOCG6921只在精母细胞表达,而不在精原细胞表达。ELOCG17821和ELOCG31141在果蝇雄成虫精巢表达,ELOCG17821也在精母细胞表达。突变jamesbond(ELOCG6921)会使果蝇精母细胞在分裂末期较早阶段卵裂沟的内移停止或显著减缓,并使收缩环从皮层分离、收缩或塌陷。这说明VLCFA及其酯类衍生物能软化膜成分,对精母细胞形成具有重要作用[38],突变jamesbond还能显著抑制雄果蝇生育力,并通过改变信息素成分影响其他雄果蝇生育力[39]。定向RNA干扰果蝇精巢生殖细胞发育后期胞囊中的noa(ELOCG3971),也会导致雄果蝇不育。并且,noa缺失还会使果蝇运动功能受损,生存力显著下降[40]。
1.3.2 ELO在信息素合成中的作用ELO影响合成的信息素种类主要包括烃类、酯类和醇类化合物。Chertemps等[41]首次报道了昆虫的ELO基因,命名为elo68α,编码蛋白质能延伸C14:1Δ9和C16:1Δ9,在雄果蝇生殖系统中特异性表达,对其一个氨基酸位点定点突变会使雄蝇射精管球(EjB)中性信息素(SPs)cis-vaccenyl acetate(cVA)的含量降低。之后,Chertemps等[3]又报道了一种在雌果蝇中特异性表达的ELO,命名为eloF,能在酿酒酵母中表达并将FAs延伸至C30。RNA干扰eloF会使雌果蝇C29二烯烃减少和C25二烯烃增加,延长果蝇交配时长,减少交配尝试和交配次数。而将eloF在雄果蝇中表达未产生与雌果蝇相似的表皮烃类(雄果蝇中表皮烃类主要为C23、C25一烯烃,而在雌果蝇中主要为C27、C29二烯烃)。另外,jamesbond在EjB和雄蝇生殖系统特异性表达、缺失会导致雄果蝇不再生成信息素(3R, 11Z, 19Z)-3-acetoxy-11, 19-octacosadien-1-ol(CH503)[39]。
1.3.3 ELO对昆虫表皮功能的影响Juárez[2]研究了德国小蠊Blatella germanica表皮中脂肪酸延伸酶的功能,以C16:0脂酰辅酶A为底物时,主要产物为C18脂酰辅酶A;以C18:0脂酰辅酶A为底物时,C20脂酰辅酶A为主要产物。Falcón等[42]分析意大利蜂Apis mellifera表皮转录组,发现有10个ELO,功能有待深入研究。此外,在果蝇绛色细胞中的VLCFA通路中,干扰其中一个ELO,会出现与突变绛色细胞中ACCCG11198相似的表型[13]。
1.3.4 其它昆虫ELO的研究目前,除了在果蝇中对ELO进行研究之外,黄粉虫的ELO基因和功能亦有报道。有2个ELO被克隆,其中TmELO1能够在酵母中将脂肪酸延伸至C24,而TmELO2改变了≤C18脂肪酸的组分比例。另外,通过干扰老熟幼虫的基因,TmELO1会导致黄粉虫死亡率上升,说明其对黄粉虫老熟阶段存活具有至关重要的作用[43]。
1.4 脂肪酸去饱和酶(desat或FAD)去饱和酶(desat或FAD)是能催化脂酰辅酶A在特定键位上形成双键的酶,每个双键的形成需一个氧气分子和两个电子参与。去饱和酶对脂肪酸碳链长和双键形成位置都具有特异性[44]。已发现的desat可分为两种,分别为可溶性酰基-酰基载体蛋白和完整膜蛋白,可溶蛋白主要存在于植物质粒体中[45],膜蛋白又可分为acyl-CoA去饱和酶、‘front-end’-acyl-CoA去饱和酶和acyl-lipid去饱和酶这3类[44]。昆虫desat已有进行了广泛研究,在果蝇中研究较系统,其他研究集中于desat在信息素合成中的作用。
1.4.1 果蝇中desat的研究第一个报道的昆虫desat为果蝇desat1[46],随后desat1被发现具有Δ9功能特异性,最优底物为棕榈酸[47]。desat1突变会导致雌雄果蝇角质层不饱和烃含量降低。另外,desat1未突变的雄蝇不能分辨desat1突变的雌雄果蝇的性别,突变desat1的雄蝇不能分辨未突变desat1果蝇的性信息素(SPs),并且desat1被发现在产生和感知SPs的组织中表达,说明SPs的产生和感知是由desat1基因编码的不同蛋白质调节的,体现了desat1的多效性[48]。之后,Houot等[49]进一步研究了desat1对SPs的产生和感知的影响,证明desat1对信息素通信的多效性依赖于其转录活性的调节。Bonsquet等[50]的研究还发现,desat1除了在FB和绛色细胞表达外,在前脑中部(含丰富神经细胞)、malpighi小管和直肠乳突(与含水量有关的组织)也有表达,因而desat1除了参与通信,还能通过FB和绛色细胞或通过激素来调控果蝇脂质代谢,并参与调控虫体水量。desat1还被发现在生殖系统组织中表达,包括精囊、附腺、卵巢和EjB。作者认为,由于一些具有desat1活性的组织能产生性信号,因此可能存在一个共同进化过程,形成了desat1调节区域,使desat1在产生SPs的化学感觉器官和脑神经元中表达。
除了desat1外,果蝇desat2和desatF的研究发现,desat2的最优底物为豆蔻酸,desat2在果蝇基因组中存在,但在Canton-S种果蝇中不能转录表达,而在Tai雌蝇中能转录表达并可生成5, 9-十七碳二烯烃,体现了黑腹果蝇二烯烃多态性[47]。而desatF只在雌果蝇中表达,RNA干扰desatF会导致雌蝇表皮二烯烃减少而单烯烃增加,同时增加交配时长,减少雄蝇求偶和交配尝试次数。另外,desatF在含有丰富二烯烃的D. sechellia中也有表达,但在只有单烯烃的D. simulans中不表达,说明desatF是果蝇产生二烯烃的关键基因。desatF增强子区域的修饰是果蝇属特异性SPs多样性的基础[51-52]。
此外,Wang等[53]通过向果蝇喂食FAD的抑制剂CAY10566导致果蝇不饱和FAs减少,生长减缓,第一次蜕皮之后蜕皮激素调节蛋白不再诱导表达,正常蜕皮不能进行,并于几天后死亡,与desat1突变型果蝇表型相似。
1.4.2 desat与信息素多样性的关系除果蝇之外,主要研究了昆虫desat在信息素多样性中所起的作用。发现不同位点的desat会影响昆虫信息素的多样性。Albre等[54]从4种卷叶蛾中得到6个desat,其中2个desatΔ9和1个desatΔ10参与信息素合成。desatΔ10表达量的差异造成不同卷叶蛾间信息素成分的多样性:Ctenopseustis obliquana和Planotortrix octo利用desatΔ10合成的(Z)-8-tetradecenyl acetate是信息素主要成分,这两种的desatΔ10在信息素腺中的表达量显著高于C. herana和P. excessana,说明信息素存在种间差异且与desat的调节有关。之后,Albre等[55]又在卷叶蛾Planotortrix的P.octo中发现desatΔ5表达,参与合成SPs (Z)-8-tetradecenyl acetate,而在P. excessana信息素腺desatΔ5不表达,从而产生与P.octo不同的SPs (Z)-5-tetradecenyl acetate和(Z)-7-tetradecenyl acetate。另外,Hagstrm等[56]在新西兰独有的斜纹卷蛾Ctenopseustis obliquana和C. herana中发现一种只对豆蔻酸的Δ5有去饱和功能的desat7,能参与生成SPs (Z)-5-tetradecenyl acetate。此外,在玉米螟Ostrinia中,不同种间的desat作用双键位置不同(Δ11或Δ14),使产生的信息素种类不同[57]。种间desat表达差异也导致了昆虫信息素的多样性。在玉米螟中,Ostrinia nubilalis和O. furnacalis间信息素的差异原因之一是在O. furnacalis信息素腺中存在一种表达的desat,而该desat在O. nubilalis中不表达[57]。
此外,desat还通过形成同分异构体信息素产生信息素多样性。一种卷叶蛾Choristoneura rosaceana利用desatΔ11以豆蔻酸为底物合成(E)-11-tetradecenoate和(Z)-11-tetradecenoate(35:65),而另一种卷叶蛾C. parallela也利用desatΔ11以豆蔻酸为底物但合成几乎纯的(E)-11-tetradecenoate。这两个不同的desat共有24个不同的氨基酸位点,其细胞溶质羧基端蛋白258E对于C. rosaceana Z的合成非常重要。将C. rosaceana desatΔ11该位点的谷氨酸突变为天冬氨酸,就具备了与C. parallela desatΔ11相似的活性;而将C. parallela desatΔ11该位点突变为谷氨酸造成了其产物比例变化(E:Z=64:36)[58]。此外,玉米螟Ostrinia desatΔ11的种间氨基酸位点差异也造成了信息素同分异构比例的不同[59]。
还有一种情况也会导致信息素的多样性:在烟草天蛾Manduca sexta SPs中含有不饱和脂肪酸(UFAs)衍生物[60]。MsexD2具有Z11位和E10、E12位去饱和活性,分别生成单不饱和与双不饱和SPs成分的前体[61],MsexD3、MsexD5对MsexD2形成的双不饱和脂肪酸的E/Z14位引入双键,不仅SPs存在同分异构体,还引入了3个不饱和键[60]。
1.4.3 desat的功能多样性及调控机制除信息素多样性的研究之外,关于desat的其它功能及调控也进行了报道。
在尺蠖属Ascotis selenaria信息素腺中发现一个与desatΔ11相似但进化方式不同的基因Asdesat1,跨膜区域已经退化,无去饱和功能,但在该虫信息素生物合成过程中起作用[62]。在花萤Chauliognathus lugubris中存在一种特殊的desat,在防御腺体及其附腺中表达,参与合成防御信息素8Z-dihydromatricaria acid,用于驱赶禽类和保护卵[63]。熊蜂属Bombus雄蜂唇腺会分泌一种特殊标志信息素,由萜类和含一个或两个双键的不同碳链长的脂肪族化合物组成。其中的双键由desat产生,desatΔ4在C14具有Δ4活性,desatΔ9在C14~C18具有Δ9活性。desatΔ9参与Bombus lucorum和B. lapidaries标志信息素的脂肪酸成分合成[64]。
转录组和基因组中发现大量desat的存在。在意大利蜂表皮转录组中发现了6个desat相关基因,其在蜜蜂表皮中高表达[42]。在蚂蚁Formica exsecta转录组中发现6个desatΔ9,认为对蚂蚁间的交流具有重要作用[65]。家蚕转录组中鉴定得到14个desat。其中Bmdesat1和Bmdesat5~8具有Δ11活性,Bmdesat3~4具有Δ9活性[66]。赤拟谷盗Tribolium castaneum基因组中发现有15个desat,其中11个已被克隆,8个desat具有去饱和活性[66]。小菜蛾Plutella xylostella desatΔ11在各发育阶段表达,desatΔ9只在雌成虫期表达,都受信息素生物合成活化神经肽(PBAN)调控[67]。而在甘蓝夜蛾Mamestra brassicae中的一种desatΔ11在雌蛾信息素腺高表达,却不受PBAN调控[68]。
1.5 FARFAR能将脂肪酸转化为脂肪醇,脂酰辅酶A在FAR作用下,由NAD(P)H提供氢离子,形成脂肪醇[4]。
目前在昆虫中报道的FAR主要作用为参与信息素以及昆虫表皮醇类及蜡酯的合成。经FAR催化产生信息素同分异构体造成了不同地域蛾类在性行为上的差异以及生殖隔离:欧洲玉米螟Ostrinia nubilalis雌蛾的信息素用于吸引雄蛾,其成分包含两种同分异构体信息素Z-11-tetradecenyl acetate(Z-)和E-11-tetradecenyl acetate(E-),Z-与E-的比例差异造成了生殖隔离现象,FAR变异是造成信息素Z-和E-比例不同的关键因素。Z亚种的FAR能把Z-前体大量转化为醇而对E-前体转化较少;而在E亚种中,结果正好相反[69]。另外,家蚕BmFAR[70]、玉米螟Ostrinia scapulalis FARXII[71]、3个巢蛾属Yponomeuta pgFAR[72]、4种相关物种Heliothis virescens与H. subflexa、Helicoverpa armigera与H. assulta的pgFAR[73]和草地夜蛾Spodoptera littoralis Slit-FAR1[74]都特异性地在雌虫信息素腺中表达,被认为是昆虫合成信息素的关键酶。而意大利蜂AmFAR1在其头部大量表达,并能合成脂肪醇(C14-C22,最优底物为C18)[4]。蜡酯由脂肪醇与脂肪酸反应得到,扶桑绵粉蚧表面含有丰富的白色蜡酯成分,Li等[75]发现扶桑绵粉蚧进行螺虫乙酯处理后,扶桑绵粉蚧中PsFar Ⅰ表达量上调,同时发现其表面变黄及蜡酯量减少,因而作者推测FAR可能涉及扶桑绵粉蚧对螺虫乙酯的解毒以及表面蜡酯合成。
2 小结与展望综上所述,脂肪酸合成通路及其关键基因对昆虫脂质积累、信息通信、生殖发育、表皮功能、滞育发生等方面都具有重要的作用,通路中关键基因的缺失会使昆虫在生长发育过程中产生不同程度的影响。目前的研究结果表明,ACC主要对昆虫脂质积累、生殖能力和表皮功能起作用;FAS主要对昆虫脂质积累以及滞育发生有影响;ELO主要对昆虫生殖细胞发育、信息素合成和表皮功能起作用;desat主要对昆虫信息素合成及通讯起作用;FAR主要参与昆虫信息素合成及表皮醇类和蜡酯的合成。目前的研究主要集中探讨单个关键基因对昆虫的影响上,将来可能会在以下几个方面有较深入研究:(1)以果蝇为模式昆虫,研究脂肪酸合成关键基因对健康的影响,研发相关药物,用于治疗肿瘤、肥胖等疾病。(2)鉴于已有以ACC为靶标的杀虫剂用于害虫防治,以及脂肪酸合成通路关键基因缺失会造成昆虫死亡、信息交流障碍和种群数量降低等影响,可以研发以FAS、ELO、desat和FAR为靶标的新型杀虫剂,达到控制害虫的目的。(3)昆虫富含优质的不饱和脂肪酸,进一步研究其合成机制,为昆虫作为理想的脂肪酸来源提供理论依据。(4)通过昆虫分子生物学和功能基因组等途径,研究脂肪酸合成通路基因与其他通路基因的关系和对昆虫生命活动的影响。
| [1] |
Sassa T, Kihara A. Metabolism of very long-chain fatty acids:genes and pathophysiology. Biomolecules and Therapeutics, 2014, 22(2): 83-92. DOI:10.4062/biomolther.2014.017 |
| [2] |
Juárez M. Fatty acyl-CoA Elongation in Blatella germanica integumental microsomes. Archives of Insect Biochemistry and Physiology, 2004, 56(4): 170-178. DOI:10.1002/arch.v56:4 |
| [3] |
Chertemps T, Duportets L, Labeur C, et al. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(11): 4273-4278. DOI:10.1073/pnas.0608142104 |
| [4] |
Teerawanichpan P, Robertson A J, Xiao Q. A fatty acyl-CoA reductase highly expressed in the head of honey bee (Apis mellifera)involves biosynthesis of a wide range of aliphatic fatty alcohols. Insect Biochemistry and Molecular Biology, 2010, 40(9): 641-649. DOI:10.1016/j.ibmb.2010.06.004 |
| [5] |
Cuvillier O. Sphingosine in apoptosis signaling. Biochimica Et Biophysica Acta, 2003, 1585(2-3): 153-162. |
| [6] |
Hannun Y A, Obeid L M. Ceramide in the eukaryotic stress response. Trends in Cell Bioloy, 2000, 10(2): 73-80. DOI:10.1016/S0962-8924(99)01694-3 |
| [7] |
Hannun Y A, Obeid L M. The Ceramide-centric universe of lipid-mediated cell regulation:stress encounters of the lipid kind. Journal of Biolgical Chemistry, 2002, 277(29): 25847-25850. DOI:10.1074/jbc.R200008200 |
| [8] |
易杰群, 邹杰文, 张古忍. 昆虫脂肪酸及其脱饱和酶与耐寒性的关系概述. 环境昆虫学报, 2015, 37(1): 155-162. Yi J Q, Zou J W, Zhang G R. The relationship between the fatty acids and those desaturases and the cold tolerance in the insects. Journal of Environmental Entomology, 2015, 37(1): 155-162. |
| [9] |
Lehrke M, Pascual G, Glass C K, et al. Gaining weight:the keystone symposium on PPAR and LXR. Genes and Development, 2005, 19(15): 1737-1742. DOI:10.1101/gad.1341005 |
| [10] |
Iwanaga T, Tsutsumi R, Noritake J, et al. Dynamic protein palmitoylation in cellular signaling. Progress Lipid Research, 2009, 48(3-4): 117-127. DOI:10.1016/j.plipres.2009.02.001 |
| [11] |
Choi-Rhee E, Cronan JE. The biotin carboxylase-biotin carboxyl carrier protein complex of Escherichia coli acetyl-CoA carboxylase. Journal of Biological Chemistry, 2003, 278(33): 30806-30812. DOI:10.1074/jbc.M302507200 |
| [12] |
Nikolau B J, Ohlrogge J B, Wurtele E S. Plant biotin-containing carboxylases. Archives Biochemistry and Biophysics, 2003, 414(2): 211-222. DOI:10.1016/S0003-9861(03)00156-5 |
| [13] |
Parvy J-P, Napal L, Rubin T, et al. Drosophila melanogaster acetyl-CoA-Carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genetics, 2012, 8(8): e1002925. DOI:10.1371/journal.pgen.1002925 |
| [14] |
Tong L. Acetyl-coenzyme A carboxylase:crucial metabolic enzyme and attractive target for drug discovery. Cellular and Molecular Life Science Cmls, 2005, 62(16): 1784-1803. DOI:10.1007/s00018-005-5121-4 |
| [15] |
Waldrop G L, Holden H M, St Maurice M. The enzymes of biotin dependent CO2 metabolism:what structures reveal about their reaction mechanisms. Protein Science, 2012, 21(11): 1597-1619. DOI:10.1002/pro.v21.11 |
| [16] |
Lümmen P, Khajehali J, Luther K, et al. The cyclic keto-enol insecticide spirotetramat inhibits insect and spider mite acetyl-CoA carboxylases by interfering with the carboxyltransferase partial reaction. Insect Biochemistry and Molecular Biology, 2014, 55: 1-8. DOI:10.1016/j.ibmb.2014.09.010 |
| [17] |
Baker K D, Thummel C S. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metabolism, 2007, 6(4): 257-266. DOI:10.1016/j.cmet.2007.09.002 |
| [18] |
Gutierrez E, Wiggins D, Fielding B, et al. Specialized hepatocyte like cells regulate Drosophila lipid metabolism. Nature, 2007, 445(7125): 275-280. DOI:10.1038/nature05382 |
| [19] |
Sieber M H, Thummel C S. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metabolism, 2009, 10(6): 481-490. DOI:10.1016/j.cmet.2009.10.010 |
| [20] |
Qatanani M, Lazar M A. Mechanisms of obesity-associated insulin resistance:many choices on the menu. Genes and Development, 2007, 21(12): 1443-1455. DOI:10.1101/gad.1550907 |
| [21] |
Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochemical Journal, 2008, 414(1): 1-18. DOI:10.1042/BJ20080595 |
| [22] |
Alabaster A, Isoe J, Zhou G, et al. Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochemistry and Molecular Biology, 2011, 41(12): 946-955. DOI:10.1016/j.ibmb.2011.09.004 |
| [23] |
Abu-Elheiga L, Matzuk M M, Kordari P, et al. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(34): 12011-12016. DOI:10.1073/pnas.0505714102 |
| [24] |
Urbanski J M, Benoit J B, Michaud M R, et al. The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proceedings of the Royal Society B-Biological Sciences, 2010, 277(1694): 2683-2692. DOI:10.1098/rspb.2010.0362 |
| [25] |
Zhang Y X, Ge L Q, Jiang Y P, et al. RNAi knockdown of acetyl-CoA carboxylase gene eliminates jinggangmycin-enhanced reproduction and population growth in the brown planthopper, Nilaparvata lugens. Scientific Reports, 2015, 5: 15360. DOI:10.1038/srep15360 |
| [26] |
Wheeler W M. Concerning the "blood-tissue" of the insecta. Psyche:A Journal of Entomology, 2008, 6(193): 233-236-253-258. |
| [27] |
Hoffmann A A, Harshman L G. Desiccation and starvation resistance in Drosophila:patterns of variation at the species, population and intrapopulation levels. Heredity, 1999, 83(6): 637-643. DOI:10.1046/j.1365-2540.1999.00649.x |
| [28] |
Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals:Their regulation and roles in metabolism. Progress in Lipid Research, 2006, 45(3): 237-249. DOI:10.1016/j.plipres.2006.01.004 |
| [29] |
Finzel K, Lee D J, Burkart M D. Using modern tools to probe the structure-function relationship of fatty acid synthases. Chembiochem, 2015, 16(4): 528-547. DOI:10.1002/cbic.201402578 |
| [30] |
Chintapalli V R, Wang J, Dow J A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nature Genetics, 2007, 39(6): 715-720. DOI:10.1038/ng2049 |
| [31] |
Li L, Jiang Y P, Liu Z Y, et al. Jinggangmycin increases fecundity of the brown planthopper, Nilaparvata lugens (Stål) via fatty acid synthase gene expression. Journal of Proteomics, 2016, 130: 140-149. DOI:10.1016/j.jprot.2015.09.022 |
| [32] |
Robich R M, Denlinger D L. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(44): 15912-15917. DOI:10.1073/pnas.0507958102 |
| [33] |
Zhou G, Miesfeld R L. Energy metabolism during diapause in Culex pipiens mosquitoes. Journal of Insect Physiology, 2009, 55(1): 40-46. DOI:10.1016/j.jinsphys.2008.10.002 |
| [34] |
Sim C, Denlinger D L. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipens. Physiological Genomics, 2009, 39(3): 202-209. DOI:10.1152/physiolgenomics.00095.2009 |
| [35] |
Liu W, Li Y, Zhu F, et al. Juvenile hormone facilitates the antagonism between adult reproduction and diapause through the methoprene tolerant gene in the female Colaphellus bowringi. Insect Biochemistry and Molecular Biology, 2016, 74: 50-60. DOI:10.1016/j.ibmb.2016.05.004 |
| [36] |
Tan Q Q, Liu W, Zhu F, et al. Fatty acid synthase 2 contributes to diapause preparation in a beetle by regulating lipid accumulation and stress tolerance genes expression. Scientific Reports, 2017, 7: 40509. DOI:10.1038/srep40509 |
| [37] |
Leonard A E, Pereira S L, Sprecher H, et al. Elongation of long-chain fatty acids. Progress in Lipid Research, 2004, 43(1): 36-54. DOI:10.1016/S0163-7827(03)00040-7 |
| [38] |
Szafer-Glusman E, Giansanti M G, Nishihama R, et al. A role for very-long-chain fatty acids in furrow ingression during cytokinesis in Drosophila spermatocytes. Current Biology, 2008, 18(18): 1426-1431. DOI:10.1016/j.cub.2008.08.061 |
| [39] |
Ng W C, Chin J S, Tan K J, et al. The fatty acid elongase Bond is essential for Drosophila sex pheromone synthesis and male fertility. Nature Communications, 2015, 6: 8263. DOI:10.1038/ncomms9263 |
| [40] |
Jung A, Hollmann M, Schäfer M A. The fatty acid elongase NOA is necessary for viability and has a somatic role in Drosophila sperm. Journal of Cell Science, 2007, 120(16): 2924-2934. DOI:10.1242/jcs.006551 |
| [41] |
Chertemps T, Duportets L, Labeur C, et al. A new elongase selectively expressed in Drosophila male reproductive system. Biochemical and Biophysical Research Communications, 2005, 333(4): 1066-1072. DOI:10.1016/j.bbrc.2005.06.015 |
| [42] |
Falcón T, Ferreira-Caliman J F, Franco Nunes F M, et al. Exoskeleton formation in Apis mellifera:Cuticular hydrocarbons profiles and expression of desaturase and elongase genes during pupal and adult development. Insect Biochemistry and Molecular Biology, 2014, 50(11): 68-81. |
| [43] |
Zheng T X, Li H S, Han N, et al. Functional characterization of two elongases of very long-chain fatty acid from Tenebrio molitor L. (Coleoptera:Tenebrionidae). Scientific Reports, 2017, 7: 10990. DOI:10.1038/s41598-017-11134-y |
| [44] |
Haritos V S, Horne I, Damcevski K, et al. Unexpected functional diversity in the fatty acid desaturases of the flour beetle Tribolium castaneum and identification of key residues determining activity. Insect Biochemistry and Molecular Biology, 2014, 51(7): 62-70. |
| [45] |
Sperling P, Ternes P, Zank T K, et al. The evolution of desaturases. Prostaglandins Leukotrienes and Essential Fatty Acids, 2003, 68(2): 73-95. DOI:10.1016/S0952-3278(02)00258-2 |
| [46] |
Wicker-Thomas C, Henriet C, Dallerac R. Partial characterization of a fatty acid desaturase gene in Drosophila melanogaster. Insect Biochemistry and Molecular Biology, 1997, 27(11): 963-972. DOI:10.1016/S0965-1748(97)00077-5 |
| [47] |
Dallerac R, Labeur C, Jallon J M, et al. A △9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(17): 9449-9454. DOI:10.1073/pnas.150243997 |
| [48] |
Marcillac F, Grosjean Y, Ferveur J F. A single mutation alters production and discrimination of Drosophila sex pheromones. Proceedings of the Royal Society B-Biological Sciences, 2005, 272(1560): 303-309. DOI:10.1098/rspb.2004.2971 |
| [49] |
Houot B, Bousquet F, Ferveur JF. The consequences of regulation of desat1 affects both pheromone emission and detection in Drosophila melanogaster. Genetics, 2010, 185(4): 1297. DOI:10.1534/genetics.110.117226 |
| [50] |
Bonsquet F, Nojima T, Houot B, et al. Expression of a desaturase gene, desat1, in neural and nonneural tissues separately affects perception and emission of sex pheromones in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(1): 249-254. DOI:10.1073/pnas.1109166108 |
| [51] |
Shirangi T R, Dufour H D, Williams T M, et al. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biology, 2009, 7(8): e1000168. DOI:10.1371/journal.pbio.1000168 |
| [52] |
Ng S H, Shankar S, Shikichi Y, et al. Pheromone evolution and sexual behavior in Drosophila are shaped by male sensory exploitation of other males. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(8): 3056-3061. DOI:10.1073/pnas.1313615111 |
| [53] |
Wang Y, da Cruz T C, Pulfemuller T, et al. Inhibition of fatty acid desaturases in Drosophila melanogaster larvae blocks feeding and developmental progression. Archives of Insect Biochemistry and Physiology, 2016, 92(1): 1-18. DOI:10.1002/arch.21286 |
| [54] |
Albre J, Lienard M A, Sirey T M, et al. Sex pheromone evolution is associated with differential regulation of the same desaturase gene in two genera of leafroller moths. PLoS Genetics, 2012, 8(1): e1002489. DOI:10.1371/journal.pgen.1002489 |
| [55] |
Albre J, Steinwender B, Newcomb R D. The evolution of desaturase gene regulation involved in sex pheromone production in leafroller moths of the genus Planotortrix. Journal of Heredity, 2012, 104(5): 627-638. |
| [56] |
Hagström ÅK, Albre J, Tooman L K, et al. A novel fatty acyl desaturase from the pheromone glands of Ctenopseustis obliquana and C. herana with specific Z5-desaturase activity on myristic acid. Journal of Chemical Ecology, 2014, 40(1): 63-70. DOI:10.1007/s10886-013-0373-1 |
| [57] |
Roelofs W L, Liu W, Hao G, et al. Evolution of moth sex pheromones via ancestral genes. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(21): 13621-13626. DOI:10.1073/pnas.152445399 |
| [58] |
Ding B J, Carraher C, Lofstedt C. Sequence variation determining stereochemistry of a △11 desaturase active in moth sex pheromone biosynthesis. Insect Biochemistry and Molecular Biology, 2016, 74: 68-75. DOI:10.1016/j.ibmb.2016.05.002 |
| [59] |
Fujii T, Yasukochi Y, Rong Y, et al. Multiple △11-desaturase genes selectively used for sex pheromone biosynthesis are conserved in Ostrinia moth genomes. Insect Biochemistry and Molecular Biology, 2015, 61: 62-68. DOI:10.1016/j.ibmb.2015.04.007 |
| [60] |
Buček A, Matoušková P, Vogel H, et al. Evolution of moth sex pheromone composition by a single amino acid substitution in a fatty acid desaturase. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(41): 12586-12591. DOI:10.1073/pnas.1514566112 |
| [61] |
Matousková P, Pichová I, Svatoá A. Functional characterization of a desaturase from the tobacco hornworm moth (Manduca sexta) with bifunctional Z11-and 10, 12-desaturase activity. Insect Biochemistry and Molecular Biology, 2007, 37(6): 601-610. DOI:10.1016/j.ibmb.2007.03.004 |
| [62] |
Fujii T, Suzuki M G, Katsuma S, et al. Discovery of a disused desaturase gene from the pheromone gland of the moth Ascotis selenaria, which secretes an epoxyalkenyl sex pheromone. Biochemical and Biophysical Research Communications, 2013, 441(4): 849. DOI:10.1016/j.bbrc.2013.10.143 |
| [63] |
Haritos V S, Horne I, Damcevski K, et al. The convergent evolution of defensive polyacetylenic fatty acid biosynthesis genes in soldier beetles. Nature Communications, 2012, 3(4): 1150. |
| [64] |
Buček A, Vogel H, Matoušková P, et al. The role of desaturases in the biosynthesis of marking pheromones in bumblebee males. Insect Biochemistry and Molecular Biology, 2013, 43(8): 724-731. DOI:10.1016/j.ibmb.2013.05.003 |
| [65] |
Badouin H, Belkhir K, Gregson E, et al. Transcriptome characterisation of the ant Formica exsecta with new insights into the evolution of desaturase genes in social Hymenoptera. PLoS One, 2013, 8(7): e68200. DOI:10.1371/journal.pone.0068200 |
| [66] |
Chen Q M, Cheng D J, Liu S P, et al. Genome-wide identification and expression profiling of the fatty acid desaturase gene family in the silkworm, Bombyx mori. Genetics and Molecular Research, 2014, 13(2): 3747-3760. DOI:10.4238/2014.May.13.2 |
| [67] |
Lee D-W, Kim Y, Koh KH. RNA interference of PBAN receptor suppresses expression of two fatty acid desaturases in female Plutella xylostella. Journal of Asia-Pacific Entomology, 2011, 14(4): 405-410. DOI:10.1016/j.aspen.2011.05.004 |
| [68] |
Köblös G, Dankó T, Sipos K, et al. The regulation of △11-desaturase gene expression in the pheromone gland of Mamestra brassicae(Lepidoptera; Noctuida)during pheromonogenesis. General and Comparative Endocrinology, 2015, 19(2): 217-227. |
| [69] |
Lassance J M, Groot A T, Liénard M A, et al. Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature, 2010, 466(7305): 486-489. DOI:10.1038/nature09058 |
| [70] |
Moto K, Yoshiga T, Yamamoto M, et al. Pheromone gland-specific fatty-acyl reductase of the silkmoth, Bombyx mori. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(16): 9156-9161. DOI:10.1073/pnas.1531993100 |
| [71] |
Antony B, Fujii T, Moto K, et al. Pheromone-gland-specific fatty-acyl reductase in the adzuki bean borer, Ostrinia scapulalis (Lepidoptera:Crambidae). Insect Biochemistry and Molecular Biology, 2009, 39(2): 90-95. DOI:10.1016/j.ibmb.2008.10.008 |
| [72] |
Liénard M A, Hagstöm Å K, Lassance J M, et al. Evolution of multicomponent pheromone signals in small ermine moths involves a single fatty-acyl reductase gene. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(24): 10955-10960. DOI:10.1073/pnas.1000823107 |
| [73] |
Hagström Å K, Liénard M A, Groot A T, et al. Semi-selective fatty acyl reductases from four heliothine moths influence the specific pheromone composition. PLoS One, 2012, 7(5): e37230. DOI:10.1371/journal.pone.0037230 |
| [74] |
Carot-Sans G, Muñoz L, Piulachs M D, et al. Identification and characterization of a fatty acyl reductase from a Spodoptera littoralis female gland involved in pheromone biosynthesis. Insect Molecular Biology, 2015, 24(1): 82-92. DOI:10.1111/imb.2015.24.issue-1 |
| [75] |
Li X L, Zheng T X, Zheng X W, et al. Molecular characterization of two fatty acyl-CoA reductase genes from Phenacoccus solenopsis (Hemiptera:Pseudococcidae). Journal of Insect Science, 2016, 16(1): 49. DOI:10.1093/jisesa/iew038 |
 2017, Vol. 37
2017, Vol. 37




